Abstract
Background: The aim of this study was to investigate the effects of carbon-dioxide treatment on heart rate variability (HRV) parameters: mean RR interval (RRI), standard deviation of RR intervals (SDNN), root mean square of successive RR differences (RMSSD); and Porta and Guzik indices, as measures of heart rate asymmetry. Materials and Methods: Twenty patients were enrolled (mean±SD, age=59±7.8 years). Measurements were performed before CO2 treatment, at the beginning of treatment, at 15 min of treatment, immediately after and 1 h after the treatment. Results: Significant increase in SDNN was found 1 h after the treatment when compared to that before it (p=0.011). There were no significant changes in other parameters. Conclusion: CO2 treatment can influence the autonomic nervous system identified by SDNN changes. However, larger studies are required to confirm these results.
Keywords: Carbon dioxide treatment, rehabilitation, balneotherapy, heart rate variability
The transcutaneous administration of carbon dioxide (CO2), hereafter referred to as ‘CO2 treatment’ has been used for curative purposes for centuries. The first paper investigating the medicinal use of CO2 was published by Brandi et al. in 1932 (1). CO2 passes freely through membranes and has a well-known vasodilatory effect. Both in vitro and in vivo studies have demonstrated a rightward shift of the oxygen–haemoglobin dissociation curve after administration of CO2. Sakai et al. described this as an “artificial Bohr effect”. This is responsible for the decrease in pH and increase in partial pressure of oxygen in the tissues, through facilitated O2 release, which were shown in vivo (2). The findings of Minamiyama and Yamamoto confirmed these effects using intra-vital video microscopy to demonstrate subcutaneous vasodilation after CO2 administration. In addition, CO2 was shown to increase blood flow in the observed subcutaneous vessels (3). CO2 treatment is used to cure several diseases, such as peripheral arterial and venous disorders (e.g. claudication, and lower limb ulcer), heart diseases (e.g. hypertension, and heart failure) and immunological disorders (e.g. Raynaud’s syndrome) (4,5). In short, CO2 treatment is a non-invasive, highly efficient, low-cost treatment capable of easing the symptoms of arterial and venous diseases possibly due to vasodilatation and reduction of oxidative stress. However, as far as we are aware of, there is no evidence for alteration of the autonomic nervous system after CO2 treatment.
Heart rate variability (HRV) is controlled by the sympathetic and parasympathetic nervous systems, and is influenced by several other factors (e.g. autocrine, paracrine, endocrine, and mechanical stretch). The sinus node is the final summing element in controlling the heart rate. HRV analysis is a non-invasive method providing information on the autonomic regulation of cardiac activity (6,7). In recent decades, several studies have shown an association between cardiovascular diseases and HRV. HRV analysis is considered a useful tool for predicting the risk of sudden cardiac death after myocardial infarction (8,9). Numerous studies have investigated HRV among diabetic patients: the most useful finding is that reduced HRV can indicate diabetic neuropathy before clinical symptoms actually appear (10-12). A recent study suggested that HRV can be a potential new tool to predict complications after cardiac surgery (13). In short, HRV assessment is a non-invasive method used to assess the heart’s ability to respond and adapt to external or internal stress.
The aim of this study was to investigate the instantaneous effects of a single CO2 treatment on HRV and heart rate asymmetry (HRA) parameters.
Materials and Methods
The present study was performed at our ISO 9001-accredited Cardiology Rehabilitation Inpatient Unit from July 2017 to January 2018 in Zsigmondy Vilmos SPA Hospital, Harkány. Non-diabetic, abstinent, hypertensive patients, suffering from coronary heart disease were enrolled. Patients who had previously received CO2 treatment were excluded. Moreover, patients who had suffered myocardial infarction, stroke or had undergone open heart surgery less than a year before the study were also excluded. Additionally, individuals diagnosed with any kind of cancer or kidney injury were also not enrolled. Informed written consent was obtained from each patient. The study protocol was approved by the Regional Ethics Committee at the University of Pécs, Pécs, Hungary (Permission No. 5919), in accordance with the 2008 Helsinki declaration.
