Abstract
Background/Aim: The treatment of human glioma tumor is still an unmet medical need. Natural products are always promising resources for discovery of anticancer drugs. Lauryl gallate (LG) is one of the derivatives of gallic acid, widely present in plants, that has been shown to induce anticancer activities in many human cancer cell lines; however, it has not been studied in human glioma cell lines. Thus, the effects of LG on human glioblastoma U87 cells were investigated in the present in vitro study. Materials and Methods: Cell morphology and viability were examined by phase-contrast microscopy. Annexin V/Propidium iodide (PI) double staining were performed and assayed by flow cytometry to confirm that viable cell number reduction was due to the induction of apoptosis. Furthermore, U87 cells were exposed to LG in various concentrations and were analyzed by caspase activity assay. To further confirm that LG induced apoptotic cell death, the expression of apoptosis-associated proteins in LG-treated U87 cells was tested by western blot. Results: LG induced morphological changes and decreased viability in U87 cells. Annexin V/PI double staining revealed that LG induced apoptotic cell death in U87 cells in a dose-dependent manner. The increased activities of caspase-2, -3, -8 and -9 demonstrated that LG induced U87 cell apoptosis through a caspase-dependent pathway. In terms of molecular level, LG increased pro-apoptotic proteins Bax and Bak and decreased anti-apoptotic protein Bcl-2 in U87 cells. Furthermore, LG also suppressed the expression of p-Akt, Pak1, Hif-1α and Hif-2α, β-catenin and Tcf-1 in U87 cells. Conclusion: These results suggest that LG induced apoptotic cell death via the caspase-dependent pathway in U87 cells
Keywords: Lauryl gallate, apoptotic cell death, caspase pathway, human glioblastoma U87 cells
Glioma is recognized as the most common primary tumor of the central nervous system, comprising about 50~60% of malignant brain tumors (1). Glioblastoma multiforme (GBM) is the most common and most malignant type of glioma in adults (2). Patients with GBM have a median survival time of 1 year, while <5% of patients survive for 5 years, worldwide (3). Currently, the standard treatment of GBM includes surgery, radiation therapy, chemotherapy, or other adjuvant therapy or combinations of radiotherapy and chemotherapy or combined modalities (4-6). The median survival after brain tumor resection is 8-13 months. However, surgery with radiotherapy and chemotherapy provided significant benefit in median overall survival (21.3 months). Despite many advances in brain tumor therapy, the maximal benefit of treatment is limited and almost all patients relapse (7,8). Thus, many investigators are focused on discovering new compounds for the treatment of GBM, particularly ingredients derived from natural products.
It is well accepted that compounds capable of inducing cancer cell death by apoptosis would be the best strategy for anticancer therapy. Apoptosis is a normal process of programmed cell death that includes a cascade of events leading to signal transduction (9), cell shrinkage and nuclei condensation (10,11), and degradation of cellular protein and DNA (12). It is well documented that apoptosis is activated via two major routes, the death receptor or extrinsic pathway and the mitochondrial or intrinsic pathway; however, mitochondria play a critical role in the commitment of cells to apoptosis in both pathways (13). So far, many natural compounds have been identified to induce pro-apoptotic effects, and they could be considered as promising candidates for novel cancer therapeutics (14-16).
Lauryl gallate (LG) is a derivative of gallic acid, a natural plant triphenol. LG acts as an antioxidant via inhibiting xanthine oxidase and subsequently preventing the generation of superoxide radicals (17), as an anti-bacterial factor through inhibiting the growth of Gram-positive Bacillus subtilis (18), and also as anti-viral against African swine fever virus in Vero cells (19). It is noteworthy that LG has only limited toxicity to normal cells, and thus, it has been used as an antioxidant food additive since long (20). Previous in vivo studies have shown that LG not only prevents the formation of dimethylbenzanthracene-induced skin tumours, but also selectively kills tumor cells on established tumours in mice (21).
However, there is no report on the effect of LG in human brain tumor cells. Thus, in the present study, LG was investigated as a potential therapeutic agent for human brain tumor. In particular, the induction of cell apoptosis was examined in U87 human glioblastoma cells treated with LG in vitro. The results demonstrated that LG decreased the total viable cell number via the induction of apoptotic cell death through mitochondrial pathways and the activation of caspase-3.
Materials and Methods
Chemicals and reagents. LG, propidium iodide (PI), Tris-HCl, trypsin, trypan blue and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). LG was dissolved in DMSO as a stock for further experiments. Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), fetal bovine serum (FBS) and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, California, USA).
Cell culture. The U87 human glioblastoma cell line was obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan) and were cultured in DMEM medium supplemented with 10% FBS, 0.1 mg/ml streptomycin, and 100 U/ml penicillin and were incubated at 37˚C in a humidified atmosphere of 5% CO2 (22).
Cell morphological changes and viability assay. U87 cells (2×104 cells/well) were maintained in 12-well plate for 24 h and were treated with LG (0, 0.1, 0.3, 0.5 μM) for 24 and 48 h. After treatment, cells morphological changes evaluated by photographed under contrast-phase microscopy. Cells were harvested and were stained with PI (5 μg/ml) for cell viability by flow cytometry (Becton-Dickinson, San Jose, CA, USA) as previously described (23).
