Abstract
Aim: The aim of the present study was to confirm therapeutic efficacy and find probable mechanism of action of amentoflavone in hepatocellular carcinoma (HCC) in vivo. Materials and Methods: Luciferase reporter vector pGL4.50_transfected SK-Hep1 (SK-Hep1/luc2) tumor-bearing mice were treated with vehicle or amentoflavone (100 mg/kg/day by gavage) for 14 days. Tumor growth, amentoflavone toxicity, and extracellular signal-regulated kinase (ERK)/nuclear factor-kappaB (NF-ĸB) signaling in tumor progression were evaluated with digital caliper, bioluminescence imaging, computed tomography, body weight, pathological examination of liver, and immunohistochemistry staining. Results: Amentoflavone significantly inhibited tumor growth, ERK/NF-ĸB activation, and expression of tumor progression-associated proteins as compared to vehicle-treated group. In addition, body weight and liver morphology of mice were not influenced by amentoflavone treatment. Conclusion: These results suggest that amentoflavone inhibits HCC progression through suppression of ERK/NF-ĸB signaling.
Keywords: Amentoflavone, hepatocellular carcinoma, extracellular signal-regulated kinase, nuclear factor-kappaB, bioluminescence imaging
Hepatocellular carcinoma (HCC) is the commonest and highly lethal primary malignant liver tumor worldwide. Epigenetic and genetic alterations contribute to HCC formation and multiple oncogenic proteins modulate HCC progression (1). Protein overexpression of cyclin-D1, vascular endothelial growth factor (VEGF), matrix metallopeptidase (MMP) 9, cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein (C-FLIP), induced myeloid leukemia cell differentiation protein (MCL)-1, and x-linked inhibitor of apoptosis protein (XIAP) trigger HCC cell proliferation, angiogenesis, metastasis, and escape from apoptosis resulting in cancer progression and poor prognosis (2-6). Extracellular signal-regulated kinase (ERK), the downstream component of RAS/RAF/[mitogen-activated protein kinase/ERK kinase (MEK)]/ERK signaling pathway, is involved in HCC progression by activating expression of transcriptional factors-regulated oncogene-encoded proteins (7). ERK activation was found to be enhanced in human HCC as compared to non-neoplastic hepatic cells (8).
Nuclear factor-kappaB (NF-ĸB), a heterodimer composed of p50 and p65 subunits, is a transcription factor critical for tumor progression. Many oncogenic proteins including cyclin-D1, VEGF, MMP-9, C-FLIP, MCL-1, and XIAP are encoded by NF-ĸB-targeted genes. Phosphorylated ERK (pERK) mediates NF-ĸB activation (2-3,9). In addition to regulation of tumor progression, some studies presented ERK/NF-ĸB signaling as also mediating therapeutic resistance in HCC. Vorinostat, a histone deacetylase inhibitor, and radiation both inhibit tumor growth, while triggering ERK/NF-ĸB signaling in Huh7 HCC cells in vitro and in vivo. Moreover, blockage of ERK/NF-ĸB signaling sensitizes HCC to vorinostat or radiation (10,11). Inhibitors of ERK/NF-ĸB signaling have the potential to act as a new agent offering benefits for patients with HCC.
Recently, studies have suggested that flavonoids isolated from plants have anti-HCC activity or can sensitize HCC to therapeutic agents. Curcumin, a flavonoid extracted from turmeric (Curcuma longa), has been shown not only to activate apoptosis induced by endoplasmic reticulum (ER) stress in HCC but also to enhance the anti-HCC effect of radiation via inhibition of NF-ĸB activation (12,13). Amentoflavone, a flavonoid isolated from Selaginella tamariscina, has been indicated to overcome sorafenib resistance through restoration of sorafenib-induced apoptosis in HCC (14). There is evidence that curcumin possesses anti-HCC activity through inhibition of signal transduction which modulates HCC progression (15). However, the anti-HCC effect of amentoflavone is still ambiguous. Therefore, the aim of the present study was to investigate the therapeutic efficacy and mechanism of amentoflavone in treating HCC in mice.
