Abstract
Background/Aim: To predict survival outcomes of different patients with the same stage of disease is difficult. The possible correlation between 18F-fluorodeoxyglucose (18F-FDG) uptake parameters and survival outcomes was investigated in oral squamous cell carcinoma patients by multivariate analysis adjusted for the pathological stage according to the 8th edition of the tumor-node-metastasis (TNM) classification of the Union for International Cancer Contro. Patients and Methods: 18F-FDG-uptake parameters of 28 patients were assessed by positron emission tomography with computed tomography (PET/CT). Results: A peak of standardized uptake value of primary tumor (p-SUVpeak) of ≥14.1 was significantly correlated with shorter overall survival by univariate and multivariate analyses adjusted for the pathological TNM stage. A p-SUVpeak of ≥14.1 was significantly associated with shorter local recurrence-free survival and disease-free survival. Conclusion: A higher p-SUVpeak on pretreatment 18F-FDG-PET/CT is a prognostic parameter of identifying lower survival outcomes.
Keywords: Oral squamous cell carcinoma, 18F-FDG-PET/CT, SUVpeak, overall survival, UICC8th
The tumor-node-metastasis (TNM) staging system is applied worldwide as a predictor of various carcinomas, including oral squamous cell carcinoma (OSCC), but it is difficult to predict survival outcomes of different patients with the same stage (1,2). Parameters of 18F-2-deoxyglucose (18F-FDG) uptake, as assessed by positron emission tomography with computed tomography (PET/CT), in head and neck cancer, were shown to be associated with overall survival (OS) by multivariate analysis adjusted for TNM stage of the 7th edition of the Union for International Cancer Control (UICC7th) (3,4). However, no study has yet investigated the association between survival outcomes and 18F-FDG-uptake parameters in OSCC by multivariate analysis adjusted for TNM stage based on the 8th edition of the Union for International Cancer Control (UICC8th) published in 2017 (1).
In OSCC, the presence of pathological lymph node metastasis (pN+) is the most classical predictor, and the presence of extranodal extension (ENE+) is widely accepted as being predictive of poor survival (5,6). The maximum standardized uptake value (SUVmax), which is commonly used as 18F-FDG-uptake parameter, was reported to predict the risk of both pN+ and ENE+ (7,8). However, usefulness of volumetric 18F-FDG-uptake parameters, such as metabolic tumor volume (MTV), total legion glycolysis (TLG), peak of standardized uptake value (SUVpeak), to predict both pN+ and ENE+ remains unknown.
Therefore, the aim of this study was to identify associations between 18F-FDG-uptake parameters and survival outcomes in OSCC by univariate and multivariate analyses adjusted for the pathological TNM stage based on UICC8th, and determine whether 18F-FDG-uptake parameters, including volumetric parameters, are correlated with either pN+ or ENE+.
Patients and Methods
Patients and clinicopathological parameters. Both primary tumor surgery and neck dissection without preoperative treatment underwent in 50 patients with newly diagnosed OSCC with clinical lymph node metastasis at the Aichi Cancer Center Hospital between January 2008 and July 2013, as previously described (2,9). Among these 50 patients, 28 received pretreatment 18F-FDG-PET/CT at East Nagoya Imaging Diagnosis Center were enrolled in this study. The study was approved by the Institutional Review Board, and informed consent was obtained from each patient prior to treatment examination. The procedures of initial TNM staging based on UICC7th, treatments, pathological examinations, follow-up as well as the assessments of pathological parameters for tumor size and thickness, pathological tumor volume (PTV), lymph node density are described elsewhere (2,9,10). Depth of invasion was assessed from deepest point of invasion to horizon of the adjacent mucosal basement membrane according to AJCC8th staging manual (11). Restaging was performed both from pathological reports and depth of invasion based on UICC8th, as described by Sano (12). The clinicopathological parameters are listed in Table I.
Table I. Clinicopathological parameters of 28 patients with oral squamous cell carcinoma .
OSCC: Oral squamous cell carcinoma; UICC7th: the 7th edition of Union for International Cancer Control; ENE: extranodal extension; UICC8th: the 8th edition of Union for International Cancer Control.