Apoptotic cell death assay. U87 cells (2×104 cells/well) were incubated with LG (0, 0.1, 0.25, 0.5, and 1 μM) 24 h. Cells were harvested and were double stained with Annexin V/PI for total apoptotic cell death analysis by flow cytometry as previously described (24).
Measurement of caspase-2,-3,-8, -9 and -12 activities. U87 cells (5×105 cells/100 mm-dish) were treated with LG (0, 0.25, 0.5, 1 μM) for 48 h. Cells were collected and resuspended in lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl and 1% Triton X-100). Cell lysate was used for measuring total protein by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) as the standard. Cell lysates (50 μg) were incubated at 37˚C with substrates for each caspase, conjugated to the fluorescent reporter molecule 7-amino-4-trifluoromethyl coumarin (AFC) [Caspase-2 (VDVAD-AFC), Caspase-3 (DEVD-AFC), Caspase-8 (IETD-AFC), Caspase-9 (LEHD-AFC), and Caspase-12 (ATAD-AFC); R&D Systems Minneapolis, MN, USA]. After incubation for 7h, cleavage of the peptide by caspase enzymatic activity released the fluorochrome (excitation/emission=405/510 nm). The level of caspase enzymatic activity was directly proportional to the fluorescence signal detected with a fluorescent microplate reader (Fluoroskan Ascent, Labsystems, Helsinki, Finland) as described previously (27).
Western blotting analysis. U87 cells (5×105 cells/100 mm-dish) were incubated with LG at various final concentrations (0, 0.25, 0.5 and 1 μM) for 24 h. Cells were harvested and gently resuspended in lysis buffer (10 mM Tris pH 7.5, 0.5 mM EDTA pH 8.0, 0.5 mM DTT, 0.5% CHAPS, 10% glycerol) supplemented with a cocktail of protease inhibitors (Thermo Fisher Scientific) and were incubated for 30 min on ice. Cell debris were removed by centrifugation at 10,000 g at 4˚C for 20 min. All supernatants were used for measuring total protein by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) as the standard. About 30 μg of each sample were separated by SDS polyacrylamide gel electrophoresis and then electrotransferred onto a PVDF membrane (Millipore, Bedford, MA, USA). The membrane was washed and incubated with blocking buffer (5% BSA, 1X Tris buffered saline, 0.1% Tween 20) for 1 h followed by incubation with primary antibodies against BAX, B-cell lymphoma 2 (BCL2), Bcl-2 antagonist/killer protein (BAK), β-actin, T-cell factor-1 (Tcf-1), Tcf-3, Tcf-4, lymphoid enhancer-binding factor 1 (LEF-1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), hypoxia-inducible factor 1α (HIF-1α), HIF-2α, phosphorylated AKT serine/threonine kinase (p-AKT), phosphorylated p21-activated kinase-1 (p-PAK-1) and β-Catenin (Cell Signaling, Danvers, MA, USA). After washed, the membranes were incubated with HRP-conjugated anti-rabbit IgG secondary antibody (1:10,000) (Cell Signaling). Immunoreactive protein was visualized and detected by ImmobilonTM Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) (23,24). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for band density quantification.
Immunocytochemistry. U87 cells were fixed with 4% paraformaldehyde in PBS and washed twice with cold PBS. The fixed cells were permeabilized and blocked with 0.1% Triton X-100 and 1% BSA simultaneously for 1 h. Cells were then incubated for 1 h with anti-β-catenin antibody (Cell Signaling) diluted in PBS containing 1% BSA. After incubation with FITC-conjugated secondary antibody (Cell Signaling, MA, USA) at room temperature for 1 h, cells were stained with DAPI (Thermo Fisher Scientific) and mounted over glass slides. Protein expression of b-catenin was evaluated using a confocal laser scanning microscope (Olympus FV1000, Tokyo, Japan).
Statistical analysis. The results were expressed as mean±standard deviation (SD). Figures are representative one of three independent experiments. Statistical analysis was performed by Student’s t-test with statistical significance of p<0.05.