Materials and Methods
Chemicals and agents. Amentoflavone (CAS Number 1617-53-4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies to pERK (Selleckchem, Houston, TX, USA), NF-ĸB p65 (Ser536) (Cell signaling, Beverly, MA, USA), MMP-9 (EMD Millipore, Billerica, MA, USA), VEGF (EMD Millipore), Cyclin-D1 (Cell signaling), MCL-1 (BioVision, Milpitas, CA, USA), C-FLIP (BioVision), XIAP (Thermo Fisher Scientific, Fremont, CA, USA) were purchased from different companies as listed. Secondary antibodies were bought from Jackson ImmunoResearch (West Grove, PA, USA). Hygromycin and primary anti-β-actin were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Vector pGL4.50 (luc2) and substrate D-luciferin were purchased from Promega (Madison, WI, USA).
Cell culture. Human HCC cell line SK-Hep1 was purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan, ROC). Cells were maintained in Dulbecco’s modified Eagle’s medium [supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin] and grown at 37˚C in a humidified incubator with 5% CO2 and 95% air (16). pGL4.50 (luc2)-transfected SK-Hep1 (SK-Hep1/luc2) cells were also maintained and grown in the same conditions as SK-Hep1 except for the addition of 100 μg/ml hygromycin to the medium.
Plasmid transfection and stable clone selection. pGL4.50 (luc2) was transfected into 2×106 SK-Hep1 cells in 10-cm dish using jetPEI-DNA transfection reagent (Polyplus Transfection, Illkirch, Bas-Rhin, France). Plasmid transfection and selection of stable clone followed the procedure as given in a previous study (3).
Development of HCC-bearing mice. Six-week-old nude mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan, ROC). A total of 1×107 SK-Hep1/luc2 cells were resuspended in 100 μl serum-free DMEM and mixed with 50 μl matrigel (Corning, New York, USA). The cell suspension was then inoculated subcutaneously into the right leg of nude mice (n=5 for each group) (17).
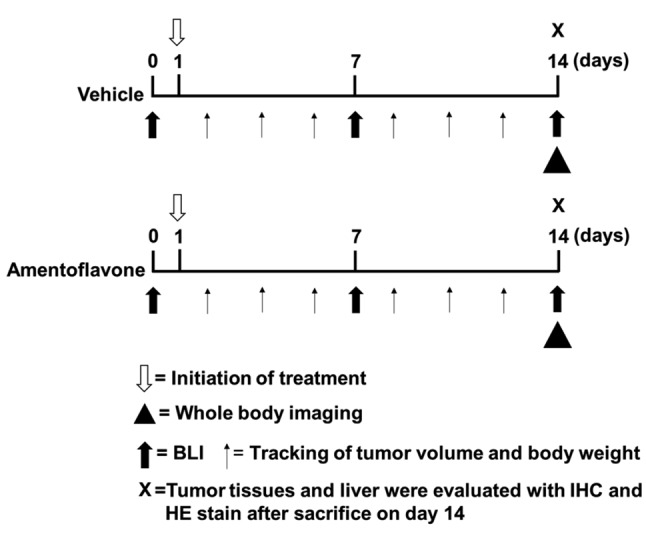
Experimental protocol (Figure 1). After the mean tumor volume reached 100 mm3, mice were treated by gavage with vehicle (140 μl phosphate-buffered solution and 10 μl dimethyl sulfoxide) or amentoflavone (100 mg/kg) daily for 14 days (n=5 for each group). Digital calipers were used to measure tumor growth, and tumor volume was calculated using the formula 0.523 × length × width × thickness of tumor. Bioluminescent imaging (BLI) and computed tomography (CT) for small animals were also used to monitor tumor growth. The body weights of mice were taken daily during treatment. Mice were sacrificed after treatment for 14 days. Tumor and normal liver tissue were taken for immunohistochemistry (IHC) staining and pathological examination. Three independent experiments were repeated. The protocol follows institutional animal care and use guidelines. The animal experiment was approved by Taipei Medical University Institutional Animal Care and Use Committee (LAC-2017-055).
Figure 1. Schematic depiction of experimental protocol. Please refer to Materials and Methods section for details. BLI: Bioluminescent imaging, IHC: immunohistochemistry, HE: hematoxylin and eosin.