Image assessment. The procedures of both 18F-FDG-PET/CT scan (Biograph True Point PET/CT/40 with True V: Siemens Health Medical Solutions Inc., Malvern, PA, USA) and semiquantitative evaluation for 18F-FDG-uptake parameters (Advantage Workstation 4.6 software program the PET VCAR: GE Healthcare, Chalfont, UK) were described previously (10). The mean±standard deviation (SD) of blood sugar level at staging was 114.0±34.3 mg/dl, and the mean±SD duration from 18F-FDG-PET/CT to surgery was 19.1±9.1 days. The SUVmax, SUVpeak, MTV, TLG of the primary tumor were defined as p-SUVmax, p-SUVpeak, p-MTV, p-TLG, respectively. The p-MTV and p-TLG were calculated by adopting a threshold fraction of 45% of the p-SUVmax from the volumetric region of interest on three-dimensional images. The p-SUVpeak was calculated as the average standardized uptake value within a 1-cm3 spherical volumetric region of interest that included the maximum number of pixels.
Diagnosis of pN+ and ENE+. The 18F-FDG-uptake parameters of lymph nodes with pN+ and/or ENE+ were reassessed based on a report by Dequanter et al. (7) with minor modifications. Regions of pN+ and/or ENE+ were recorded in reference to the system of the Japan Neck dissection Study Group and classified based on submental and submandibular, jugular, or posterior triangle lymph nodes (5). A site of abnormal accumulation of 18F-FDG on PET/CT was interpreted as the presence of lymph node metastasis, while normal accumulation was considered as metastasis-free. The SUVmax, SUVpeak, MTV, TLG of the lymph node were defined as ln-SUVmax, ln-SUVpeak, ln-MTV, ln-TLG, respectively. For the semiquantitative analysis, the 18F-FDG-uptake parameters of the lymph node of interest, which included ln-SUVmax, ln-SUVpeak, ln-MTV, ln-TLG, were measured from the site of abnormal accumulation. If there were multiple lymph nodes within a certain region, the node with the highest ln-SUVmax was selected. At each region, all PET/CT results were compared with pathological results, which were considered as gold standard.
Statistical analysis. Statistical analyses were performed using JMP software (version 9; SAS Institute: Cary, NC, USA). Simple regression analysis was used to identify correlations between PTV and 18F-FDG-uptake parameters of primary tumor. OS was defined as the duration from FDG-PET/CT to death or last contact and assessed by Kaplan–Meier method. OS based on pathological stage of UICC8th (I-IVA, IVB) were compared by log-rank test. The correlations between OS and the cut-off values for various 18F-FDG-uptake parameters of the primary tumor were identified by log-rank test (2,9). Multivariate analysis adjusting pathological stage of UICC8th (IVB; I-IVA) was performed to identify correlations between 18F-FDG-uptake parameters of primary tumor and OS by a Cox’s proportional hazards model. The relationships between p-SUVpeak groups (≥14.1; <14.1) and clinicopathological parameters were assessed by Mann-Whitney U- or chi-squared test. The Kaplan-Meier method was used to identify differences between the p-SUVpeak groups in local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS). The area under the curve (AUC) of receiver-operating characteristic curve, sensitivity, 1-specificity were calculated to detect the best cut-off values for 18FDG-uptake parameters of lymph node compare pN+ versus pathological lymph node metastasis (pN-), and ENE+ versus ENE- by logistic regression analysis. p<0.05 was considered statistically significant.
Results
PTV and 18F-FDG-uptake parameters. Among all patients, the mean±SD values of PTV, p-SUVmax, p-SUVpeak, p-MTV, p-TLG were 8.87±9.06 cm3, 17.8±6.62 g/ml, 12.1±4.62 g/ml, 5.64±4.70 cm3, 66.1±61.0 g, respectively. PTV was significantly correlated with both p-MTV (p<0.01) and p-TLG (p<0.01), but not with either p-SUVmax or p-SUVpeak.