Results
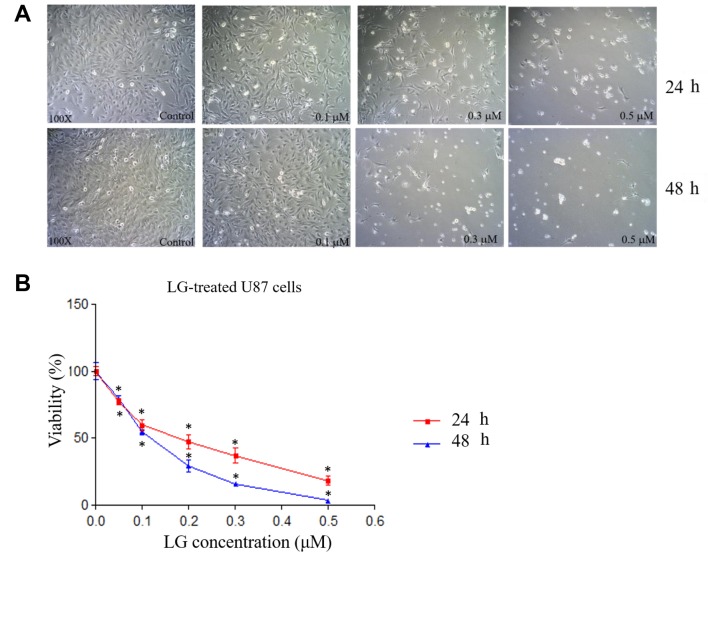
LG induced cell morphological changes and decreased cell viability in U87 cells. U87 cells treated with LG were examined for morphological changes at 24 and 48 h. Results indicated that LG induced cell morphological changes at both treated times based on cell debris and shrinking (Figure 1A). Cell viability was also analyzed, and results showed decreased total viable cell number of U87 cells in a dose-dependent manner (Figure 1B). The IC50 value of LG in U87 cells was about 0.2 μM for 24 h treatment.
Figure 1. Lauryl gallate (LG) induced cell morphological changes and decreased cell viability in U87 cells. U87 cells were treated with LG (0,0.05, 0.1, 0.2, 0.3, 0.5 μM) for 24 and 48 h. A: Cell morphological changes were examined and photographed at 24 and 48 h. B: Total cell viability was measured. *p<0.05, significant difference between LG-treated groups and the control as analyzed by Student’s t test.
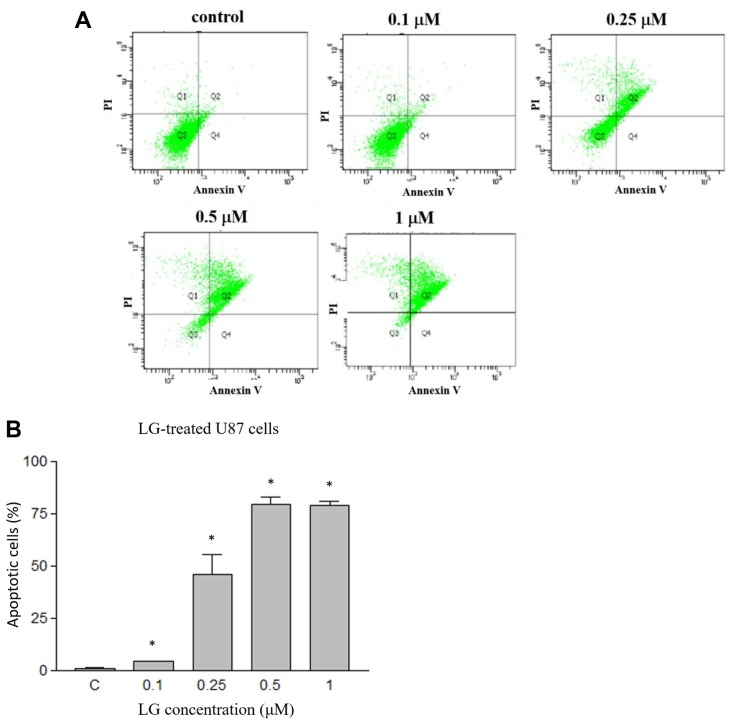
LG induced apoptotic cell death in U87 cells. To examine how LG induced U87 cell death, U87 cells were treated with LG (0, 0.1, 0.25, 0.5 and 1 μM) for 24 h and then examined by Annexin V/PI double staining followed by flow cytometry. The results indicated that LG induced earlier and late apoptotic cell death (Figure 2A). Furthermore, LG induced apoptotic cell death in U87 cells in a dose-dependent manner (Figure 2B). As shown in Figure 2, percentage of Annexin V/PI double positive U87 cells were 1.3±0.4%, 4.6±0.2%, 46.1±16.3%, 79.5±5.9% and 78.9±4.0% after a 24-h treatment with 0, 0.1, 0.25, 0.5 and 1 μM LG, respectively, indicating that LG induced apoptosis in U87 cells.
Figure 2. Lauryl gallate (LG) induced apoptotic cell death in U87 cells. U87 cells were treated with LG (0, 0.05, 0.1, 0.2, 0.25, 0.3, 0.5 and 1.0 μM) for 24 h and apoptotic cell death was determined by Annexin V/PI double staining and analyzed by flow cytometry assay. A: representative profiles; B: calculated percentage of apoptotic cell death. *p<0.05, significant difference between LG-treated groups and the control as analyzed by the Student’s t test.