CO2 gas was administered for 35 minutes in a plastic bag sealed at mid-thoracic level, as previously described by Fabry et al. (4). HRV data acquisition was performed using a hand-held, battery-powered device based on a PIC18F46J50 microcontroller (Microchip Technology Inc., Chandler, AZ, USA) and INA333 instrumentation amplifier chip (Texas Instruments, Dallas, TX, USA). The device contains analogue circuitry for a single-channel (three-electrode) electrocardiograph (ECG) amplifier among others. The recordings were transferred to a PC on an SD card for analysis. After extracting the 1-ms temporal resolution ECG signal from the stored data, automatic detection of RR intervals and manual checking was performed by ECGRdet v2.1 software (L. Hejjel, Pécs, Hungary). A 300-s-long artefact-free part of the recording was then selected manually. Particular attention was paid to selecting a portion containing exclusively normal sinus interbeat intervals in order to obtain correct parameters of HRV. Varian v2.2 software (L. Hejjel, Pécs, Hungary) was used to calculate the standard time-domain parameters: mean RR interval (meanRRI), standard deviation of normal-to-normal RR intervals (SDNN) and root mean square of successive RR differences (RMSSD), as well as HRA parameters: Porta index and Guzik index. The hardware and software (ECGRdet and Varian) were developed by L. Hejjel (14-16). Non-invasive blood pressure measurements were made in the middle of each of the five stages of the study. Average respiration frequency was calculated by fast Fourier transformation of the tachogram by Varian v2.2.
During the 3-week-long in-ward rehabilitation, patients underwent a combination of physiotherapies, and received nine CO2 treatment (35 min per session). In order to follow the changes of HRV parameters, analysis was performed immediately before CO2 treatment, immediately after the beginning of the CO2 treatment, 15 min after the beginning of the CO2 treatment, immediately after the end of the CO2 treatment, and 1 h after the end of the CO2 treatment. All the measurements were performed with the patients in supine position 15 min after orthostatic adaptation and from 8am to 10pm in order to avoid positional or diurnal influences, respectively. The patients did not receive any medication besides their regular antihypertensive drugs.
Statistical analysis. Statistical analysis was carried out with StatistiXL package (Broadway-Nedlands, Western Australia) in MS Excel (Microsoft Corp., Redmond, WA, USA). Calculated HRV and HRA measures were assessed by Friedman test and post-hoc Wilcoxon test with Holm–Bonferroni correction. The 2-5th stages were compared to the first one. Before correction p-values of less than 0.05 were considered statistically significant. Charts were constructed using OriginPro 2017 (OriginLab Corp., Northampton, MA, USA
Results
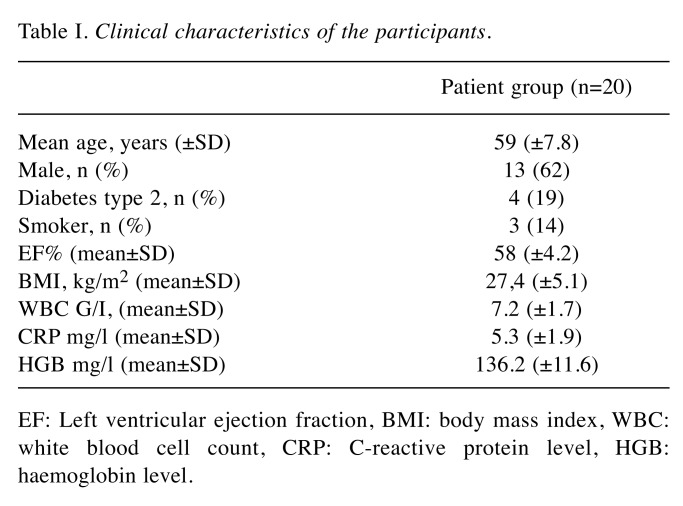
Twenty-three patients were enrolled, out of whom three were excluded due to inappropriate ECG signal quality, resulting in 20 patients remaining in the study (age, mean±SD=59±7.8 years). Clinical characteristics of the participants are shown in Table I.
