Abstract
The kidney is a major clearance organ of the body and is responsible for the elimination of many xenobiotics and prescription drugs. With its multitude of uptake and efflux transporters and metabolizing enzymes, the proximal tubule cell (PTC) in the nephron plays a key role in the disposition of xenobiotics and is also a primary site for toxicity. In this minireview, we first provide an overview of the major transporters and metabolizing enzymes in the PTCs responsible for biotransformation and disposition of drugs. Next, we discuss different cell sources that have been used to model PTCs in vitro, their pros and cons, and their characterization. As current technology is inadequate to evaluate reliably drug disposition and toxicity in the kidney, we then discuss recent advancements in kidney microphysiological systems (MPS) and the need to develop robust in vitro platforms that could be routinely used by pharmaceutical companies to screen compounds. Finally, we discuss the new and exciting field of stem cell–derived kidney models as potential cell sources for future kidney MPS. Given the push from both regulatory agencies and pharmaceutical companies to use more predictive “human-like” in vitro systems in the early stages of drug development to reduce attrition, these emerging models have the potential to be a game changer and may revolutionize how renal disposition and kidney toxicity in drug discovery are evaluated in the future.
Introduction
The kidneys perform essential functions in humans by maintaining the composition of blood and its pH; preventing the buildup of waste products; and keeping levels of electrolytes, such as sodium, potassium, and phosphate, stable. In normal adults, the two kidneys daily filter about 150–180 liters of blood to produce 1 to 2 liters of urine, which is comprised of wastes and extra fluid. The kidneys are also responsible for the elimination of numerous drugs, endogenous metabolites important to maintain physiologic homeostasis, exogenous and endogenous toxins, nutrients, and so forth. The elimination of exogenous and endogenous compounds through the kidney occurs as a net result of glomerular filtration, tubular secretion, kidney metabolism, and reabsorption. Evaluation of the mechanisms involved in the elimination of drugs and other exogenous and endogenous molecules can provide valuable understanding of their clearance, the potential for drug-drug interactions (DDIs), the potential for development of kidney and other organ toxicity, and thus on the effect of the elimination of an investigational drug on its pharmacokinetics (PK) in patients with compromised kidney functions.
In the past several decades, considerable progress has been made in our understanding of the mechanisms by which drugs and xenobiotics are eliminated by kidneys. The discovery and identification of several important tubular apical and basolateral transporters and their role in the elimination and reabsorption of xenobiotics and endogenous substrates have spearheaded this renaissance (Morrissey et al., 2013; Nigam et al., 2015; Miners et al., 2017). Furthermore, metabolizing enzymes in the kidneys also play an important role in the clearance of xenobiotics and endogenous compounds (Lash, 1994; Lock and Reed, 1998; Lohr et al., 1998; Knights et al., 2013). Therefore, it is important to evaluate early in development the following:1) the mechanism of clearance of new chemical entities (NCEs), 2) DDI as a victim if the metabolism and transport are modulated by coadministered drugs, 3) DDI as a perpetrator, and 4) potentials for toxicity. In a recent DDI guidance, the Food and Drug Administration has recommended performing clinical DDI studies to understand whether the NCE could be a victim of kidney transporter inhibition if it undergoes active renal secretion or there are concerns about renal toxicity. The guidance further recommends DDI studies as a perpetrator if in vitro studies demonstrate that the NCE has the potential for inhibition of cytochrome P450 (P450) enzymes and transporters, including kidney transporters, regardless of the investigational drug’s route of elimination [Food and Drug Administration (FDA) 2017a,b].
Besides the liver, the kidney is one of the most frequent targets for drug-induced toxicity. Of the top 200 prescribed drugs, 32% undergo renal elimination (Morrissey et al., 2013). About 20%–30% of intensive care unit patients and ∼5% of hospitalized patients develop acute kidney toxicity, and nearly 20% of these toxicities are attributed to nephrotoxic drugs (Li et al., 2014), possibly because the kidney is an organ that is exposed to a lot of drugs, metabolites, and endogenous compounds by being the recipient of the 25% of cardiac output and an organ of elimination of many of these compounds (Tiong et al., 2014). Unfortunately, nephrotoxicity is identified late in the development programs, with only 2% of drug attritions happening in preclinical studies but 19% during phase 3 studies (Redfern et al., 2010). This inability to successfully remove nephrotoxic compounds from development early in the program could be attributable to a lack of appropriate preclinical models to investigate kidney toxicity (Li et al., 2014; Tiong et al., 2014).
Accumulating evidence indicates an important role of the kidney in the metabolism, transport, and clearance of xenobiotics, proteins, hormones, and endogenous compounds; consequently, there has been accelerated growth in the past decade in the development of technologies to investigate the disposition of NCEs targeted to treat human diseases and potential for toxicities. This article highlights the evolution of novel technologies developed to investigate the mechanism of disposition of drugs by the kidneys and the potential for toxicities, together with an overview of the role of kidney metabolism and transport and their involvement in kidney toxicity.
Overview of Metabolism and Transport of Drugs in Kidney and their Role in Kidney Toxicity
The metabolism of drugs in the kidney has been described extensively (Lash, 1994; Lock and Reed, 1998; Lohr et al., 1998; Knights et al., 2013; Gundert-Remy et al., 2014; Miners et al., 2017). Numerous enzymes play a role in the metabolism and clearance of endogenous and exogenous compounds, including P450 enzymes, non-P450 enzymes, such as uridine-diphosphate-glucuronosyltransferases (UGTs), esterases, glutathione-s-transferases, sulfotransferases, and some other enzymes highlighted in Table 1. Therefore, kidneys can execute a diverse array of metabolic reactions, such as oxidation, reduction, hydrolysis, and conjugation. Many of these reactions facilitate the elimination of drugs. Although these reactions are considered a detoxification mechanism, certain metabolism reactions lead to the formation of reactive species, resulting in kidney toxicities. For example, glutathione S-conjugates can undergo further metabolism to cysteine S-conjugates that can be eliminated as nontoxic mercapturic acids or catalyzed by β-lyase to unstable thiols. These thiols are highly reactive and can covalently bind to cellular macromolecules, causing cytotoxicity and carcinogenicity. In addition to β-lyase, cysteine conjugate S-oxidase and other enzymes can bioactivate chemicals to produce nephrotoxic species (Lash, 1994).
TABLE 1.
List of drug-metabolizing enzymes commonly associated with biotransformation of drugs and xenobiotics in the kidney (Lash, 1994; Lock and Reed, 1998; Lohr et al., 1998; Knights et al., 2013)
| Phase 1 Enzymes | Conjugating Enzymes | Hydrolytic and Other Metabolizing Enzymes |
|---|---|---|
| Cytochrome P450a CYP2B6, CYP3A5, CYP4A11, CYP4F2, CYP4F8, CYP4F11, CYP4F12 | UDP-Glucuronocyltransferasesb UGT1A5, UGT1A6, UGT1A7, UGT1A9, UGT2B7 | Epoxide hydrolase |
| Microsomal flavin adenine dinucleotide-containing monooxygenase | N-acetyl transferase | Catalase |
| Alcohol and aldehyde dehydrogenase | N-methyl transferase | NADPH-quinone oxidoreductase |
| Prostaglandin synthetase | Glutathione S-transferase | Superoxide dismutase |
| Monoamine oxidase | Glutathione disulfide reductase | |
| Steroid 21 hydroxylase | Sulfotransferases | Esterases, carboxyesterases |
| Thiol S-methyl transferase | ||
| Cysteine conjugate β-lyase | ||
| Glutathione peroxidase | ||
| Glutamyl transferase, cysteinylglycinase |
Major P450 enzymes identified in the kidney.