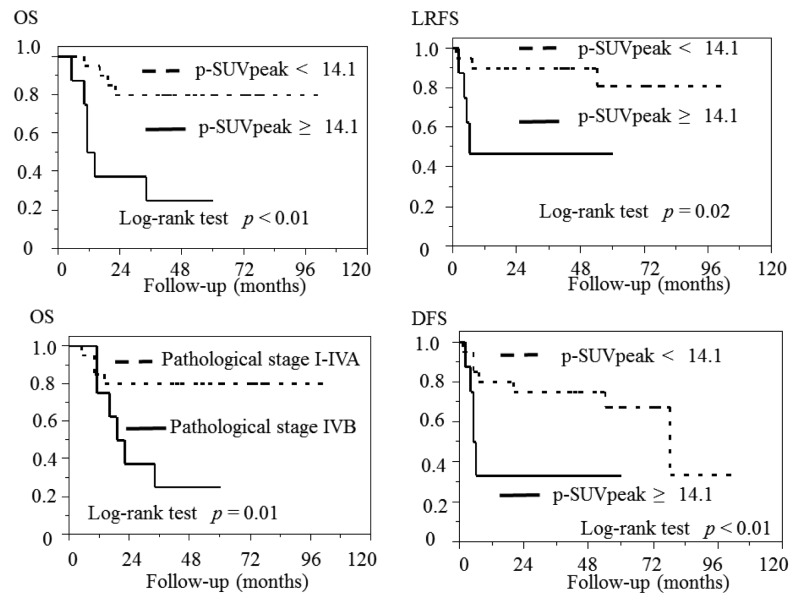
Clinical course. The mean±SD duration of follow-up among the whole population, the 18 patients who survived, the 10 patients who died was 45.3±27.0, 62.1±17.0, 15.2±8.23 months, respectively. The 3-year rates of OS, LRFS, RRFS, DMFS, DFS were 64.3%, 78.3%, 71.1%, 76.5%, 63.8%, respectively. Pathological stage IVB of UICC8th was significantly correlated with shorter OS than pathological stage I-IVA of UICC8th (p=0.01).
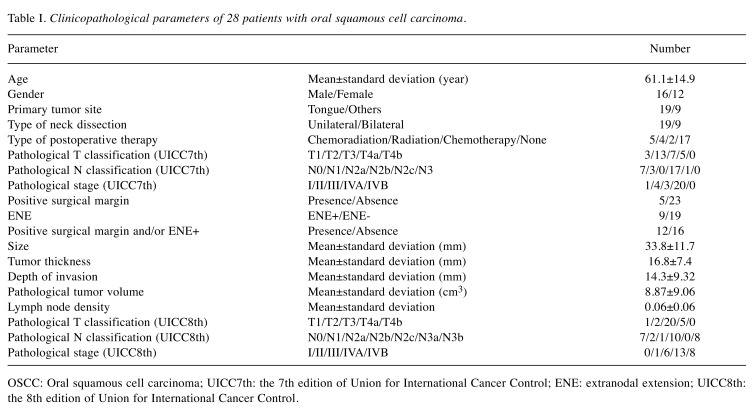
Cut-off values of 18F-FDG-uptake parameters. The lowest p values were p-SUVmax=19.5 (p=0.03), p-SUVpeak=14.1 (p<0.01), p-MTV=11 (p=0.049), p-TLG=110 (p=0.049). (Figure 1).
Figure 1. Probability values of OS analysis using different cut-off levels for p-SUVmax, p-SUVpeak, p-MTV, p-TLG values. OS: Overall survival;p-SUVmax: maximum standardized uptake value of primary tumor; p-SUVpeak: peak standardized uptake value of primary tumor; p-MTV: metabolictumor volume of primary tumor; p-TLG: total lesion glycolysis of primary tumor.