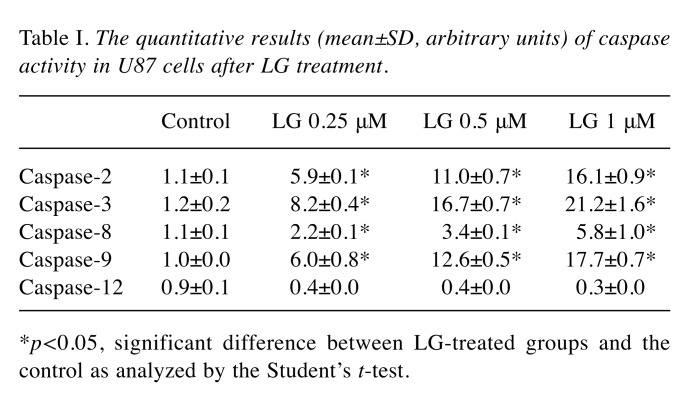
LG affected the enzymatic activity of Caspase-2, -3, -8, -9 and -12 in U87 cells. To further investigate the involvement of caspases in LG-induced apoptosis, U87 cells were treated with LG (0, 0.1, 0.25, and 1 μM) for 48 h and the activities of caspase-2, -3, -8, 9 and -12 were evaluated for 7 h. Results indicated that LG significantly promoted the activities of caspase-2, -3, -8 and -9 (LG-induced caspase activity: caspase-3>-9>-2>-8) in a dose-dependent manner, but not the activity of caspase-12. Treatment of U87 cells with various concentrations of LG for 48 h resulted in significant increases in the activities of caspase-2, -3, -8, and -9 (Figure 3 and Table I).
Figure 3. Lauryl gallate (LG) affects caspase-2, -3, -8, -9 and -12 activities in U87 cells. U87 cells treated with LG (0, 0.1, 0.25, and1 μM) for 48 h, and the activities of caspase-2, -3, -8, 9 and -12 weremeasured. *p<0.05, significant difference between LG-treated groupsand the control as analyzed by Student’s t test.
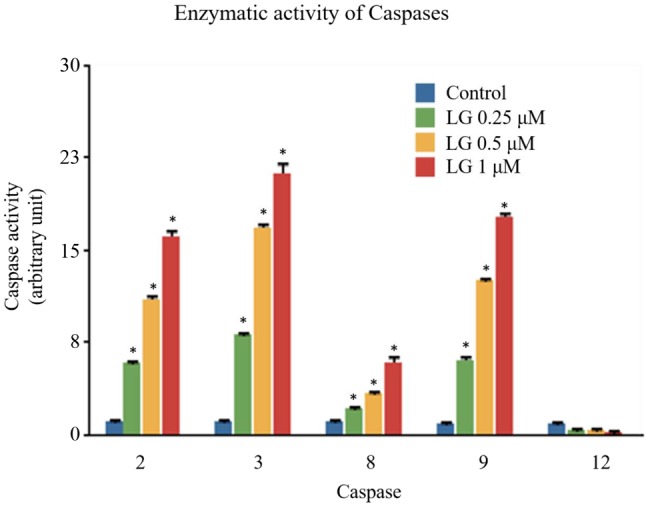
Table I. The quantitative results (mean±SD, arbitrary units) of caspase activity in U87 cells after LG treatment.
*p<0.05, significant difference between LG-treated groups and the control as analyzed by the Student’s t-test.
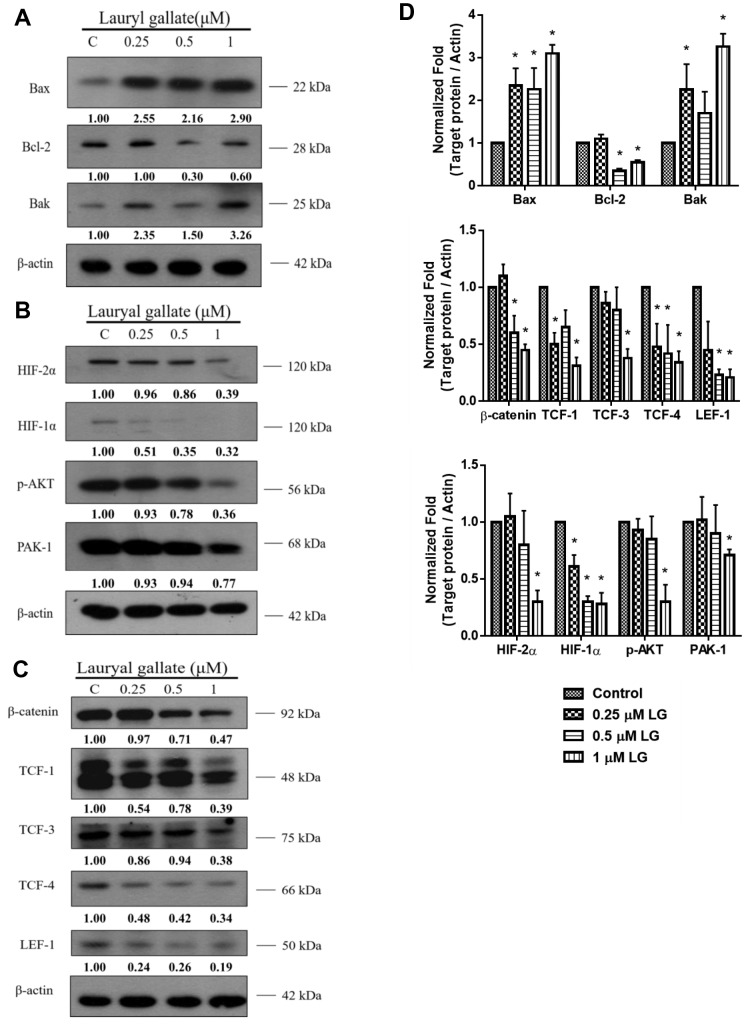
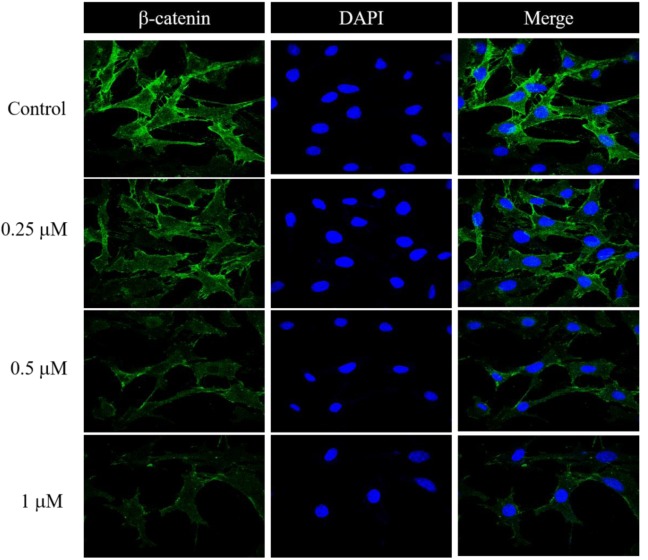
LG altered apoptosis-associated protein expression in U87 cells. To further confirm the molecular mechanisms of LG-induced apoptotic cell death in U87 cells, the expression of apoptosis-associated proteins was examined in LG-treated cells. Results indicated that LG significantly increased pro-apoptotic protein expression of BAX and BAK, while decreased the expression of anti-apoptotic protein BCL-2 (Figure 4A). Furthermore, we found that cells treated with LG (only at 1 μΜ) had significantly decreased expression of HIF-1α and -2α, p-AKT and PAK-1, which are associated with cell survival (Figure 4B). In addition, this study also showed that LG decreased the expression of β-Catenin, TCF-1, TCF-3, TCF-4 and LEF-1 in U87 cells (Figure 4C). The quantitative results of protein expression under LG exposure in U87 cells are shown in Figure 4 D. The expression of β-Catenin in U87 cells was further examined by confocal laser microscopy systems after exposed to LG (0, 0.25, 0.5 and 1 μM) for 24 h. Results confirmed that LG decreased the expression of β-catenin in U87 cells and this effect was dose-dependent (Figure 5).