Table I. Clinical characteristics of the participants.
EF: Left ventricular ejection fraction, BMI: body mass index, WBC: white blood cell count, CRP: C-reactive protein level, HGB: haemoglobin level.
During the entire measurement, there were no significant systolic or diastolic blood pressure changes detected in any patient.
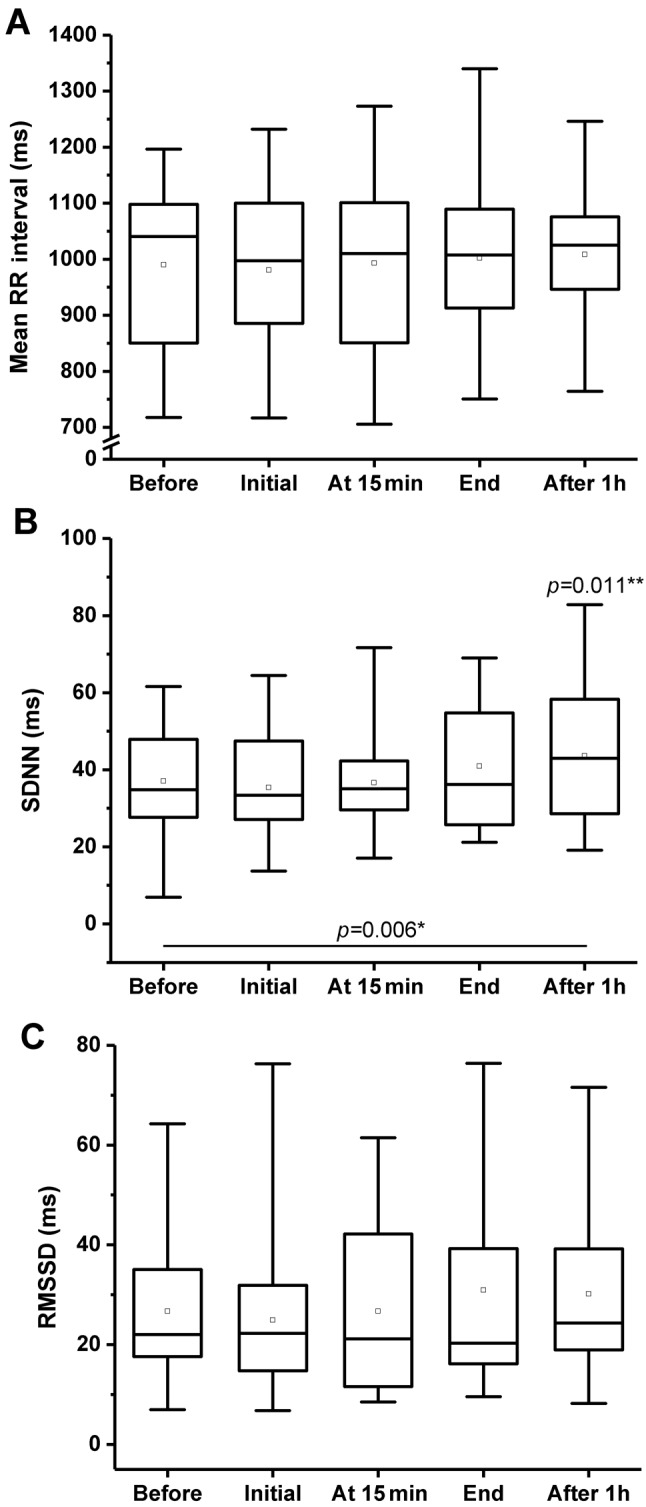
Regarding the meanRRI, there were no significant changes found (Figure 1A). A significant increase in SDNN was detected 1 h after the CO2 treatment (Friedman test: p=0.006, Wilcoxon test: p=0.011) (Figure 1B). No significant changes were demonstrated in the RMSSD parameter (Figure 1C).