UGT enzymes expressed in human kidney.
Nephrotoxicity owing to biotransformation in the kidney has been well documented. Chloroanilines are commonly used as chemical intermediates to manufacture dyes, agricultural chemicals, drugs, and industrial compounds. Among trichloroanilines (TCAs), 3,4,5-trichloroaniline is the most potent nephrotoxicant. Using isolated renal cortical cells from rats, Racine et al. (2014) demonstrated that pretreatment with P450 inhibitor piperonyl butoxide, cyclooxygenase inhibitor indomethacin, or peroxidase inhibitor mercaptosuccinate reduced TCA-mediated cytotoxicity; however, no effect was seen after pretreatment with flavin-containing monooxygenase (FMO) inhibitors, indicating that bioactivation of TCA to toxic metabolites by P450s, cyclooxygenase, and peroxidase contributed to the TCA cytotoxicity (Racine et al., 2014). Acyclovir and other antiviral drug-induced nephrotoxicities have caused concerns in humans (Izzedine et al., 2005). In vitro studies using cultured human proximal tubular cells (PTCs) suggested that the acyclovir-induced toxicity was associated with the formation of acyclovir aldehyde in the kidney by alcohol dehydrogenase (Gunness et al., 2011). Cisplatin causes dose-limiting nephrotoxicity in rodents and humans (Wainford et al., 2008). Inhibition of γ glutamyltransferase (GGT) prevented cisplatin induced nephrotoxicity in vivo in rats and C57BL6 mice, indicating that this enzyme plays a critical role in cisplatin nephrotoxicity. In vitro studies using isolated rat and human renal PTCs demonstrated that amino peptidase-N, renal dipeptidase, and C-S lyase were not involved in cisplatin-induced toxicity.
Renal transporters also play an important role in the disposition of drugs and the development of kidney toxicity; the latter by accumulation of compounds and/or their metabolites. These transporters are part of two superfamilies of carrier proteins, ATP-binding cassette (ABC) and solute carrier (SLC) (Morrissey et al., 2013; Nigam et al., 2015; Miners et al., 2017). The SLCs generally transport substances either down their concentration gradient or against their concentration gradient, coupled with movement of a second substance down its concentration gradient. In the kidney, the most multispecific SLC transporters appear to be organic anion transporters (OATs): OAT1 (SLC22A6) and OAT3 (SLC22A8) and organic cation transporter (OCT2, SLC22A2). A recent publication suggests that another less commonly cited transporter, OAT2, expressed in both the liver and kidney, is involved in the elimination of several drugs, including creatinine and cGMP in the kidney (Shen et al., 2017). Accumulating evidence further indicates the importance of several other SLC families of transporters, such as multidrug and toxin extrusion proteins (MATEs, SLC37), peptide transporters (PEPT, SLC15), and organic carnitine/zwitterionic transporters (OCTN, SLC22A4 and SLC22A5). ABC transporters use energy generated by the hydrolysis of ATP to transport molecules across cell membranes. The most commonly linked ABC transporters of significance for pharmaceuticals’ transport are P-glycoprotein (P-gp, ABCB1), breast cancer resistance protein (BCRP, ABCG2), and multidrug resistance proteins (MRP, ABCC), such as MRP2 and MRP4. Table 2 shows the nomenclature, common names, and localization of key transporters in the PTCs. OAT1, OAT3, and OCT2 on the basolateral membrane and P-gp, MATEs, MRP2, and MRP4 on the apical membrane are the major PTC transporters known to interact with many renally secreted drugs (Morrissey et al., 2013). Renal transporter–mediated elimination of drugs or metabolites has been extensively investigated, and the results indicate that, in general, drugs with molecular weight less than 400 Da are substrates of several renal transporters (Varma et al., 2017).
TABLE 2.
A list of transporters commonly associated with the transport of drugs and xenobiotics in the kidney proximal tubule
| Common Name (Symbol) | Gene Family-Derived Name | Localization |
|---|---|---|
| Organic cation transporter 2 (OCT2) | SLC22A2 | Basolateral |
| Organic anion transporter 1 (OAT1) | SLC22A6 | Basolateral |
| Organic anion transporter 3 (OAT3) | SLC22A8 | Basolateral |
| Organic anion transporter 4 (OAT4) | SLC22A11 | Apical |
| Urate anion exchanger 1 (URAT1) | SLC22A12 | Apical |
| Multidrug and toxin extrusion protein 1 (MATE1) | SLC47A1 | Apical |
| Multidrug and toxin extrusion protein 2K (MATE2K) | SLC47A2 | Apical |
| Oligoeptide transporter 1 (PEPT1) | SLC15A1 | Apical |
| Oligoeptide transporter 2 (PEPT2) | SLC15A2 | Apical |
| Multidrug resistance protein 1 (P-gp) | ABCB1 | Apical |
| Breast cancer resistance protein (BCRP) | ABCG2 | Apical |
| Multidrug resistance associated rrotein 2 (MRP2) | ABCC2 | Apical |
| Multidrug resistance associated protein 4 (MRP4) | ABCC4 | Apical |
Whereas renal transporters generally enhance renal elimination, certain transporters, such as OAT4, PEPT2, and sodium-glucose cotransporter-2 (SGLT2), located on the apical side of the PTCs, facilitate reabsorption of their substrates (Burckhardt, 2012; DeFronzo et al., 2017; Tchernitchko et al., 2017). As renal elimination of endogenous and xenobiotic compounds is determined by its net vectorial transport, the PK parameters will be affected when an NCE and/or its metabolites inhibit any of the transporters responsible for the elimination of these molecules. The subsequent imbalance can result in adverse effects on the kidney or any other organ. Because of the significance of the several kidney transporters in regulating drug clearance, regulatory authorities that govern the approval of pharmaceuticals for humans require evaluation of the potential for an NCE to be a perpetrator of DDI and, if renal active secretion is significant (>25% of total clearance), to evaluate the potential to be a victim of DDIs [European Medicines Agency (EMA), 2012; FDA, 2017a,b). Inhibition of these transporters can affect not only exposure to the coadministered drugs but also the elimination of endogenous and exogenous toxins, therefore posing significant risks to patients. More recently, the International Transporter Consortium published recommendations to help guide clinical studies on the currently accepted drug transporter interactions (Giacomini et al., 2010). A list of probe substrates and inhibitors for in vitro and in vivo DDI assessment can be found in the FDA and pharmaceuticals and medical devices agency DDI guidelines (EMA, 2012; FDA, 2017a,b).
Emerging data associate membrane transporters to kidney disease and toxicity if a drug is accumulated in the kidney to a certain level (Dresser et al., 2001; Nies et al., 2011; Miners et al., 2017). Accumulation in the kidney can arise when uptake of the drug into the kidney PTCs is faster than efflux out of the kidney, a process generally mediated by transporters. Nephrotoxicity occurs when a NCE is a substrate of one or multiple transporters that are inhibited by concomitant medication or if the NCE inhibits the transport of toxicants or coadministered drug(s), resulting in an increase in intracellular and/or circulating concentration of substrate(s) of the transporter involved. For example, the nephrotoxicity of cisplatin has been associated with renal accumulation, determined by the substrate specificity of the OCT2 and MATE families of transporters (Yokoo et al., 2007). OCT2 mediates the transport of cisplatin into PTC from blood, whereas MATE1/2K transports cisplatin from PTC to urine. Several genetic variants with reduced functionality of OCT2 can lower uptake/accumulation of cisplatin in PTCs, thus reducing cisplatin-induced nephrotoxicity. Tenofovir is primarily a substrate of OAT1 and, to a lesser extent, OAT3, together they transport the nucleotide antiviral from blood into the PTC. The drug is subsequently transported out of PTC into urine by MRP4. The nephrotoxicity of tenofovir was due to accumulation of the drug in the PTC (Moss et al., 2014). Ritonavir, an inhibitor of multiple transporters expressed in the kidney, including MRPs, increases the propensity for developing nephrotoxicity in patients who are taking ritonavir in combination with tenofovir compared with patients treated with tenofovir alone (Moss et al., 2014). Cidofovir, a substrate for OATs, has been associated with dose-limiting nephrotoxicity (Ho et al., 2000). Probenecid, an inhibitor of OAT-mediated uptake of cidofovir by PTCs, decreases the prevalence of cidofovir-induced nephrotoxicity. OAT1-mediated uptake and accumulation of cephaloridine, a β-lactam antibiotic, has been connected to the cytotoxicity to the PTC. The toxicity could be completely prevented by probenecid (Tune and Fravert, 1980).