In vivo BLI. Before image acquisition, mice were anesthetized using 1-3% isoflurane and injected intraperitoneally with 150 mg/kg D-luciferin 15 min. Tumor growth was detected by using BLI once per week as previously described (10). The photon signal from tumor was detected with Xenogen IVIS imaging system 200 series (Xenogen, Alameda, CA, USA) at an acquisition period of 10 s. Photon signals from regions of interest (ROIs) of tumor were calculated with Living Image version 2.20 (Xenogen).
In vivo whole-body imaging. Mice tumor were scanned by animal single-photon-emission computed tomographic scanner (Mediso Ltd., Budapest, Hungary) on day 14 after treatment. Mice were anesthetized using 1-3% isoflurane and scanned for 10 min (operation parameters for tumor monitoring: tube energy 55 kVp × 145 μA, 360 projections, voxel size 145×145×145 μm).
IHC staining. After 14 days treatment, mice were sacrificed. Tumors were resected and fixed in 4% paraformaldehyde (PFA) at 4˚C. Paraffin-embedded tumor specimens were sliced into 5-μm thickness by Bio-Check Laboratories Ltd (New Taipei City, Taiwan, ROC). IHC staining was used to evaluate protein expression in tumors and instruction provided by manufacturer was followed. Tumor tissues mounted on slides were stained with antibodies [anti-pERK, anti-NF-ĸB p65 (Ser536), anti-MMP-9, anti-VEGF, anti-cyclin-D1, anti-MCL-1, anti-C-FLIP, and anti-XIAP] and then scanned by TissueFAXS (TissueGnostics, Vienna, Austria) at 100× magnification. Image J version 1.50 (National Institutes of Health, Bethesda, MD, USA) was used to quantify IHC staining.
Pathological examination. Mice were sacrificed on day 14 after treatment. Livers were extracted and fixed in 4% PFA at 4˚C. Paraffin-embedded liver specimens were sliced into 5-μm thickness and hematoxylin and eosin (HE) staining was performed by Bio-Check Laboratories Ltd (New Taipei City, Taiwan, ROC). Liver tissues mounted on slides were stained with hematoxylin and eosin, and scanned by TissueFAXS (TissueGnostics, Vienna, Austria) at 100× magnification.
Statistical analysis. The Student’s t-test was used for verifying statistical significance of differences between amentoflavone-and vehicle-treated groups. Data were presented as means±standard error. Differences with p-values of less than 0.05 were considered statistically significant.
Results
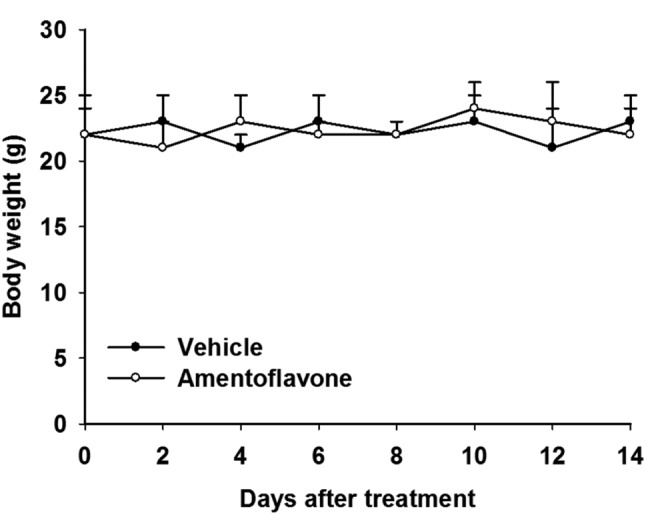
Acute toxicity analysis of amentoflavone in SK-Hep1/luc2 tumor-bearing mice. Body weight tracking of mice was used to verify whether amentoflavone induced acute toxicity in mice. SK-Hep1/luc2 tumor-bearing mice were treated with vehicle (140 μl PBS plus 10 μl DMSO by gavage) or amentoflavone (100 mg/kg/day by gavage) for 14 days. During the treatment period, body weights within and between vehicle and amentoflavone groups did not significantly change or differ from each other (Figure 2).
Figure 2. Effect of amentoflavone on body weight of SK-Hep1/luc2 tumor-bearing mice. Body weight of mice in vehicle-treated and amentoflavone-treated groups was measured on days 0, 2, 4, 6, 8, 10, 12, and 14 after treatment.