Figure 1. Time domain measurements before, during (at beginning and at15 min), and after (immediately and 1 h later) CO2 treatment. A: Meantime between two consecutive R-waves on the ECG (RR interval). B:Standard deviation of RR intervals (SDNN). C: Root mean square ofsuccessive RR interval differences (RMSSD). Boxes: Lower and upperquartiles, horizontal line: median value, whiskers: minimum and maximumvalues, square: mean value. *Friedman test p-value, **post-hoc Wilcoxon’spaired sample test p-values, only significant differences are indicated.
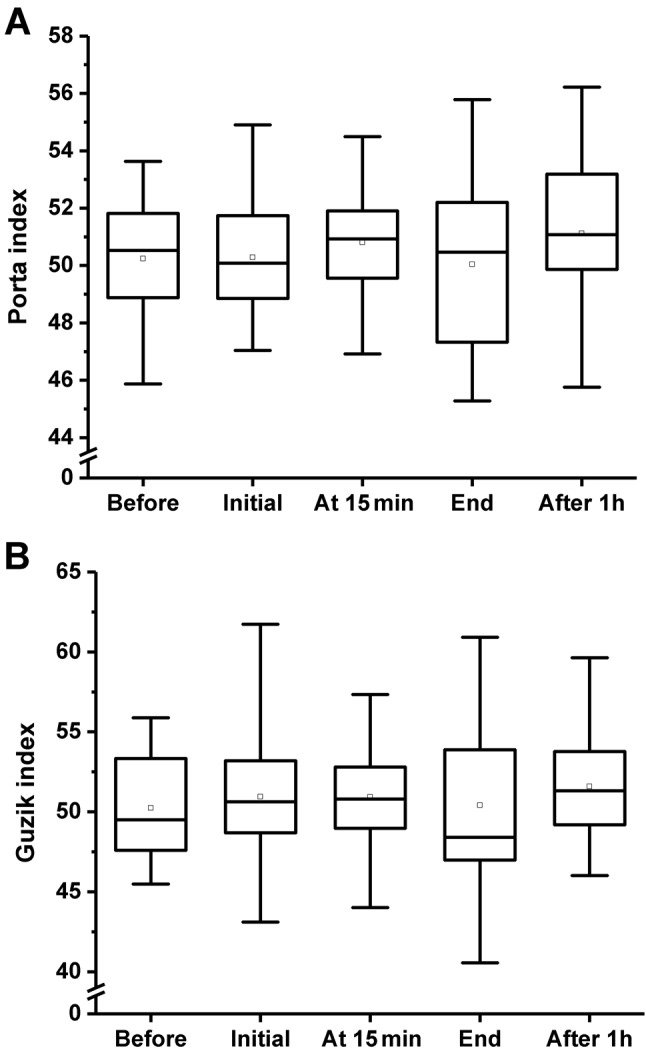
Regarding the Porta and Guzik indices, no statistically significant differences were found during the monitoring. The effects of CO2 treatment on Porta and Guzik indices are shown in Figure 2.
Figure 2. The effect of CO2 treatment on Porta (A) and Guzik (B) indicesbefore, during (at the beginning and at 15 min), and after (immediately and1 h later) CO2 treatment. Boxes: Lower and upper quartiles, horizontalline: median value, whiskers: minimum and maximum values, square: meanvalue. Friedman test resulted in p=0.555 for both parameters.
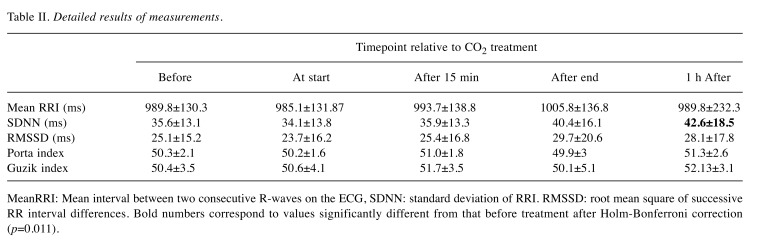
The detailed results for HRV parameters measured before, during and after CO2 treatment are shown in Table II.