Figure 4. Lauryl gallate (LG) altered apoptosis-associated proteinexpression in U87 cells. The expression of apoptosis-associated proteinsfrom LG-treated cells were examined by western blotting as describedin the Materials and Methods. A: Bax, Bak and Bcl-2; B: Hif-1α and -2α, p-Akt and Pak-1; C: β-Catenin, Tcf-1, Tcf-3, Tcf-4 and Lef-1. Thevalues above each lane represent the relative density of the bandnormalized by the density of actin-beta. D: Bar charts show westernblot results. C, Control. *p<0.05, significant difference between LGtreatedgroups and the control as analyzed by Student’s t test.
Figure 5. Lauryl gallate (LG) suppressed the expression of β-catenin in U87 cells. U87 cells treated with 0, 0.25, 0.5 and 1μM for 24 h wereexamined for the expression of β-catenin. After incubation, cells were collected for staining with anti-β-catenin antibody and were examined andphotographed by confocal laser microscopy systems.
Discussion
LG has been shown to have antitumor activities in vitro and in vivo (21,26) and exert its cytotoxic effects through the induction of apoptotic cell death in many human cancer cell lines (21,26-27). However, there is no available evidence on LG apoptotic effects on glioma cells in vitro. In the present study, we used U87 human glioblastoma cells to demonstrate the potential of LG as an anti-cancer agent in vitro.
Initially, the cytotoxic effects of LG on U87 cells were examined and LG was found to induce cell morphological changes and decrease the total viable cell number in a dose-dependent manner. In order to investigate whether the LG-induced U87 cell death was due to apoptosis or not, Annexin V/PI double staining, which has generally been used to measure apoptosis of cancer cells (28), was performed. Results indicated that LG reduced the total cell number via the induction of apoptotic cell death in U87 cells. These effects were also dose-dependent. Based on our evidence and the literature that suggests the induction of cancer cell apoptosis as a promising strategy for anticancer therapy (29-32), LG is a promising candidate as an anticancer drug for glioma.