Whereas small molecules are excreted via active transport with the aid of transporters, glomerular filtration, and passive diffusion, the overall excretion of proteins involves an interplay between excretion and reabsorption. Many large, soluble biomolecules are reabsorbed in the PTCs by endocytosis as part of the renal physiology that deals with the retrieval of filtered proteins, thus preventing them from disappearing from the body through the urine. This is also essential for the recovery of vitamins, hormones, enzymes, and some drugs. The endocytic receptors megalin and cubilin have been identified as essential receptors responsible for the reabsorption of proteins (Christensen and Gburek, 2004; Nielsen et al., 2016).
Megalin is a 600-kDa glycosylated receptor containing a single transmembrane domain (23 amino acids) and an intracellular C-terminal cytoplasmic tail (209 amino acids). It is predominantly expressed in the apical membranes of the PTCs. The cytoplasmic domain of megalin regulates receptor trafficking and endocytosis. Cubilin is a 460-kDa glycosylated extracellular protein that interacts with other membrane proteins for membrane localization and endocytosis. In PTCs, cubilin interacts with megalin, forming a multireceptor complex with megalin, driving internalization of the complex and bound ligand. This has been supported by in vitro uptake studies of the cubilin ligands transferrin and apolipoprotein A-I showing that the uptake was inhibited by anti-megalin antibodies and megalin antisense nucleotides (Kozyraki et al., 2001; Nielsen et al., 2016). It has been further shown that cubilin also depends on a single transmembrane protein, amnionless, for membrane localization and endocytosis. Nielsen et al. (2016) extensively reviewed the role of megalin and cubilin in the reabsorption of proteins in the kidney and provided an extensive list of ligands of these receptors. Once the proteins are reabsorbed, they are subsequently degraded in lysosomes. The free amino acids are transported across the basolateral membrane by amino acid transporters.
Since reabsorption of the filtered proteins in the proximal tubule is an important physiologic and pathophysiological function by regulating biologically important substances like vitamins, hormones, enzymes, and others, the importance of the proper functioning of megalin and cubilin cannot be overstated. If not duly reabsorbed, the excess proteins in the tubular fluid, irrespective of their discrete biologic activities, are sufficient to initiate a cascade of events leading to tubular injury, interstitial inflammation, fibrosis, and eventual renal scarring (Kozyraki et al., 2001; Christensen and Gburek, 2004).
Whereas all kidney transporters have their own importance and can play a vital role in the disposition of drugs, an ideal PTC model should demonstrate at least functionality of OAT1, OAT3, and OCT2 on the basolateral side and MATEs, P-gp, BCRP, MRP2, and MRP4 on the apical side to be used in assessing the nephrotoxic liabilities of NCEs. In addition, especially if biologics or large molecules are being assessed, it should demonstrate megalin- and cubilin-mediated uptake potential of the large molecule. GGT enzyme, one of PTCs’ antioxidant defense mechanisms, should also be present at physiological levels in the model. Finally, many of the metabolic enzymes listed in Table 1 should also be a part of an ideal in vitro PTC model.
Common Sources of Kidney Cells
In the context of drug development, regulatory agencies have specified prominent transport proteins of which assessment of a drug’s inhibitory and substrate potentials is required (EMA, 2012; FDA, 2017a,b). Often, in vitro transporter-involved DDI studies are performed using single-transporter transfected cell lines, such as human embryonic kidney cell line 293 (HEK293) or Madin-Darby canine kidney (MDCK). Singly transfected cells allow for specific interactions to be elucidated, but correlation to in vivo interactions is not always straightforward. Primary renal cell cultures provide a more realistic model but are limited by donor availability and variability. Recently, the abundance of renal transporters, with the exceptions of BCRP and MATE-2K, within the human kidney cortex has been quantified (Prasad et al., 2016). Although large donor-to-donor and site-specific variabilities were seen, this information helps fill a large gap in the understanding of renal transporters and may be used to characterize in vitro cell lines and inform more accurate in vitro to in vivo extrapolation (IVIVE) models. The use of kidney cell lines to evaluate transporter interactions in the context of nephrotoxicity have been previously reviewed (Fisel et al., 2014; George et al., 2017). This section highlights common sources of renal models with respect to drug transporters, nephrotoxicity, and drug disposition.
Renal Proximal Tubule Cells/Telomerase Reverse Transcriptase.
One major challenge with human-derived cells is their limited capacity to be expanded in culture. Renal proximal tubule epithelial cells (RPTECs/PTCs) with ectopic expression of telomerase reverse transcriptase immortalizes human-derived RPTECs for up to 90 population doublings (Wieser et al., 2008). RPTEC/TERT1 cells stably express RNA and protein for OCT2, OCT3, OCTN2, OAT1, OAT3, OAT4, MATE1, and MATE2K. Functional transport of the fluorescent cation 4-Di-1-ASP by OCT/MATE was displayed in RPTEC/TERT1 cells; however, OAT function was questionable because of an inability to transport p-aminohippuric acid (PAH) (Aschauer et al., 2015a). The lack of OAT function may be overcome by stably transfected lines available through American Type Culture Collection (ATCC, 2016), although currently limited data are available. RPTEC/TERT1 cells have been used for in vitro kidney toxicity assessments via genetic analysis (Aschauer et al., 2015b) and of environmental toxicants (Simon et al., 2014; Simon-Friedt et al., 2015). Recently, highly differentiated three-dimensional (3D) tubules of RPTEC/TERT1 cells were developed by sandwiching them between Matrigel layers, leading to a branched network of cell-free lumen; however, OAT1 expression was negligible, even though other characteristics of a well differentiated epithelium, such as polar expression of Na+/K+ ATPase and ZO-3, were present (Secker et al., 2017). Although RPTEC/TERT1 cells provide an advantage of reduced culture time, they are limited by low OAT1 expression/function and require specialized culture media conditions. RPTEC/TERT1 cells can be licensed through Evercyte GmBH.
Conditionally Immortalized Human PTEC.