Table II. Detailed results of measurements .
MeanRRI: Mean interval between two consecutive R-waves on the ECG, SDNN: standard deviation of RRI. RMSSD: root mean square of successive RR interval differences. Bold numbers correspond to values significantly different from that before treatment after Holm-Bonferroni correction (p=0.011).
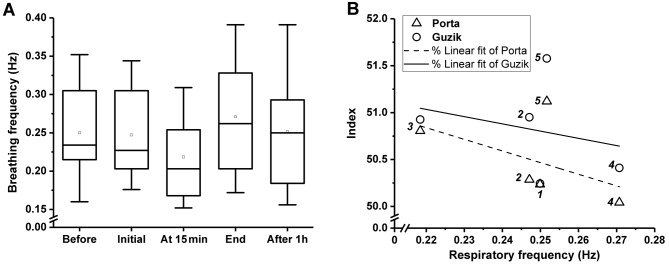
The breathing frequency dropped from 0.25 to 0.22 Hz after 15 min of treatment and elevated to 0.27 Hz immediately after CO2 treatment (Figure 3A). There was no correlation (insignificant p-values) between respiration frequency and HRA parameters (Figure 3B).
Figure 3. A: Mean values of breathing frequency derived from spectral analysis of the tachograms. Boxes: Lower and upper quartiles, horizontalline: median value, whiskers: minimum and maximum values, square: mean value. Friedman test resulted in p=0.555. B: Correlation of breathingfrequency and heart rate asymmetry parameters with non-significant linear fit. The Arabic numbers in Italics next to data points correspond to thefive measurements made. The wide divergence of the Porta and Guzik indices at the beginning of the treatment, immediately after and 1 h after thetreatment is remarkable, as well as the elevated indices at the 5th measurement compared to the 1st and 2nd in spite of the similar breathingfrequency.
Discussion
In the present study, the effects of CO2 treatment on the autonomic nervous system were investigated using HRV and HRA analysis. Our protocol was aimed at minimizing the effects of diurnal changes, body position, vocalization and movements on HRV and HRA parameters. To rule out inter-observer errors, all measurements were performed by the same investigator.
To the best of our knowledge this is the first study investigating the effects of CO2 treatment on HRV and HRA parameters.
The meanRRI remained stable during the study, reflecting steady state or balanced sympathetic and parasympathetic actions. SDNN showed a mild but continuous increase during the study, which reached statistical significance 1 h after the CO2 treatment by post-hoc test. Since SDNN reveals global variability including both sympathetic and parasympathetic effects (6,17), some autonomic influence occurs in spite of the steady-state meanRRI. The beat-to-beat variability parameter RMSSD is a measure of vagal actions on heart rate (6,17). At the beginning of the treatment a slight drop was seen, followed by a monotonous increase not reaching the level of significance. This probably occurred due to the complex cardiovascular actions of CO2, the high inter-individual variation and the relatively small sample size. Consequently, an increase in both sympathetic and parasympathetic activity can occur during CO2 treatment, resulting in unchanged heart rate, systolic and diastolic blood pressure. In line with our previous finding, the changes in overall HRV suggest that CO2 is not simply a vasodilator but is capable of altering the function of the cardiovascular system, possibly by activating endogenous mechanisms leading to vasodilatation (18). Bickel et al. found increased sympathetic activity during positive pressure CO2 pneumoperitoneum by HRV analysis possibly due to hypercarbia and tension of the peritoneum; however, in the present study there was no peritoneal tension (19).