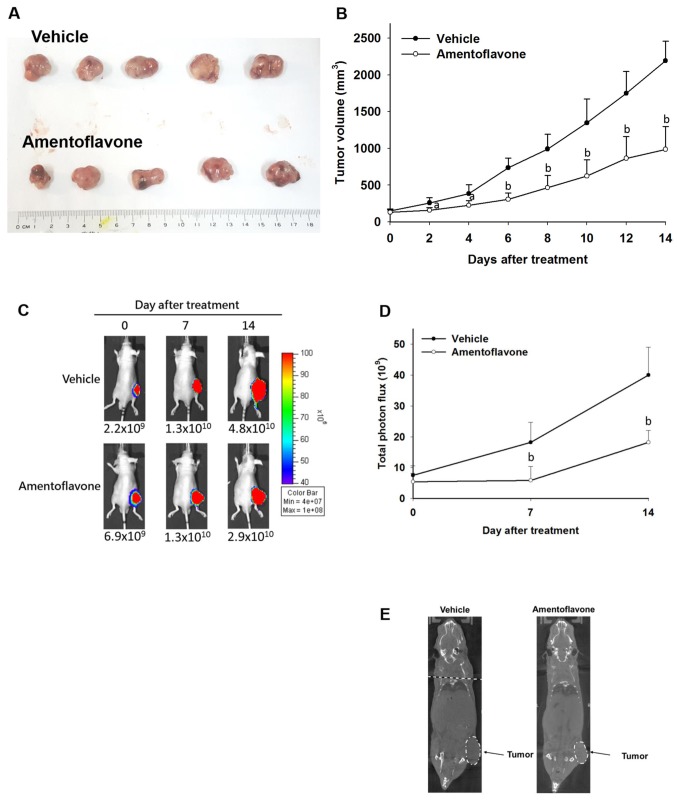
Amentoflavone inhibits tumor growth in SK-Hep1/luc2 tumor-bearing mice. Digital calipers were used to measure tumor volume of each group three times per week. During the treatment period, tumor volume of the amentoflavone-treated group was smaller than that of the vehicle-treated group. Figure 3A and B indicate amentoflavone significantly reduced the tumor volume compared to the vehicle-treated group. Amentoflavone significantly inhibited tumor growth, reducing tumor volume by 50% compared with the vehicle-treated group on day 14 after treatment.
Figure 3. Effect of amentoflavone on tumor growth of SK-Hep1/luc2 tumor-bearing mice. During the treatment period, tumor growth of mice was monitored using digital caliper, bioluminescent imaging (BLI), and animal computed tomography. A: Photograph of tumor removed from mice sacrificed on day 14 after treatment. B: Tumor volume was measured with digital caliper. C: Tumor growth was monitored with BLI and photographsof mice are shown. D: Quantification of total photon flux of BLI. E: Images of whole-body computed tomographic scan on day 14 after treatment. Significantly different at ap<0.05 and bp<0.01 compared to the vehicle-treated group.
On day 14 after treatment, 15 min after luciferin injection, tumor growth was assessed using BLI. Luciferase protein was constitutively expressed in living SK-Hep1/luc2 cells. Total photo flux of SK-Hep1/luc2 tumor in the amentoflavone-treated group was significantly reduced by 70 and 55% on day 7 and 14 after treatment (Figure 3C and D) as compared to the vehicle-treated group.
Whole-body imaging was acquired by using animal CT (Figure 3E). Under CT imaging the SK-Hep1/luc2 tumor size of the vehicle-treated group was greater than that of the amentoflavone-treated group.