Researchers have generated conditionally immortalized PTCs by noninvasively obtaining renal material from the urine and transfecting those cells by using a temperature-sensitive mutant U19tsA58 of simian virus 40 large T antigen (SV40T) and telomerase reverse transcriptase vectors (Wilmer et al., 2010). Transfection with SV40T allows the cells to proliferate at lower temperatures of 33°C, and these cells can be cultured for up to 10 days at 37°C. These cells were rigorously characterized and demonstrated multiple characteristics of the kidney epithelium, such as the presence of tight junction proteins (ZO-1), PTC-specific brush-border enzyme (aminopeptidase N), aquaporin 1, megalin/cubilin endocytic receptors, P-gp, MRP4, and OCT2; however, these cells lacked the organic anion transporters OAT1 and OAT3. Later, Nieskens et al. (2016) generated OAT-overexpressing lines, namely, conditionally immortalized proximal tubule epithelial cells (ciPTEC)-OAT1 and ciPTEC-OAT3. These overexpressing ciPTECs demonstrated the functionality of OATs by showing dose-dependent cytotoxicity to the antivirals tenofovir, cidofovir, and adefovir and inhibition of fluorescein uptake when the cells were incubated with prototypic substrates and inhibitors of these transporters. Fedecostante et al. (2018) developed 3D kidney models on decellularized rat scaffolds by recellularizing with ciPTEC-OAT1–overexpressing cells. These cells showed a strong mRNA expression of a multitude of renal transporters, such as OCT2, OAT1, P-gp, BCRP, MATEs, and MRP4. More important, the expression levels of the recellularized scaffold were always stronger than its 2D counterpart. When challenged with nephrotoxicants such as cisplatin, tenofovir, and cyclosporine A, the recellularized scaffold showed dose-dependent cytotoxicity; for cisplatin, the 3D platform showed a higher sensitivity than the 2D monolayer because of higher levels of OCT2 and MATEs in the 3D model. While this is indeed a novel platform, more studies with different types of nephrotoxicants are needed to show its actual potential for drug discovery.
Transfected Cell Lines.
HEK293, MDCK, pig kidney–derived cells (LLC-PK1), and Chinese hamster ovary cell lines are commonly transfected with a single transporter for use in in vitro testing. These cell lines have been transfected with all relevant drug transporters to determine uptake and inhibition parameters of NCEs. Transfected cell lines are easily obtained, simple to maintain, and provide relatively reproducible data. These cell lines could be used to characterize substrate selectivity and species differences explaining renal toxicity (Zou et al., 2018). Hence, transfected cell lines are the most common tools used to evaluate transporter interactions in vitro in the kidney; however, lack of physiology limits their utility in toxicity testing or for predictive screens.
Epithelial Cell Lines from Normal Adult Human Kidney.
In 1994, human renal cortex–derived cells were transfected with the human papilloma virus 16 E6/E7 genes to create the immortalized human kidney (HK-2) cell line (Ryan et al., 1994). HK-2 cells have since been used to show that P-gp is suppressed by vancomycin (Im et al., 2017), to elucidate the biotransformation and toxicity of acyclovir (Gunness et al., 2011), to assess in vitro biomarkers of cisplatin nephrotoxicity (Sohn et al., 2013), and to evaluate general nephrotoxicity of compounds (Wu et al., 2009; Li et al., 2017). P-gp (Tramonti et al., 2001) and monocarboxylate transporter (MCT) (Wang et al., 2006) have been fully characterized in HK-2 cells; however, an extensive genetic analysis of HK-2 cells revealed that OAT1, OAT2, OAT3, OCT2, MRP2, and BCRP were not expressed by HK-2 cells, bringing into question their overall utility in assessing renal transporter function or transporter-related toxicities (Jenkinson et al., 2012). Thus, HK-2 cells may be limited in future nephrotoxicity assessments as they were shown to be inferior to primary RPTECs/PTCs for in vitro nephrotoxic biomarker (KIM-1, neutrophil gelatinase-associated lipocalin, and macrophage colony-stimulating factor) production (Huang et al., 2015). Additionally, we are unaware of any reports regarding expression or function of MATEs in HK-2 cells.
RPTECs.
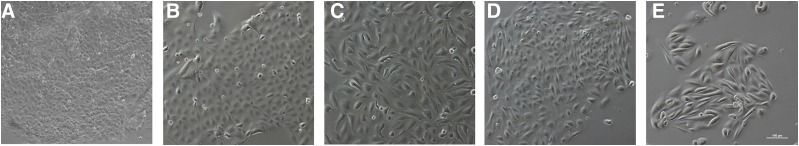
RPTECs or PTCs, whether cryopreserved or freshly isolated, are often thought of as the “gold standard” for in vitro kidney work. Freshly isolated RPTECs express mRNA for all the major renal transporters, predominantly in the proximal tubule. The function of P-gp, BCRP, MRP2, OCT2, and OAT1/3 was also established (Brown et al., 2008). It should be noted that determination of specific transporter effects is challenging. For instance, functional studies confirm contributions of both OAT1- and OAT3-mediated transport of substrates such as PAH and statins (Burckhardt and Burckhardt, 2003; Windass et al., 2007; Brown et al., 2008). Commercial entities, such as Solvo Biotechnology, ATCC, and others, offer a wide range of cryopreserved and freshly isolated proximal tubule cells. As large donor-to-donor and site-specific variabilities of renal transporters were shown in isolated healthy kidneys (Prasad et al., 2016) and large differences in native tissue versus cell lines (Hilgendorf et al., 2007), it can be assumed that large variability in transporter expression and function exists within available RPTECs. This may be further exacerbated by disease states (Motohashi et al., 2002; Habu et al., 2003; Feng et al., 2010). Cryopreserved and freshly isolated RPTECs each come with their own challenges. The availability of primary tissue is limited, and many protocols exist for purifying proximal tubules from other renal cells (Gozalpour and Fenner, 2018). Cryopreservation can assist with availability issues, and cryopreserved RPTECs have been shown to express kidney tubule specific markers (Adler et al., 2016) and are more differentiated than cell lines like HK-2 (David et al., 2004); however, cryopreserved RPTECs lack strong transporter mRNA expression compared with freshly isolated renal tissue (Van der Hauwaert et al., 2014). RPTECs have been used extensively in nephrotoxicity investigations and were reviewed recently (Gozalpour and Fenner, 2018). RPTECs were used to describe biotransformation-related nephrotoxicity of cisplatin (Wainford et al., 2008) and TCA (Racine et al., 2014). In Fig. 1, phase-contrast images of a few different cell types show minor variations in their morphology. Although both fresh and cryopreserved PTCs show greater physiologic relevance to the native kidney compared with cell lines in terms of the transporters, their expression can quickly downregulate in conventional static culture models. Hence, advanced dynamic models are needed.
Fig. 1.
Morphologic comparisons of commonly used kidney cell lines and primary cells derived from induced pluripotent stems cells or human tissue. (A) LLC-PK1, (B) MDCK, (C) HK-2, (D) iPSC-derived tubule epithelial cells, (E) proximal tubule epithelial cells grown from human kidney biopsy (scale bar = 100 µm).
In general, the limitations associated with these in vitro models to predict kidney toxicity are associated with the cell types because not all necessary transporters, metabolizing enzymes, or biomarkers are expressed at physiologic levels. Therefore, selecting the right endpoints becomes essential for a particular in vitro model. Many of the available PTC cell types discussed herein have also been used for nephrotoxicity prediction in both 2D and 3D systems. Tiong et al. (2014) reviewed the applicability of PTC cell types and culture systems for nephrotoxicity testing. One striking limitation is that few studies include more than 10 compounds. Thus, it becomes extremely difficult to generate any reasonable statistics related to the predictive performance of the assay and for researchers to assess properly the utility of the model. To our knowledge, only two groups have looked at a compound set large enough to generate the different parameters related to the predictive performance of the assay, such as sensitivity, specificity, area under the receiver operating characteristic curve (AUC-ROC), and meaningful positive predictive and negative predictive values. Few different reports have been published that evaluate the nephrotoxic potential of more than 40 compounds (Li et al., 2013, 2014; Kandasamy et al., 2015). The researchers compared the predictive performance of HK-2 cells, LLC-PK1 cells, primary PTCs (HPTCs), and stem cell–derived PTCs (HPTC-like). They showed that the primary PTCs cells, which retain multiple characteristics of the in vivo counterpart, showed the highest accuracy (AUC-ROC: 0.85), whereas HK-2 cells, which have limited transporter expression (Jenkinson et al., 2012), showed the lowest accuracy (AUC-ROC: 0.71). Another report recently compared the nephrotoxic potential of 39 mechanically distinct nephrotoxicants using primary PTCs and showed that heme oxygenase 1 combined with cell count yielded the highest predictive performance in their assay (AUC-ROC: 0.92) (Adler et al., 2016); however, tenofovir could not be detected as being nephrotoxic in their model, likely because of the lack of apical-to-basolateral polarity, which was seen when primary PTCs are grown on a flat surface.