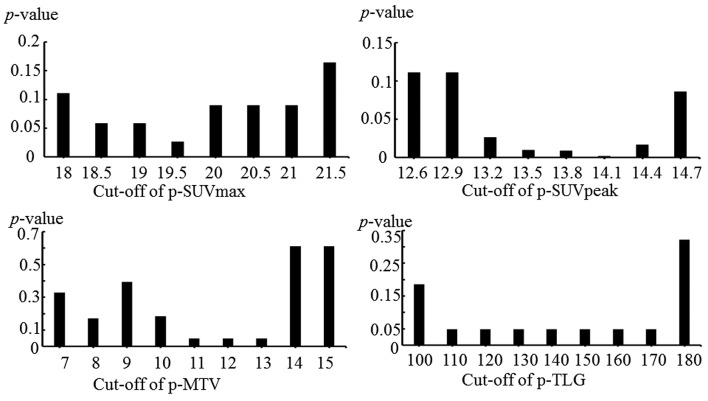
Multivariate OS analysis. Multivariate analysis adjusted for 18F-FDG-uptake parameters and pathological stage of UICC8th, p-SUVpeak ≥14.1 (p=0.047) was significantly associated with shorter OS, while p-SUVmax of ≥19.5, p-MTV≥11, p-TLG≥110 were not (Table II).
Table II. Multivariate analysis of overall survival by a Cox’s proportional hazards model.
UICC8th: The 8th edition of Union for International Cancer Control; p-SUVmax: maximum of standardized uptake value of primary tumor; p- SUVpeak: peak of standardized uptake value of primary tumor; p-MTV: metabolic tumor volume of primary tumor; p-TLG: total lesion glycolysis of primary tumor.
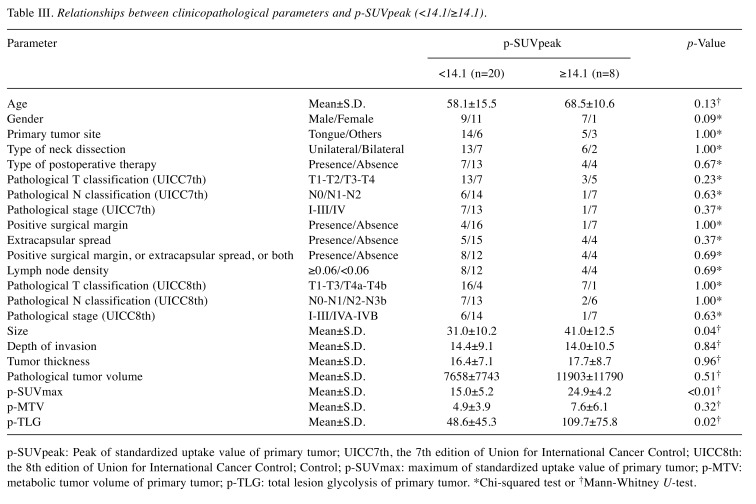
The p-SUVpeak groups. The relations between clinicopathological parameters of the two groups (p-SUVpeak ≥14.1; <14.1) are shown in Table III. A p-SUVpeak ≥14.1 was significantly associated with larger tumor size (p=0.04), greater p-SUVmax (p<0.01) and p-TLG (p=0.02). A p-SUVpeak ≥14.1 was significantly associated with shorter LRFS (p=0.02) and DFS (p<0.01). However, there were no significant differences in RRFS or DMFS. The Kaplan–Meier curves are shown in Figure 2.
Table III. Relationships between clinicopathological parameters and p-SUVpeak (<14.1/≥14.1).
p-SUVpeak: Peak of standardized uptake value of primary tumor; UICC7th, the 7th edition of Union for International Cancer Control; UICC8th: the 8th edition of Union for International Cancer Control; Control; p-SUVmax: maximum of standardized uptake value of primary tumor; p-MTV: metabolic tumor volume of primary tumor; p-TLG: total lesion glycolysis of primary tumor. *Chi-squared test or †Mann-Whitney U-test.