Regarding the Porta and Guzik indices, no statistically significant differences were found during the investigation, once again probably due to the small sample size. However, an increase in both these indices at the end of the treatment and a mild drop immediately after the treatment was observed, although they remained higher even after 1 h compared to the basal level (Table II). According to our previous study, the Porta and Guzik indices are strongly correlated to each other in healthy volunteers even at different inspiration/expiration ratios (16), whereas in the current investigation, their divergence was observed at the beginning of CO2 treatment, immediately after and 1 h after the treatment (Figure 3B). The physiopathology of HRA parameters is not well known; therefore, we extracted the average breathing frequency from the tachograms by fast Fourier analysis in order to link the observed HRA changes to breathing rate. Faster breathing corresponds to a shorter expiration period relative to inspiration, theoretically resulting in higher HRA indices, based on the work of Klintworth et al. (16). On the contrary, in our study, an opposing change of breathing frequency compared with Porta and Guzik indices was observed (Figure 3B), resulting in a negative regression line. Unfortunately, the correlation was not significant due to the outlier at the final measurement, when in spite of the normalization of the breathing rate, the HRA indices further increased (Figure 3B). This phenomenon again reflects both an instantaneous and a sustained vegetative action of CO2 treatment, which cannot be explained by our monitored or computed parameters, therefore precise respiration monitoring (via transthoracic impedance or a sensitive chest strap) is recommended in subsequent investigation. This mighty be explained at the second measurement of CO2 treatment by the Bohr effect, when enough CO2 accumulates in the body to facilitate O2 release from haemoglobin (2,3), resulting in slower breathing as a response to higher oxygen tension in the chemoreceptor area. After finishing the treatment, the rapid diffusion and exhalation of CO2 causes the Bohr effect to cease, which stimulates the breathing centre, resulting in a transient higher breathing immediately after the treatment (Figure 3A).
The relatively small number of participants may be the reason for the insignificant differences in RMSSD and HRA parameters.
In conclusion, the reported study protocol is adequate for investigating the effects of CO2 treatment on HRV and HRA parameters. According to the results of this pilot study, CO2 treatment can influence the autonomic nervous system. However, larger studies with respiration monitoring are required to confirm these results and to discover the mechanism.
Conflict of Interest
The Authors declare that they have no conflict of interest in regard to this study.
Funding
OriginPro v2017 software was funded by 3/2016 AOK-KA grant from the Medical Faculty of the University of Pécs, Hungary. Balázs Németh was supported by the ÚNKP-17-3-III New National Excellence Program of the Ministry of Human Capacities, Hungary.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the Regional Research Ethics Committee and with the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the Regional Ethics Committee of University of Pécs, Pécs, Hungary (Permission No.: 5919.)
Acknowledgements
The Authors express their special thanks to all the nurses of the Cardiology Ward for their invaluable help during this study. The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
References
- 1.Brandi C, Aniello CD, Grimaldi L, Bosi B, Dei I, Lattarulo P, Alessandrini C. Carbon dioxide therapy in the treatment of localized adiposities: Clinical study and histopathological correlations. Aesthetic Plast Surg. 2001;25:170–174. doi: 10.1007/s002660010116. [DOI] [PubMed] [Google Scholar]