It is well known that cancer cell apoptosis can be induced through caspase- dependent and -independent pathways (33,34). Thus, in order to investigate whether LG induced apoptosis in U87 cells via the activation of caspases, the enzymatic activities of caspases -2, -3, -8, -9 and 12 were measured. It was demonstrated that LG increased the activities of all caspases, except casaspe-12, suggesting that LG-induced cell apoptosis in U87 cells is mediated through caspase-dependent pathways. It has previously been shown that the metabolite of cholesterol, pregnenolone, induced caspase-dependent apoptosis in glioma cells in vitro, involving Fas-Fas ligand interaction, followed by the activation of caspase-8 and -9 at 24 h, and caspase-3/7 at 48 h (35). The pregnenolone-induced intrinsic apoptosis may be through disruption of lipid rafts (35). Although LG does not share similar structure with pregnenolone, it might influence the properties of lipid rafts followed by induction of intrinsic apoptosis because of its amphipathic structure. However, further investigations should be performed to demonstrate the mechanism.
Moreover, the expression of pro-apoptotic and anti-apoptotic proteins in LG-treated U87 cells was analyzed. It is known that the BCL-2 family proteins are involved in mitochondrial membrane permeability, which is important for the induction of the intrinsic apoptotic pathway, and they can be classified into pro-apoptotic (BAX) and anti-apoptotic (BCL-2) proteins (36,37). Western blot showed that LG increased the expression of pro-apoptotic proteins BAX and BAK (Figure 4A) and suppressed the level of anti-apoptotic protein such as BCL-2 (Figure 4A) in U87 cells. These results suggested that LG-induced apoptosis of U87 might be through the intrinsic pathway. Furthermore, it has been reported that increased BAX/BCL-2 ratio can affect the mitochondrial membrane potential leading to apoptosis via caspase-dependent and/or caspase-independent pathways (38). It has also been documented that the BAX/BCL-2 ratio affects cell-fate determination, and might be involved in the formation of various apoptosis-related diseases, as well (39,40). Thus, the increase of BAX/BCL-2 ratio induced by LG in U87 cells might alter their mitochondrial membrane potential.
The present results also showed that LG decreased the protein levels of HIF-1α and -2α, p-AKT and PAK-1 in U87 cells (Figure 4B). HIF-1α is a transcription factor and a critical regulator for cellular oxygen balance, since it is also involved in cellular survival during hypoxia (41). Numerous studies have shown that HIF-1α inhibition induces apoptosis in many cancer cell lines, including glioblastoma (42,43). Furthermore, previous studies have reported that downregulation of MCL-1 and HIF-1α is implicated in curcumin-mediated apoptosis in infantile hemangioma endothelial cells (44). AKT is also involved in cell survival (45,46); It is activated by phosphorylation (47) and, herein, it was shown that LG inhibited the expression of p-AKT (Figure 4B). Inhibition of AKT phosphorylation may increase susceptibility of cells to apoptosis, which has been previously reported in pancreatic beta-cells (48).
The present results also showed that LG suppressed the expression of PAK1 in U87 cells in a dose-dependent manner. Previous studies on melanoma and gastric cancer have reported that PAK-targeted therapeutics might have anti-tumor effects (49,50). Currently, many investigators are focused on identifying selective PAK inhibitors for specific tumors (51). Moreover, previous studies have demonstrated that TCF-1 has higher expression in some cancer cells and suppression of β-Catenin signaling has been shown to suppress the pancreatic tumor growth by disrupting nuclear β-Catenin/TCF-1 complex (52). Herein, it was demonstrated that the expression of β-Catenin and TCF-1, -3 and -4 and LEF-1 was decreased in LG-treated U87 cells. We also used confocal laser microscopy systems to examine the β-Catenin expression of LG-treated cells and results confirmed that LG suppressed the expression of β-Catenin in U87 cells, in a dose-dependent manner (Figure 5).
To our knowledge, this is the first study to show that LG induces apoptotic cell death in U87 glioblastoma cells in vitro. The effects of LG were mediated via caspase-dependent pathways, as shown by increased expression of pro-apoptotic proteins and decreased expression of anti-apoptotic BCL-2. Thus, our results suggest that LG is a promising candidate for anti-tumor therapy for GMB.
Acknowledgements
This work was supported by the grants –MOST 106-2627-M-001-001 (C. S. Chiang) and TCVGH-1053102A (C. C. Liu).