Another limitation, particularly of 2D cultures, is the lack of appropriate toxicity biomarkers. Commonly nonspecific cell health markers, such as ATP, apoptosis/necrosis, mitochondrial function, and transepithelial electrical resistance, are used. The 2D cultures of stem cell–derived PTCs (HPTC-like) showed that more specific endpoints, such as inflammatory cytokines (interleukin-6) or chemokines (interleukin-8), demonstrated better predictive performance (AUC-ROC: 0.94) than a commonly used cytotoxic endpoint such as ATP depletion endpoints (AUC-ROC: 0.65) in primary PTCs (Li et al., 2013, 2014).
Importance of Fluid Shear Stress for KPT Cells
In vivo, there is a constant flow of glomerular filtrate over the PTCs. In addition to providing several different growth factors and hormones to the PTCs, the filtrate subjects the PTCs to shear stress, which is detected by the mechanosensory cilia located on the apical side of the cells. Although the exact mechanism of this mechanotransduction phenomenon is somewhat controversial (Delling et al., 2016), it has been convincingly shown in numerous studies that fluid-induced shear stress leads to enhanced endocytosis in renal cells (Raghavan et al., 2014; Long et al., 2017), cytoskeletal reorganization (Duan et al., 2008), and the presence of continuous tight junction (ZO-1) and adherens junction proteins (E-cadherin) (Duan et al., 2007), characteristics that suggest improved cellular maturity. Hence, over the past decade, there has been a great interest in mimicking the complex in vivo architecture and microenvironment of the kidney. By using the techniques initially developed for the semiconductor fabrication industry, numerous research groups and startup companies have now developed microfluidic devices using novel materials that can provide biomechanical cues to the kidney cells in vitro. In addition to the kidney, numerous other organs, such as the liver (Domansky et al., 2010), heart (Mathur et al., 2015), brain (Park et al., 2015), lung (Huh et al., 2010), among others, are also being developed.
Kidney MPS Technologies for Drug Discovery
Microfluidic Kidney MPS.
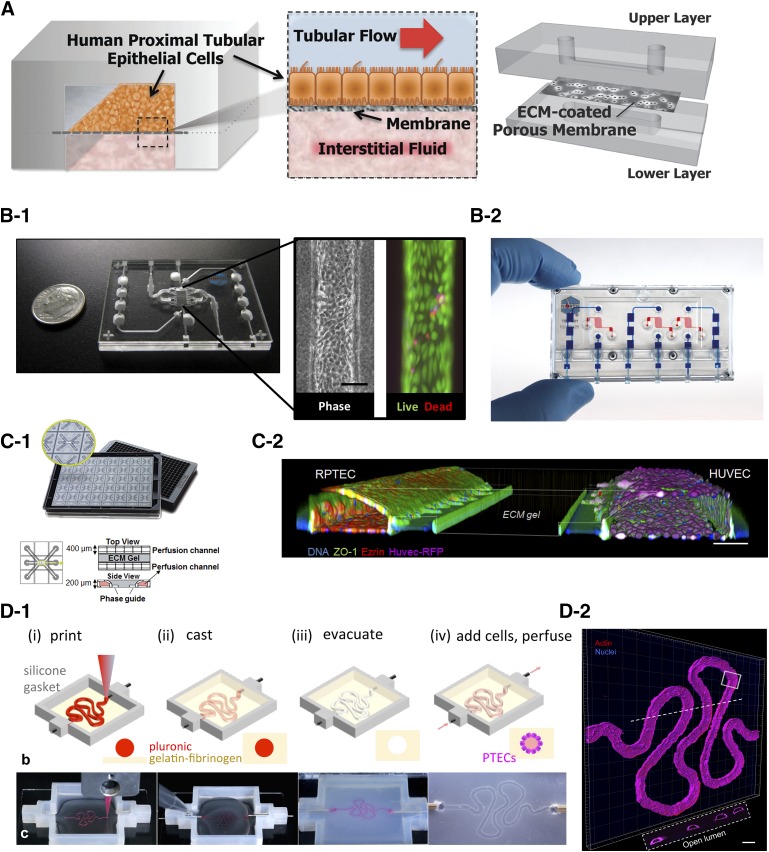
One of the first kidney-on-a-chip models with PTCs was developed by Ingber’s group. A polyester membrane coated with collagen IV was sandwiched between two polydimethylsiloxane (PDMS) slabs as shown in Fig. 2A (Jang et al., 2013). The bottom surface served as a medium or drug reservoir, and human primary PTCs were seeded on the top surface. After reaching confluence, a fluidic shear stress of 0.2 dyn/cm2 was applied to the cells on the top surface, which led to their maturation as demonstrated by increased expression of aquaporin 1 and Na+/K+ ATPase and an increased number of primary cilia compared with the static Transwell model. Furthermore, there was a marked increase in the expression of SGLT2, which led to increased glucose transport. Enhanced uptake of albumin was also reported, possibly owing to enhanced megalin-cubilin functionality. These researchers were also able to model cisplatin toxicity, which is transported to the PTCs via OCT2. PTCs seeded in the microfluidic chips showed increased fidelity to cisplatin by showing reduced lactate dehydrogenase release and apoptosis. Additionally, cimetidine, a known inhibitor of OCT2, offered significant protection to the PTCs from cisplatin-mediated toxicity in the microfluidic chip, but only partial protection was offered in the static model. Increased functionality of P-gp was also noted in the dynamic models compared with the static model. Thus, this work showed that shear stress helped the PTCs achieve a more mature phenotype, and a kidney-on-a-chip could better model nephrotoxicity by improved expression of both uptake and efflux transporters. Recently, development of a glomerulus-on-a-chip from human induced pluripotent stem was also reported by the same group (Musah et al., 2017).
Fig. 2.
Examples of kidney microfluidic devices (A–C) and bioprinted device (D). (A) Bilayer PDMS device with a sandwiched porous ECM-coated polyester membrane creating two compartments: top compartment for cell seeding with physiologic flow and bottom compartment serves as a reservoir. Reproduced with permission from the Royal Society of Chemistry and Jang et al. (2013). (B-1) Single-channel 3D MPS platform showing phase contrast and live/dead image of PTCs at day 28 within the microfluidic channel. Reproduced with permission from Elsevier and Weber et al. (2016) (B-2) Three-channel device of the 3D MPS platfrom shown in (B-1). Image provided by Nortis Bio (C-1) Overview of the platform for epithelium and endothelial tubule coculture. The three-lane OrganoPlate contains 40 microfluidic chips in a 384-well plate. Photos show top and bottom of the three-lane plate with zoom of a single chip. An ECM is patterned by two PhaseGuides. (C-2) A 3D reconstruction of a confocal stack shows the tubular morphology of the RPTEC and human umbilical cord endothelial cell cultures (HUVEC) alongside the ECM gel. The HUVEC cells express RFP (purple), and the tubes are stained for ZO-1 (green), ezrin (red), and DNA (blue). Scale bar, 100 µm. Image provided by Mimetas (D-1). Schematics and images showing the different steps of fabricating a 3D convoluted PTC channel. (D-2) A confocal 3D rendering of PTCs in the channel: actin (red) and nuclei (blue). Reproduced with permission from Homan et al. (2016). The work was published under a CC BY license (Creative Commons Attribution 4.0 International License; https://creativecommons.org/licenses/by/4.0/). No changes were made to the original figure.