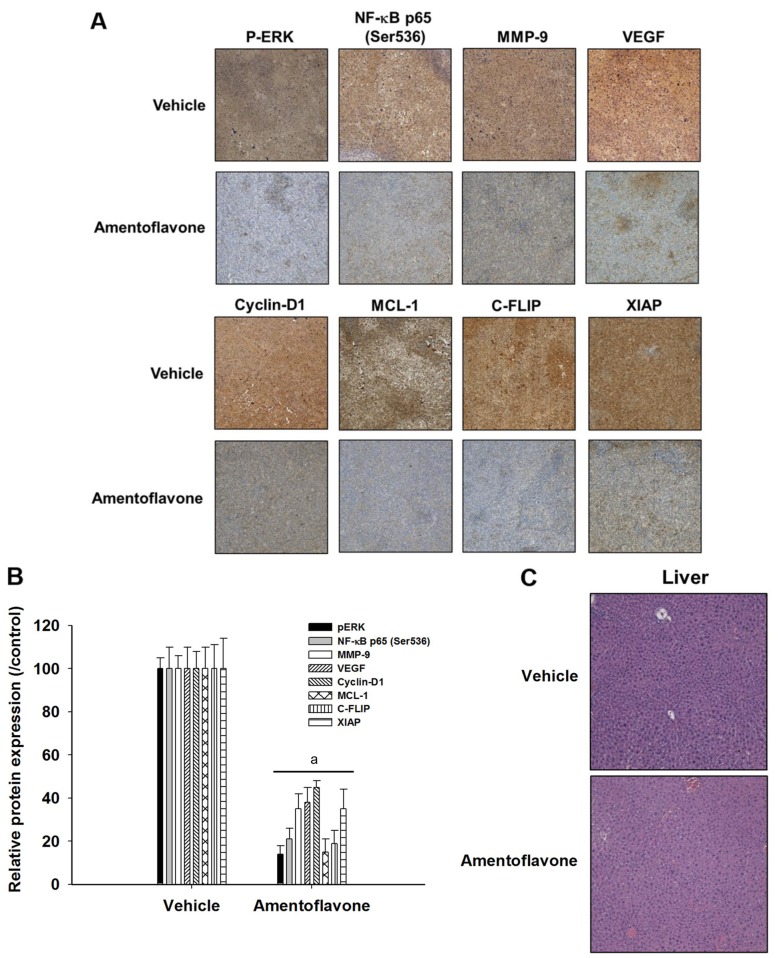
Amentoflavone reduces expression of ERK/NF-ĸB-modulated tumor progression associated proteins in SK-Hep1/luc2 tumor-bearing mice. IHC was used to verify the effect of amentoflavone on expression of tumor progression-associated proteins modulated by ERK/NF-ĸB. Figure 4A and B indicate amentoflavone significantly reduced protein expression of p-ERK, NF-ĸB p65 (Ser536), MMP-9, VEGF, cyclin-D1, MCL-1, C-FLIP, and XIAP in SK-Hep1/luc2 tumor tissues by 55-85% compared to the vehicle-treated group. Hepatocyte morphology was not affected by vehicle or amentoflavone treatment (Figure 4C).
Figure 4. Effect of amentoflavone on tumor progression-associated proteins modulated by extracellular signal-regulated kinase (ERK)/nuclear factorĸB (NF-ĸB) in SK-Hep1/luc2 tumor-bearing mice. Protein levels of phosphorylated (P)ERK, NF-ĸB p65 (Ser536), matrix metallopeptidase 9 (MMP- 9), vascular endothelial growth factor (VEGF), cyclin-D1, myeloid leukemia cell differentiation protein (MCL-1), cellular FADD-like IL-1β-converting enzyme-inhibitory protein (C-FLIP), and X-linked inhibitor of apoptosis protein (XIAP) in tumor tissues were investigated by immunohistochemistry. A: Image at magnification of 100×. B: Quantification of protein level. C: Pathological examination of liver tissues obtained from mice sacrificed on day 14 after treatment was evaluated by hematoxylin-eosin staining. aSignificantly different at p<0.01 compared to the vehicle-treated group.
Discussion
Active anti-apoptotic signaling is key to drug resistance which causes treatment failure in HCC. Anti-apoptotic proteins such as MCL-1, C-FLIP, and XIAP attenuate the cytotoxicity of anticancer drugs through blockage of intrinsic and extrinsic apoptotic pathways in HCC (16). Sorafenib is an oral multi-kinase inhibitor for treatment of HCC. Overexpression of MCL-1 diminishes sorafenib-induced apoptosis (18). High expression of both C-FLIP (inhibitor of caspase-8 activation) and XIAP (inhibitor of caspase-3 activation) was found to be associated with poor survival in patients with HCC (4,6). Cyclin-D1, a regulator of cell cycle at the G1 to S transition, was also overexpressed in HCC tissues. High expression of cyclin-D1 was also correlated with tumor stage in HCC (19). Silencing of C-FLIP, XIAP, cyclin-D1, or MCL-1 by small interfering ribonucleic acid (siRNA) or short hairpin RNA inhibited cell proliferation and triggered apoptosis in HCC cell lines (4,6,20).