Figure 2. Pathological stage of IVB based on the UICC8th was associated with significantly shorter OS (p=0.01), and p-SUVpeak of ≥14.1 was associatedwith significantly lower OS (p<0.01), LRFS (p=0.02), DFS (p<0.01). UICC8th: The 8th edition of the Union for International Cancer Control, p-SUVpeak: peak standardized uptake value of primary tumor; OS: overall survival; LRFS: local recurrence-free survival; DFS: disease-free survival.
pN+ and ENE+. The relations between the 18F-FDG-uptake parameters and pathological findings were assessed in 88 cervical regions. The numbers of regions classified as pN+ and ENE+ totaled 31 and 11, respectively. The numbers of regions not classified as pN+ and ENE+ totaled 57 and 77, respectively. The best cut-off values to detect pN+ were ln-SUVmax=3.33 (p<0.01, AUC =0.84), ln-SUVpeak=1.82 (p<0.01, AUC=0.82), ln-MTV=0.27 (p=0.43, AUC=0.66), ln-TLG=1.03 (p<0.01, AUC=0.74). The best cut-off values to detect ENE+ were ln-SUVmax=3.61 (p<0.01, AUC=0.80), ln-SUVpeak=1.07 (p<0.01, AUC=0.79), ln-MTV=0.37 (p=0.55, AUC=0.66), ln-TLG=3.1 (p=0.23, AUC=0.74). The highest AUC to detect pN+ and ENE+ were ln-SUVmax=3.33 (sensitivity=80.7%, specificity=77.2%) and ln-SUVmax=3.61 (sensitivity=81.8%, specificity=67.5%), respectively.
Discussion
The present study showed, for the first time, significant associations between high p-SUVpeak values and lower OS in OSCC by using multivariate analysis adjusted for pathological stage based on UICC8th. Also, high p-SUVpeak was significantly correlated with reduced LRFS and DFS rates.
In head and neck cancer, including OSCC, 18F-FDG-uptake parameters were significantly correlated with OS (3,4,10). For example, high p-SUVpeak was predictive of shorter OS in OSCC by multivariate analysis adjusting pathological stage of UICC7th (4). Our findings of a significant association between 18F-FDG-uptake parameters and OS are consistent with these studies (3,4,10). Moreover, the present study identified correlations between survival and 18F-FDG uptake parameters in OSCC by multivariate analysis adjusting TNM stage of UICC8th. The present results suggest that pre-treatment SUVpeak is a predictor of shorter OS.
The present findings also demonstrated that greater tumor size, p-SUVmax, p-MTV, p-TLG, which were poor predictors of OSCC (4,13,14), were significantly correlated with higher p-SUVpeak. Moreover, higher SUVpeak was significantly correlated with worse LRFS and DFS in this study, and we suggest that when SUVpeak is relatively high, more aggressive treatment strategies such as postoperative chemoradiotherapy are needed to improve survival outcomes.
In esophageal cancer, ln-MTV was significantly correlated with pN+ (15). In head and neck cancer, 18F-FDG-uptake was used to evaluate treatment response of lymph node metastasis after chemoradiotherapy (16), and the significant association between 18F-FDG-uptake and chemosensitivity for cisplatin was reported (17). To the best of our knowledge, the usefulness of volumetric 18F-FDG-uptake parameters to predict pN+ and ENE+ has not been investigated. In this study, ln-SUVmax in OSCC had the highest AUC=0.82 to detect both pN+ and ENE+ than ln-MTV, ln-TLG, ln-SUVpeak.
The limitations of the present study include its retrospective design and relatively small number of subjects; thus, prospective analysis with a larger number of subjects is needed to verify these results.
Conclusion
A high p-SUVpeak was correlated with shorter OS in OSCC by multivariate analysis adjusting pathological stage of UICC8th.
Conflicts of Interest
The Authors declare no conflict of interest regarding this study.
Acknowledgements
This study was supported from JSPS KAKENHI Grant Number of 16K11253.