University of Washington researchers collaborated with Nortis Inc. to develop a novel kidney-on-a-chip platform where a single straight microfluidic channel was molded inside a collagen I matrix using a microfiber (Fig. 2B1 and 2) (Weber et al., 2016). Primary human PTCs were seeded inside a collagen IV–coated channel, allowed to adhere for 24 hours, and then perfusion of media was initiated inside the lumen. Cells inside the lumen displayed characteristic features of mature PTCs with ZO-1 staining localized to the apical surface and basolateral expression of Na+/K+ ATPase, suggesting polarized epithelia. KIM-1, an important kidney injury biomarker, was present at low levels on the kidney-on-a-chip device but is often expressed at high levels in a 2D static model, suggesting that the architecture of the device, coupled with flow, helped with a quiescent phenotype of the cells and prevented epithelial mesenchymal transition. Other characteristics, such as strong GGT activity and strong functionality of SGLT2, were also noted in the kidney microphysiological system (MPS). The researchers also demonstrated metabolism potential of the MPS by showing bioactivation of vitamin D. Calcifediol, a vitamin D prehormone produced in the liver, was converted to the active form of vitamin D, calcitriol, through the action of CYP27B1 and to its inactive metabolites via CYP24A1. Finally, the researchers demonstrated that in the MPS device, probenecid, a competitive inhibitor of OAT1/3 and MRP2/4, decreased the apparent permeability of PAH, whereas no change in the apparent permeability was seen in the static 2D Transwell model. The researchers then coupled the kidney MPS with a liver MPS to elucidate the nephropathy of a commonly used Chinese herb, aristolochic acid (AA) (Chang et al., 2017). AA is a potent nephrotoxin; however, it often requires bioactivation and formation of reactive metabolites, a process that happens in the liver. AA showed modest cell death and KIM-1 expression when directly added to the kidney MPS before hepatic metabolism; however, when AA was first added to the liver MPS, which was coupled to the kidney MPS, increased cell death and KIM-1 expression occurred. Metabolites of AA were actively secreted out of the liver MPS via the action of MRPs and taken up by the PTCs via the action of OAT4 located on the apical side of the kidney MPS to cause nephrotoxicity. This and similar MPS systems could be used to model the toxicity of drugs in the future, develop pharmacokinetic/pharmacodynamic relationships, and be used to investigate organ-organ interactions.
Although the above-mentioned kidney MPS platforms show increased maturation of kidney cells compared with their static counterparts, most are currently being fabricated from PDMS, a polymer notorious in the absorption of small, lipid-soluble hydrophobic compounds. Hence, for the widespread use of these kidney MPS platforms, alternative materials need to be used for their fabrication to prevent, or at least minimize, drug absorption. van Midwoud et al. (2012) demonstrated that UV-ozone–treated polycarbonate and cyclic olefin copolymer showed excellent biocompatibility, ease of device fabrication, and transparency, characteristics required for microfluidic devices; moreover, these thermoplastics significantly minimized adsorption of compounds and thus could be used as alternatives for PDMS in the next generation of kidney MPS. Furthermore, most of these kidney MPS platforms currently lack kidney microvasculature, which plays an extremely important role in vivo by delivering nutrients to the tubular cells and maintenance of a healthy kidney epithelium (Jen et al., 2011). As they also participate in tubular secretion and reabsorption of drugs and xenobiotics, they are prone to their insults as well (Basile, 2007). Thus, to model drug clearance and toxicity to the kidney cells accurately, it is vital that the next generation of kidney MPS incorporate kidney vasculature. Some other challenges such as laboratory-to-laboratory variability in the fabrication of the device, its robustness, and the need for experienced personnel to assemble the device should also be addressed for the MPS technology to gain a strong foothold in research in industry. Finally, the current throughput of these devices prevents its widespread use, especially in a pharma setting where hundreds of compounds are routinely screened. To increase the throughput of these devices while still allowing flow-based studies for kidney, Mimetas, a Dutch startup company, recently launched an Society for Biomolecular Screening SBS-compatible, PDMS-free microtiter 384-well plate MPS called OrganoPlates. These OrganoPlates use phase-guide technology, which enables the patterning of liquids and gels (Vulto et al., 2011). Four wells of the plate together form one microfluidic chip, thus leading to 96-microfludic chips (two-channel) on a single plate. An extracellular matrix (ECM) is first pipetted in the gel channel, which gets patterned because of the phase-guide. This matrix serves as the support layer for the cells in the liquid channel, where they can adhere and proliferate to form a tube-like structure. The liquid channel can be perfused using a gravity driven flow eliminating the need for costly pumps. The resulting tubule of cells can be used for several functions, such as investigating barrier integrity after a chemical insult, transporter-based drug clearance and toxicity, and others, making it an extremely versatile platform. Based on this unique platform, Mimetas, along with several collaborators from the European Union were part of a Crack-iT NephroTube challenge to develop new models for assessing nephrotoxicity in vitro. Furthermore, by reducing the number of devices in the plate, a third channel could be added to grow the kidney endothelial cells, thereby mimicking the vasculature of the kidney (Fig. 2C-1 and 2). Although this new platform could offer much potential and will be useful for testing the nephrotoxic liability of compound, its validation is critical to show its strength over current existing 2D static models. Most of the preceding models have focused on characterizing few uptake and efflux transporters; however, a better characterization of the functionality of transporters is needed for each of these models so that the end user knows the strengths and shortcomings of these models in relation to xenobiotic handling and thus can use these platforms accordingly. A large data set of mechanistically distinct nephrotoxicants also needs to be validated in these models. In general, MPS technologies exhibit more physiologically relevant phenotypes for many kidney makers compared with their static 2D counterparts; however, currently limited information exists involving the use of kidney specific biomarkers to assess nephrotoxic liabilities of drugs in MPS to allow for IVIVE. Also, as most of these MPS are fabricated using some form of lithography or other types of cleanroom-based manufacturing processes, inclusion of sensors/biosensors which can allow different readouts in real-time per user needs would also be a great addition to the next generation of devices to obtain an efficient in vitro kidney model.
Bioprinted Kidney MPS.
A new emerging technology of bioprinting is now being increasingly used to develop complex 3D in vitro models (Bajaj et al., 2014; Murphy and Atala, 2014). Homan et al. (2016) demonstrated the biofabrication of a 3D human renal PTC model with complex geometry and an open lumen lined by PTCs, which allowed active perfusion and their maintenance in the construct for over 2 months (Fig. 2D-1 and 2). The PTCs in the 3D chip showed enhanced cell height, microvilli length and density, improved albumin uptake, and megalin expression compared with their 2D controls with and without perfusion. Additionally, the researchers showed dose-dependent toxicity of a common nephrotoxin, cyclosporine A, in their 3D PTC model. As this model was bioprinted, it can be batch produced and can also incorporate user-defined size and geometry. Other relevant cell types of the kidney, such as endothelial cells, can be cultured in conjunction with the PTCs to accurately model the kidney barrier. Thus, this platform has the potential to be developed into a truly unique in vitro system for nephrotoxicity screening and xenobiotic handling. Another example of a 3D bioprinted kidney model was demonstrated by Organovo, a San Diego–based company that specializes in the development of 3D printed tissues (King et al., 2017). The unique aspect of their model was the incorporation of kidney interstitial cells, such as renal fibroblasts and endothelial cells in addition to PTCs, thus allowing the development of a diseased model when challenged with tumor growth factor-β, a master regulator of fibrosis. In addition, the researchers also demonstrated improved kidney phenotype, which could be maintained for up to a month in culture, and strong expression of important kidney markers, including renal transporters. Importantly, expression of the four transporters most relevant for xenobiotic handling in the kidney, OAT1/3 (low), OCT2, and P-gp, was maintained over the course of a month and verified to be similar compared with that in the kidney cortex byliquid chromatography-tandem mass spectroscopy. The ability of this model to be used for nephrotoxicity screening would require its validation with a large library of compounds to demonstrate its potential.