Metastasis contributes to the high mortality rate of HCC. MMP-9 and VEGF are metastasis-associated proteins. MMP-9, also called gelatinase B, mediates degradation of the basement membrane, leading to tumor cell invasion and metastasis. VEGF regulates tumor angiogenesis, which is mandatory for metastasis (21). High expression of both VEGF and MMP-9 is potentially predictive of metastatic recurrence after curative resection of HCC (22,23). siRNA targeting VEGF and MMP-9 inhibited angiogenesis, tumor growth, and invasion in HCC in vitro and in vivo (24,25). Previous studies indicated blockage of ERK/NF-ĸB signaling inhibits tumor growth and protein expression of MMP-9, VEGF, cyclin-D1, MCL-1, C-FLIP, and XIAP in Huh7 and SK-Hep1 HCC cell lines in vitro and in vivo. Both sorafenib and regorafenib, oral multi-kinase inhibitors used for treatment of HCC, have been shown to inhibit HCC progression through suppression of ERK/NF-ĸB signaling (10,17). Figures 2 and 3 demonstrate amentoflavone significantly inhibited tumor growth and reduced expression of tumor progression-associated proteins modulated by ERK/NF-ĸB in SK-Hep1/luc2 tumor-bearing mice.
In conclusion, the present study demonstrated that amentoflavone inhibits HCC progression through suppression of ERK/NF-ĸB activation in vivo. We suggest that amentoflavone as a potential anti-HCC agent which may provide better therapeutic benefit for patients with HCC.
Conflicts of Interest
The Authors disclose no potential conflicts of interest in regard to this study.
Acknowledgements
The project was supported by the National Health Research Institutes and Taipei Medical University Hospital (grant no. MG-105-SP-07 and MG-106-SP-07). This study was also supported by grants RD2017-016 (National Yang-Ming University Hospital, Yilan, Taiwan) and CTU106-P-16 (Central-Taiwan University of Science and Technology, Taichung, Taiwan). The Authors acknowledge the technical services provided by Clinical Medicine Research Laboratory of National Yang-Ming University Hospital. We would also like to thank Translational Laboratory, Department of Medical Research, Taipei Medical University Hospital, Taipei, Taiwan for equipment support.
References
- 1.Nishida N, Goel A. Genetic and epigenetic signatures in human hepatocellular carcinoma: a systematic review. Curr Genomics. 2011;12:130–137. doi: 10.2174/138920211795564359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YC, Wu RH, Wang WS. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-ĸB inactivation in SK-Hep1 cells. Oncol Lett. 2017;14:461–467. doi: 10.3892/ol.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-ĸB activation in hepatocellular carcinoma cells. Oncol Rep. 2017;37:1036–1044. doi: 10.3892/or.2016.5328. [DOI] [PubMed] [Google Scholar]
- 4.Che Y, Ye F, Xu R, Qing H, Wang X, Yin F, Cui M, Burstein D, Jiang B, Zhang DY. Co-expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am J Pathol. 2012;180:1798–180. doi: 10.1016/j.ajpath.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, Crevenna R, Monia BP, Peck-Radosavljevic M, Wacheck V. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Du X, Bao G, He X, Zhao H, Yu F, Qiao Q, Lu J, Ma Q. Expression and biological significance of C-FLIP in human hepatocellular carcinomas. J Exp Clin Cancer Res. 2009;28:24–31. doi: 10.1186/1756-9966-28-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Zhao GD, Shi Z, Qi LL, Zhou LY, Fu ZX. The RAS/RAF/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett. 2016;12:3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR, Schmid KW, Baba HA. Activation of the ERK and AKT signaling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48:83–90. doi: 10.1016/j.jhep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Pacifico F, Leonardi A. NF-kappaB in solid tumors. Biochem Pharmacol. 2006;72:1142–1152. doi: 10.1016/j.bcp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Hsu FT, Liu YC, Chiang IT, Liu RS, Wang HE, Lin WJ, Hwang JJ. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-ĸB signaling. Int J Oncol. 2014;45:177–188. doi: 10.3892/ijo.2014.2423. [DOI] [PubMed] [Google Scholar]