References
- 1.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67:139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20 doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- 4.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 5.Sabbagh AJ, Alaqeel AM. Focal brainstem gliomas. Advances in intra-operative management. Neurosciences (Riyadh) 2015;20:98–106. doi: 10.17712/nsj.2015.2.20140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Bian EB, Li J, Li J. New advances of microRNAs in glioma stem cells, with special emphasis on aberrant methylation of microRNAs. J Cell Physiol. 2014;229:1141–1147. doi: 10.1002/jcp.24540. [DOI] [PubMed] [Google Scholar]
- 7.Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26:825–853. doi: 10.1016/j.hoc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.de Groot JF, Mandel JJ. Update on anti-angiogenic treatment for malignant gliomas. Curr Oncol Rep. 2014;16:380. doi: 10.1007/s11912-014-0380-6. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 10.Orlov SN, Dam TV, Tremblay J, Hamet P. Apoptosis in vascular smooth muscle cells: role of cell shrinkage. Biochem Biophys Res Commun. 1996;221:708–715. doi: 10.1006/bbrc.1996.0661. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Maeno E, Okada Y. Prerequisite role of persistent cell shrinkage in apoptosis of human epithelial cells. Sheng Li Xue Bao. 2007;59:512–516. [PubMed] [Google Scholar]
- 12.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi A, Masuda A, Sun M, Centonze VE, Herman B. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm) Brain Res Bull. 2004;62:497–504. doi: 10.1016/j.brainresbull.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118–124. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radic Biol Med. 2011;51:2259–2271. doi: 10.1016/j.freeradbiomed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Yang XR, Wang YY, La KK, Peng L, Song XH, Shi XG, Zhu XF, Leung PC, Ko CH, Ye CX. Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis. J Nutr Biochem. 2012;23:1051–1057. doi: 10.1016/j.jnutbio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Kubo I, Masuoka N, Xiao P, Haraguchi H. Antioxidant activity of dodecyl gallate. J Agric Food Chem. 2002;50:3533–3539. doi: 10.1021/jf011250h. [DOI] [PubMed] [Google Scholar]
- 18.Kubo I, Fujita K, Nihei K, Nihei A. Antibacterial activity of akyl gallates against Bacillus subtilis. J Agric Food Chem. 2004;52:1072–1076. doi: 10.1021/jf034774l. [DOI] [PubMed] [Google Scholar]
- 19.Hurtado C, Bustos MJ, Sabina P, Nogal ML, Granja AG, Gonzalez ME, Gonzalez-Porque P, Revilla Y, Carrascosa AL. Antiviral activity of lauryl gallate against animal viruses. Antivir Ther. 2008;13:909–917. [PubMed] [Google Scholar]
- 20.van der Heijden CA, Janssen PJ, Strik JJ. Toxicology of gallates: a review and evaluation. Food Chem Toxicol. 1986;24:1067–1070. doi: 10.1016/0278-6915(86)90290-5. [DOI] [PubMed] [Google Scholar]
- 21.Ortega E, Sadaba MC, Ortiz AI, Cespon C, Rocamora A, Escolano JM, Roy G, Villar LM, Gonzalez-Porque P. Tumoricidal activity of lauryl gallate towards chemically induced skin tumours in mice. Br J Cancer. 2003;88:940–943. doi: 10.1038/sj.bjc.6600805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Liu Z, Yu G, Nie X, Jia W, Liu RE, Xu R. Paeoniflorin inhibits migration and invasion of human glioblastoma cells via suppression transforming growth factor beta-induced epithelial-mesenchymal transition. Neurochem Res. 2018;43:760–774. doi: 10.1007/s11064-018-2478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Yashiro M, Qiu H, Nishii T, Matsuzaki T, Hirakawa K. Establishment and characterization of multidrug-resistant gastric cancer cell lines. Anticancer Res. 2010;30:915–921. [PubMed] [Google Scholar]
- 24.Liu KC, Huang YT, Wu PP, Ji BC, Yang JS, Yang JL, Chiu TH, Chueh FS, Chung JG. The roles of AIF and Endo G in the apoptotic effects of benzyl isothiocyanate on DU 145 human prostate cancer cells via the mitochondrial signaling pathway. Int J Oncol. 2011;38:787–796. doi: 10.3892/ijo.2010.894. [DOI] [PubMed] [Google Scholar]
- 25.Teng CL, Han SM, Wu WC, Hsueh CM, Tsai JR, Hwang WL, Hsu SL. Mechanistic aspects of lauryl gallate-induced differentiation and apoptosis in human acute myeloid leukemia cells. Food Chem Toxicol. 2014;71:197–206. doi: 10.1016/j.fct.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Serrano A, Palacios C, Roy G, Cespon C, Villar ML, Nocito M, Gonzalez-Porque P. Derivatives of gallic acid induce apoptosis in tumoral cell lines and inhibit lymphocyte proliferation. Arch Biochem Biophys. 1998;350:49–54. doi: 10.1006/abbi.1997.0474. [DOI] [PubMed] [Google Scholar]
- 27.Roy G, Lombardia M, Palacios C, Serrano A, Cespon C, Ortega E, Eiras P, Lujan S, Revilla Y, Gonzalez-Porque P. Mechanistic aspects of the induction of apoptosis by lauryl gallate in the murine B-cell lymphoma line Wehi 231. Arch Biochem Biophys. 2000;383:206–214. doi: 10.1006/abbi.2000.2049. [DOI] [PubMed] [Google Scholar]