Currently, we believe that there is no “perfect” in vitro kidney model available for investigating nephrotoxicity; however, continued development of these advanced novel in vitro platforms offers a great promise for assessment of nephrotoxicity and understanding drug clearance for new molecular entities, especially now, when more research groups in academia and industry have started to work with both small and large molecules, many of which cannot be accurately modeled using current in vitro kidney platforms.
Current Challenges and Future Perspectives
Compared with other tissue and cell types, the use of human renal cells by the pharmaceutical industry is rather limited. In contrast, the use of cryopreserved and/or fresh human hepatocytes is quite common, and multiple companies are involved in sourcing and providing hepatocytes. The reasons for this are numerous, but one driving force has been the search for alternatives to whole-organ liver transplants. The first successful cadaveric organ transplant occurred in 1950 and the first living-twin organ transplant was in 1954, but these both involved the kidney (Lawler et al., 1950; Watson and Dark, 2012). In the case of the liver, the first attempted transplant was in 1963 by Dr. Thomas Starzl, but it was unsuccessful and resulted in the patient’s death (Starzl et al., 1964). Thus, greater efforts were expended on isolation and propagation of functional (and transplantable) hepatocytes versus kidney cell types. Using rats, Berry and Friend (1969) developed methods that resulted in high-yield isolates of liver parenchymal cells using a collagenase perfusion method. Thus, with decades of experience, robust protocols now exist for isolating human parenchymal (and nonparenchymal) liver cells. The availability of highly characterized hepatocytes from multiple vendors (e.g., Sekisui Xenotech, TRL/Lonza, and Thermo Fisher) has created an industry for supplying these cells to pharmaceutical companies for studying disposition and safety testing of NCEs. The demand of these cells is driven in part by the FDA (and other regulatory agencies) guidance, including the 2016 Safety Testing of Drug Metabolites Guidance for Industry, which states, “In vitro studies can use liver microsomes, liver slices, or hepatocytes from animals and humans and generally should be conducted before initiation of clinical trials” (FDA, 2016).
The early successes of kidney transplantation likely hindered research on isolation and propagation of cells in vitro; however, it is worth noting that, in comparison with the liver, a relatively simple organ containing only four basic cell types (parenchyma, endothelial, stellate, and Kupffer cells), the kidney is a very complex organ composed of dozens of specialized cell types (Kriz and Bankir, 1988; Baer et al., 1997). Because of this complexity, attempts to restore organ function in vivo using in vitro isolation techniques for purified cell types are simply not feasible.
In the field of preclinical toxicity, the pharmaceutical industry has historically relied on various proximal tubule cell lines, such as LLC-PK1, MDCK, and HK2 (Rezzani et al., 2002; Gunness et al., 2010). As discussed already, however, these cell lines are not adequate for use as predictive models. Thus, to address these issues, investigators have turned to isolating primary PTCs (Pizzonia et al., 1991; Baer et al., 1997; Qi et al., 2007). Now, several commercial entities sell human proximal tubule epithelial cells (e.g., ATCC, Lonza, and Biopredic); but use of these cells is not as widespread or as common as hepatocytes, likely for many reasons, including the lack of specific regulatory guidance on their use, limited characterization, as well as limited availability of human tissue. In the academic sector, their use is more commonly associated with access to tissue from a linked medical center, given the high costs charged by cell vendors.
To overcome or bypass the hurdle of access to primary kidney cell types, the field has turned to directed differentiation of human induced pluripotent stem cells (iPSCs); however, in comparison with other cells types (e.g., cardiomyocytes, neuronal/oligodendrocytes, and hepatocytes), research on the kidney has lagged. This situation changed in 2012 with the establishment of a differentiation protocol resulting in the derivation of renal podocyte-like cells (Song et al., 2012) and a series of other publications in quick succession (Araoka et al., 2014; Lam et al., 2014; Takasato et al., 2014). Recent research has described generation of kidney “organoids” from iPSCs (Morizane et al., 2015; Takasato et al., 2016; Takasato and Little, 2017) and using organoids to model kidney disease (Freedman et al., 2015; Cruz et al., 2017; Kim et al., 2017). More recently, standardized protocols have been published that detail the sequential steps and proportions of growth factors and timing for creating kidney organoids. In one protocol, a high-efficiency (80%–90%) system is described that generates nephron progenitor cells within 9 days of differentiation (Morizane and Bonventre, 2015), and a further 12 days is required to generate kidney organoids with “high reproducibility.” This protocol does make use of a 96-well format, lending it to potential utility in high-throughput screening systems, but the authors note that “careful attention to morphological changes indicative of differentiation” is necessary. Another, slightly more recent protocol describes first inducing pluripotent stem cells into a cell type akin to the posterior primitive streak, followed by subsequent programming into cells representing posterior and anterior intermediate mesoderm (Takasato and Little, 2017). These two cell types are then aggregated and undergo self-organization into kidney organoids. The resultant organoids are composed of all the cell types of the nephron, including the glomerulus, proximal/distal tubules, and endothelial network. A very recent publication has described a system that incorporates a robust differentiation protocol with little user input (Czerniecki et al., 2018). This system takes human pluripotent stem cells in 96- or 384-well format plates and completes the entire differentiation process using a robotic platform. This system yields kidney organoids composed of glomerular tissue, as well as proximal and distal tubule cells; its potential is tremendous given the relative ease of use, the high degree of throughput, and the efficiency of the differentiation protocol. Potential applications include preclinical toxicity screening of lead compounds on the resultant organoids, as well as applications in developmental toxicity screening. For the latter, one could envision perturbing the differentiation process with drugs or chemicals at any point in the protocol. Developmental and reproductive chemical screening is a critical component of the Tox21 Initiative, and this technology could refine the animal testing approaches that are currently used. As the authors also note, this technology has application in the drug discovery world when applied to kidney disease, for example, polycystic kidney disease. Given that there is only one FDA-approved drug for polyctstic disease (with ill-defined mechanism of action), this is clearly an unmet need for a highly prevalent genetic disorder. With recent advances in single-cell transcriptomics, it might also be possible to characterize more clearly these kidney organoids, similar to the work done by Park et al. (2018) on healthy mouse kidneys, to discover new cellular targets for kidney disease and to learn more about kidney development. Although great strides are being made in the generation of kidney organoids, this technology and expertise are still quite limited and currently are seen in only a handful of academic research laboratories. Before kidney organoids are adopted as part of the preclinical repertoire on NCE screening, several issues need to be addressed. First, kidney organoids are not a structure amenable to facilitated transport studies. Given that the primary reason an NCE induces nephrotoxicity is accumulation in the proximal tubule (Weber et al., 2017), protocols need to be established for isolation of pure tubule epithelial cells from organoids. Then these cell isolates could be used to populate 3D MPS as previously described. Second, the differentiation potential toward formation of a kidney organoid will vary depending on the cell source used to generate the iPSCs and thus would require some optimization.