- 11.Chen JC, Chuang HY, Hsu FT, Chen YC, Chien YC, Hwang JJ. Sorafenib pretreatment enhances radiotherapy through targeting MEK/ERK/NF-ĸB pathway in human hepatocellular carcinoma-bearing mouse model. Oncotarget. 2016;7:85450–85463. doi: 10.18632/oncotarget.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang WH, Chiang IT, Ding K, Chung JG, Lin WJ, Lin SS, Hwang JJ. Curcumin-induced apoptosis in human hepatocellular carcinoma J5 cells: critical role of ca(+2)-dependent pathway. Evid Based Complement Alternat Med. 2012;2012:512907. doi: 10.1155/2012/512907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu FT, Liu YC, Liu TT, Hwang JJ. Curcumin sensitizes hepatocellular carcinoma cells to radiation via suppression of radiation-induced NF-ĸB activity. Biomed Res Int. 2015;2015:363671. doi: 10.1155/2015/363671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WL, Hsieh CL, Chen JH, Huang CS, Chen WT, Kuo YC, Chen CY, Hsu FT. Amentoflavone enhances sorafenib-induced apoptosis through extrinsic and intrinsic pathways in sorafenib-resistant hepatocellular carcinoma SK-Hep1 cells in vitro. Oncol Lett. 2017;14:3229–3234. doi: 10.3892/ol.2017.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- 16.Chiang IT, Chen WT, Tseng CW, Chen YC, Kuo YC, Chen BJ, Weng MC, Lin HJ, Wang WS. Hyperforin inhibits cell growth by inducing intrinsic and extrinsic apoptotic pathways in hepatocellular carcinoma cells. Anticancer Res. 2017;37:161–167. doi: 10.21873/anticanres.11301. [DOI] [PubMed] [Google Scholar]
- 17.Weng MC, Wang MH, Tsai JJ, Kuo YC, Liu YC, Hsu FT, Wang HE. Regorafenib inhibits tumor progression through suppression of ERK/NF-ĸB activation in hepatocellular carcinoma bearing mice. Biosci Rep. 2018;pii:BSR20171264. doi: 10.1042/BSR20171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu C, Lin LI, Cheng YC, Feng ZR, Shao YY, Cheng AL, Ou DL. Cyclin E1 inhibition can overcome sorafenib resistance in hepatocellular carcinoma cells through Mcl-1 suppression. Clin Cancer Res. 2016;22:2555–2564. doi: 10.1158/1078-0432.CCR-15-0499. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Li X, Cheng Q, Ning D, Ma J, Zhang ZP, Chen XP, Jiang L. Effects of cyclin D1 gene silencing on cell proliferation, cell cycle, and apoptosis of hepatocellular carcinoma cells. J Cell Biochem. 2018;119:2368–2380. doi: 10.1002/jcb.26400. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of MCL-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YC, Wu RH, Wang WS. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-ĸB inactivation in SK-Hep1 cells. Oncol Lett. 2017;14:461–467. doi: 10.3892/ol.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu Q, Tang ZY, Ma ZC, Qin LX, Zhang LH. Serum vascular endothelial growth factor is a potential biomarker of metastatic recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol. 2006;6:565–568. doi: 10.3748/wjg.v6.i4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Cui J, Xu C, Xue T, Guo K, Gao D, Liu Y, Ye S, Ren Z. The significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence of hepatocellular carcinoma after curative resection. Ann Surg Oncol19. 2012;3:S375–84. doi: 10.1245/s10434-011-1836-7. [DOI] [PubMed] [Google Scholar]
- 24.Raskopf E, Vogt A, Sauerbruch T, Schmitz V. siRNA targeting VEGF inhibits hepatocellular carcinoma growth and tumor angiogenesis in vivo. J Hepatol. 2008;49:977–984. doi: 10.1016/j.jhep.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Zhu CP, Hu PF, Qian H, Ning BF, Zhang Q, Chen F, Liu J, Shi B, Zhang X, Xie WF. FOXA2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition. Carcinogenesis. 2014;35:2576–83. doi: 10.1093/carcin/bgu180. [DOI] [PubMed] [Google Scholar]