- 28.Liu KC, Shih TY, Kuo CL, Ma YS, Yang JL, Wu PP, Huang YP, Lai KC, Chung JG. Sulforaphane induces cell death through G2/M phase arrest and triggers apoptosis in HCT 116 human colon cancer cells. Am J Chin Med. 2016;44:1289–1310. doi: 10.1142/S0192415X16500725. [DOI] [PubMed] [Google Scholar]
- 29.Chou WH, Liu KL, Shih YL, Chuang YY, Chou J, Lu HF, Jair HW, Lee MZ, Au MK, Chung JG. Ouabain Induces Apoptotic Cell Death Through Caspase- and Mitochondria-dependent Pathways in Human Osteosarcoma U-2 OS Cells. Anticancer Res. 2018;38:169–178. doi: 10.21873/anticanres.12205. [DOI] [PubMed] [Google Scholar]
- 30.Sarosiek KA, Ni Chonghaile T, Letai A. Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol. 2013;23:612–619. doi: 10.1016/j.tcb.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheij M, Vens C, van Triest B. Novel therapeutics in combination with radiotherapy to improve cancer treatment: rationale, mechanisms of action and clinical perspective. Drug Resist Updat. 2010;13:29–43. doi: 10.1016/j.drup.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Zhang Y, Wang L, Lee S. Levistolide A induces apoptosis via ROS-mediated ER stress pathway in colon cancer cells. Cell Physiol Biochem. 2017;42:929–938. doi: 10.1159/000478647. [DOI] [PubMed] [Google Scholar]
- 33.Khazaei S, Abdul Hamid R, Ramachandran V, Mohd Esa N, Pandurangan AK, Danazadeh F, Ismail P. Cytotoxicity and Proapoptotic Effects of Allium atroviolaceum Flower Extract by Modulating Cell Cycle Arrest and Caspase-Dependent and p53-Independent Pathway in Breast Cancer Cell Lines. Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/1468957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CH, Chan HS, Tsay HS, Funayama S, Kuo CL, Chung JG. Ethyl acetate fraction from methanol extraction of Vitis thunbergii var. taiwaniana induced G0/G1 phase arrest via inhibition of cyclins D and E and induction of apoptosis through caspase-dependent and -independent pathways in human prostate carcinoma DU145 cells. Environ Toxicol. 2018;33:41–51. doi: 10.1002/tox.22491. [DOI] [PubMed] [Google Scholar]
- 35.Xiao X, Chen L, Ouyang Y, Zhu W, Qiu P, Su X, Dou Y, Tang L, Yan M, Zhang H, Yang X, Xu D, Yan G. Pregnenolone, a cholesterol metabolite, induces glioma cell apoptosis via activating extrinsic and intrinsic apoptotic pathways. Oncol Lett. 2014;8:645–650. doi: 10.3892/ol.2014.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 38.Su ZY, Tung YC, Hwang LS, Sheen LY. Blazeispirol A from Agaricus blazei fermentation product induces cell death in human hepatoma Hep 3B cells through caspase-dependent and caspase-independent pathways. J Agric Food Chem. 2011;59:5109–5116. doi: 10.1021/jf104700j. [DOI] [PubMed] [Google Scholar]
- 39.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 40.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 42.Vordermark D. In regard to DAI et al: inhibition of hypoxia inducible factor 1alpha causes oxygen-independent cytotoxicity and induces p53 independent apoptosis in glioblastoma cells. IJROBP 2003;55:1027-1036) Int J Radiat Oncol Biol Phys. 2003;57:1196. doi: 10.1016/s0360-3016(02)04507-8. [DOI] [PubMed] [Google Scholar]
- 43.Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C, Stratford IJ, Dive C. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou S, Wang Y, Yu Z, Guan K, Kan Q. Curcumin induces apoptosis and inhibits proliferation in infantile hemangioma endothelial cells via downregulation of MCL-1 and HIF-1alpha. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat Rev. 2017;59:93–101. doi: 10.1016/j.ctrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Lee CC, Lin ML, Meng M, Chen SS. Galangin Induces p53-independent S-phase arrest and apoptosis in human nasopharyngeal carcinoma cells through inhibiting PI3K-AKT signaling pathway. Anticancer Res. 2018;38:1377–1389. doi: 10.21873/anticanres.12361. [DOI] [PubMed] [Google Scholar]
- 47.Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–251. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapodistria K, Tsilibary EP, Politis P, Moustardas P, Charonis A, Kitsiou P. Nephrin, a transmembrane protein, is involved in pancreatic beta-cell survival signaling. Mol Cell Endocrinol. 2015;400:112–128. doi: 10.1016/j.mce.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Chuang YC, Wu HY, Lin YL, Tzou SC, Chuang CH, Jian TY, Chen PR, Chang YC, Lin CH, Huang TH, Wang CC, Chan YL, Liao KW. Blockade of ITGA2 induced apoptosis and inhibits cell migration in gastric cancer. Biol Proced Online. 2018;20 doi: 10.1186/s12575-018-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araiza-Olivera D, Feng Y, Semenova G, Prudnikova TY, Rhodes J, Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene. 2018;37:944–952. doi: 10.1038/onc.2017.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pramanik KC, Fofaria NM, Gupta P, Ranjan A, Kim SH, Srivastava SK. Inhibition of beta-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear beta-catenin/TCF-1 complex: critical role of STAT-3. Oncotarget. 2015;6:11561–11574. doi: 10.18632/oncotarget.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]