- 2.Sakai Y, Miwa M, Oe K, Ueha T, Koh A, Niikura T, Iwakura T, Lee SY, Tanaka M, Kurosaka M. A novel system for transcutaneous application of carbon dioxide causing an “Artificial Bohr Effect” in the human body. PLoS One. 2011;6:e24137. doi: 10.1371/journal.pone.0024137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minamiyama M, Yamamoto A. Direct evidence of the vasodilator action of carbon dioxide on subcutaneous micro-vasculature in rats by use of intra-vital. J Biorheol. 2010;24:42–46. [Google Scholar]
- 4.Fabry R, Monnet P, Schmidt J, Lusson JR, Carpentier PH, Baguet JC, Dubray C. Clinical and microcirculatory effects of transcutaneous CO2 therapy in intermittent claudication. Randomized double-blind clinical trial with a parallel design. Vasa. 2009;38:213–224. doi: 10.1024/0301-1526.38.3.213. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J, Monnet P, Normand B, Fabry R. Microcirculatory and clinical effects of serial percutaneous application of carbon dioxide in primary and secondary Raynaud’s phenomenon. Vasa. 2005;34:93–100. doi: 10.1024/0301-1526.34.2.93. [DOI] [PubMed] [Google Scholar]
- 6.Hejjel L, Gál I. Heart rate variability analysis. Acta Physiol Hung. 2001;88:219–30. doi: 10.1556/APhysiol.88.2001.3-4.4. [DOI] [PubMed] [Google Scholar]
- 7.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 8.Lombardi F, Mäkikallio TH, Myerburg RJ, Huikuri HV. Sudden cardiac death: Role of heart rate variability to identify patients at risk. Cardiovasc Res. 2001;50:210–217. doi: 10.1016/s0008-6363(01)00221-8. [DOI] [PubMed] [Google Scholar]
- 9.Huikuri HV, Mäkikallio TH, Raatikainen MJ, Perkiömäki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death: Appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation. 2003;108:110–115. doi: 10.1161/01.CIR.0000077519.18416.43. [DOI] [PubMed] [Google Scholar]
- 10.Singh JP, Larson MG, O’Donell CJ, Wilson PF, Tsuji H, Lyod-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability: the Framingham heart study. Am J Cardiol. 2003;86:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler T, Watkins PJ. Cardiac denervation in diabetes. Br Med J. 1973;4:584–586. doi: 10.1136/bmj.4.5892.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Istenes I, Körei AE, Putz Z, Németh N, Martos T, Keresztes K, Kempler MS, Erzsébet VO, Vargha P, Kempler P. Heart rate variability is severely impaired among type 2 diabetic patients with hypertension. Diabetes Metab Res Rev. 2014;30:305–312. doi: 10.1002/dmrr.2496. [DOI] [PubMed] [Google Scholar]
- 13.Nenna A, Lusini M, Spadaccio C, Nappi F, Greco SM, Barbato R, Covino E, Chello M. Heart rate variability: A new tool to predict complications in adult cardiac surgery. J Geriatr Cardiol. 2003;14:662–668. doi: 10.11909/j.issn.1671-5411.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hejjel L, Roth E. What is the adequate sampling interval of the ECG signal for heart rate variability analysis in the time domain. Physiol Meas. 2004;25:1405–1411. doi: 10.1088/0967-3334/25/6/006. [DOI] [PubMed] [Google Scholar]
- 15.Toth V, Hejjel L, Fogarasi A, Gyimesi C, Orsi G, Szucs A, Kovacs N, Komoly S, Ebner A, Janszky J. Periictal heart rate variability analysis suggests long-term postictal autonomic disturbance in epilepsy. Eur J Neurol. 2010;17:780–787. doi: 10.1111/j.1468-1331.2009.02939.x. [DOI] [PubMed] [Google Scholar]
- 16.Klintworth A, Ajtay Z, Paljunite A, Szabados S, Hejjel L. Heart rate asymmetry follows the inspiration/expiration ratio in healthy volunteers. Physiol Meas. 2012;33:1717–1731. doi: 10.1088/0967-3334/33/10/1717. [DOI] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 18.Németh B, Kiss I, Jencsik T, Péter I, Kreska Z, Kőszegi T, Miseta A, Kustán P, Boncz I, Laczo A, Ajtay Z. Angiotensin-converting enzyme inhibition improves the effectiveness of transcutaneous carbon dioxide treatment. In Vivo. 2017;31:425–428. doi: 10.21873/invivo.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bickel A, Yahalom M, Roguin N, Frankel R, Breslava J, Ivry S, Eitan A. Power spectral analysis of heart rate variability during positive pressure pneumoperitoneum: the significance of increased cardiac sympathetic expression. Surg Endosc. 2002;16:1341–1344. doi: 10.1007/s00464-001-9211-6. [DOI] [PubMed] [Google Scholar]