References
- 1.Brierley J, Gospodarowicz MD, Wittekind C. TNM classification of malignant tumors. International union against cancer. Oxford, England: Wiley-Blackwell. 2016;8th Edition [Google Scholar]
- 2.Suzuki H, Beppu S, Hanai N, Hirakawa H, Hasegawa Y. Lymph node density predicts lung metastasis in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2016;54:213–218. doi: 10.1016/j.bjoms.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Yabuki K, Sano D, Shiono O, Arai Y, Takahashi H, Chiba Y, Tanabe T, Nishinura G, Takahashi M, Taguchi T, Kaneta T, Hata M, Oridate N. Prognostic significance of metabolic tumor volume in patients with piriform sinus carcinoma treated by radiotherapy with or without concurrent chemotherapy. Head Neck. 2016;38:1666–1671. doi: 10.1002/hed.24488. [DOI] [PubMed] [Google Scholar]
- 4.Abgral R, Keromnes N, Robin P, Le Roux PY, Bourthis D, Palard X, Rousset J, Valette G, Marianowski R, Salaün PY. Prognostic value of volumetric parameters measured by 18F-FDG-PET/CT in patients with head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:659–667. doi: 10.1007/s00259-013-2618-1. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa Y, Saikawa M. Update on the classification and nomenclature system for neck dissection: revisions proposed by the Japan Neck Dissection Study Group. Int J Clin Oncol. 2010;15:5–12. doi: 10.1007/s10147-009-0019-z. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, Myers JN. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–70. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 7.Dequanter D, Shahla M, Aubert C, Deniz Y, Lothaire P. Prognostic value of FDG PET/CT in head and neck squamous cell carcinomas. Onco Targets Ther. 2015;8:2279–2283. doi: 10.2147/OTT.S85479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Z, Duan Z, Pan W, Wu C, Jia Y, Han B, Li C. Predicting extracapsular spread of head and neck cancers using different imaging techniques: a systemic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:413–421. doi: 10.1016/j.ijom.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Mukoyama N, Suzuki H, Hanai N, Sone M, Hasegawa Y. Pathological tumor volume predicts survival outcome in oral squamous cell carcinoma. Oncol Lett. 2018 doi: 10.3892/ol.2018.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Nishio M, Nakanishi H, Hanai N, Hirakawa H, Kodaira T, Tamaki T, Hasegawa Y. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol Lett. 2016;12:1493–1500. doi: 10.3892/ol.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin BA, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC cancer staging manual. Springer, New York. 2016;8th Edition [Google Scholar]
- 12.Sano D, Yabuki K, Arai Y, Tanabe T, Chiba Y, Nishimura G, Takahashi H, Yamanaka S, Oridate N. The applicability of new TNM classification for humanpapilloma virus-related oropharyngeal cancer in the 8th edition of the AJCC/UICC TNM staging system in Japan: A single-center study. Auris Nasus Larynx. 2018;45:558–565. doi: 10.1016/j.anl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Achmad A, Higuchi T, Arisaka Y, Yokoo H, Yokoo S, Tsushima Y. Effects of intratumoral inflammatory process on 18F-FDG uptake: pathological and comparative study with 18F-fluoro-α-methyltyrosine PET/CT in oral squamous cell carcinoma. J Nucl Med. 2015;56:16–21. doi: 10.2967/jnumed.114.144014. [DOI] [PubMed] [Google Scholar]
- 14.Heiduschka G, Virk SA, Palme CE, Ch’ng S, Eliot M, Gupta R, Clark J. Margin to tumor thickness ratio – A predictor of local recurrence and survival in oral squamous cell carcinoma. Oral Oncol. 2016;55:49–54. doi: 10.1016/j.oraloncology.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 15.I HS, Kim SJ, Kim IJ, Kim K. Predictive value of metabolic tumor volume measured by 18F-FDG PET for regional lymph node status in patients with esophageal cancer. Clin Nucl Med. 2012;37:442–446. doi: 10.1097/RLU.0b013e318238f703. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura G, Matsuda H, Taguchi T, Takahashi M, Komatsu M, Sano D, Sakuma N, Arai Y, Takahashi H. Treatment evaluation of metastatic lymph nodes after concurrent chemoradiotherapy in patients with head and neck squamous cell carcinoma. Anticancer Res. 2012;32:595–600. [PubMed] [Google Scholar]
- 17.Suzuki H, Nishio N, Hanai N, Hirakawa H, Tamaki T, Hasegawa Y. Correlation between 18F-FDG-uptake and in vitro chemosensitivity of cisplatin in head and neck cancer. Anticancer Res. 2015;35:1009–1016. [PubMed] [Google Scholar]