The International Consortium for Innovation and Quality in Pharmaceutical development-Pharma consortium, in collaboration with the National Center for Advancing Translational Services, has pushed for generation of preclinical species as a cell source for generating MPS for toxicity testing. The reason is the existence of extensive data sets on unanticipated toxicity of lead compounds, with species disparities. This leaves open the question, in the case of “killed” compounds, of which species is predictive of clinical trial outcomes. Having available human MPS, in conjunction with appropriate preclinical species MPS, allows one to address this question. The availability of iPSC-derived kidney cells will be a big advance in their adoption in the pharmaceutical industry, as they represent an unlimited cell supply and, with gene editing, will facilitate disease modeling, as well as the potential role(s) of polymorphic variants using isogenic control iPSC lines. For drug transport/toxicity, this latter application is particularly useful when applied to the transporters facilitating active uptake and secretion in the proximal tubule. The current caveat is that these cells, much like iPSC-derived cell types for other organs, are immature in phenotype, resembling fetal cells at best.
In the end, what will drive adoption of MPS technologies, including kidney MPS, will be the regulatory agencies worldwide. Given the widespread agreement that animals are not always reliable predictors of clinical trial outcomes, this paradigm shift will occur; it is simply a matter of time. In the United States, the FDA has recognized the potential of this technology and signed an agreement with Emulate in 2017 to evaluate this company’s MPS technologies at the agency’s Center for Food Safety and Applied Nutrition. So, depending on the outcomes of these efforts, we could see adoption of this new technology as standard practice as part of future investigational new drug filings.
Acknowledgments
The views expressed in this document are solely those of the authors and do not necessarily reflect those of the agency or the company. Takeda and EPA do not endorse any products or commercial services mentioned in this article.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- AA
aristolochic acid
- ABC
ATP-binding cassette
- ATCC
American Type Culture Collection
- AUC-ROC
area under the receiver operating characteristic curve
- BCRP
breast cancer resistance protein
- ciPTEC
conditionally immortalized proximal tubule epithelial cells
- ciPTEC-OAT1
conditionally immortalized proximal tubule epithelial cells overexpressing OAT-1
- ciPTEC-OAT3
conditionally immortalized proximal tubule epithelial cells overexpressing OAT-3
- DDIs
drug-drug interactions
- ECM
extracellular matrix
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- GGT
glutamyltransferase
- HK
human kidney
- HPTC
human primary tubular cell
- iPSC
induced pluripotent stem cells
- ITC
International Transporter Consortium
- IVIVE
in vitro–in vivo extrapolation
- KIM-1
kidney injury molecule-1
- LLC-PK1
pig kidney epithelial cells
- MATE-1
multidrug and toxin extrusion protein 1
- MATE-2K
multidrug and toxin extrusion protein 2K
- MDCK
Madin-Darby canine kidney
- MPS
microphysiological systems
- MRP2
multidrug resistance-associated protein 2
- MRP4
multidrug resistance-associated protein 4
- NCE
new chemical entities
- OAT
organic anion transporter
- OCT
organic cation transporter
- OCTN1
organic cation transporter, novel, type 1
- OCTN2
organic cation transporter, novel, type 2
- P450
cytochrome 450
- PAH
p-aminohippuric acid
- PDMS
polydimethylsiloxane
- PEPT
peptide transporter
- P-gp
P-glycoprotein
- PK
pharmacokinetics
- PTC
proximal tubule cell
- RPTEC
renal proximal tubule cells
- SGLT2
sodium-glucose cotransporter-2
- SLC
solute carrier
- SV40T
simian virus 40 large T antigen
- TCA
3,4,5-trichloroaniline
- TERT1
telomerase reverse transcriptase
- UDP
uridine-diphosphate
- UGT
uridine-diphosphate-glucuronosyltransferase
- URAT1
urate anion exchanger 1
- ZO-1
zonula occludens 1
- ZO-3
zonula occludens 3
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Bajaj, Chowdhury, Yucha, Kelly, Xiao.
Footnotes
P.B., S.K.C., R.Y., and G.X. are compensated employees of Takeda Pharmaceutical Company Limited. E.J.K. was supported in part by the National Institutes of Health [Grants UH3TR000504, UG3TR002158, and ES070033] and by the Environmental Protection Agency Assistance Agreement [Grant 83573801].
References
- Adler M, Ramm S, Hafner M, Muhlich JL, Gottwald EM, Weber E, Jaklic A, Ajay AK, Svoboda D, Auerbach S, et al. (2016) A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol 27:1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araoka T, Mae S, Kurose Y, Uesugi M, Ohta A, Yamanaka S, Osafune K. (2014) Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One 9:e84881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer L, Carta G, Vogelsang N, Schlatter E, Jennings P. (2015a) Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol In Vitro 30 (1 Pt A):95–105. [DOI] [PubMed] [Google Scholar]
- Aschauer L, Limonciel A, Wilmes A, Stanzel S, Kopp-Schneider A, Hewitt P, Lukas A, Leonard MO, Pfaller W, Jennings P. (2015b) Application of RPTEC/TERT1 cells for investigation of repeat dose nephrotoxicity: a transcriptomic study. Toxicol In Vitro 30 (1 Pt A):106–116. [DOI] [PubMed] [Google Scholar]
- American Type Culture Collection (ATCC) (2016) RPTEC/TERT1 OAT1 (ATCC® CRL-4031-OAT1™), 2016. https://www.atcc.org/Products/All/CRL-4031-OAT1.aspx
- Baer PC, Nockher WA, Haase W, Scherberich JE. (1997) Isolation of proximal and distal tubule cells from human kidney by immunomagnetic separation. Technical note. Kidney Int 52:1321–1331. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. (2014) 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16:247–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DP. (2007) The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72:151–156. [DOI] [PubMed] [Google Scholar]
- Berry MN, Friend DS. (1969) High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43:506–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CDA, Sayer R, Windass AS, Haslam IS, De Broe ME, D’Haese PC, Verhulst A. (2008) Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol 233:428–438. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Burckhardt G. (2003) Transport of organic anions across the basolateral membrane of proximal tubule cells, in Reviews of Physiology, Biochemistry and Pharmacology, pp 95–158, Springer, Berlin. [DOI] [PubMed] [Google Scholar]
- Burckhardt G. (2012) Drug transport by organic anion transporters (OATs). Pharmacol Ther 136:106–130. [DOI] [PubMed] [Google Scholar]
- Chang SY, Weber EJ, Sidorenko VS, Chapron A, Yeung CK, Gao C, Mao Q, Shen D, Wang J, Rosenquist TA, et al. (2017) Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2:95978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Gburek J. (2004) Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. Pediatr Nephrol 19:714–721. [DOI] [PubMed] [Google Scholar]
- Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, et al. (2017) Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater 16:1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, et al. (2018) High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22:929–940.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David EO, Philip GW, Robert JK, Margaret BF, Wenwu J, Frisa PS, Chee-Keong C, Chung-Fai Y, Kwok-Wah C, Martin IR, et al. (2004) Growth, immortalization, and differentiation potential of normal adult human proximal tubule cells. In Vitro Cell Dev Biol Anim 40:22–34. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Norton L, Abdul-Ghani M. (2017) Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13:11–26. [DOI] [PubMed] [Google Scholar]
- Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. (2016) Primary cilia are not calcium-responsive mechanosensors. Nature 531:656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser MJ, Leabman MK, Giacomini KM. (2001) Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J Pharm Sci 90:397–421. [DOI] [PubMed] [Google Scholar]
- Duan Y, Du Z, Yan Q, Weinstein A, Weinbaum S, Wang T. (2007) Role of fluid shear stress in cytoskeleton reorganization of mouse proximal tubule epithelium. FASEB J 21:A915. [Google Scholar]
- Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. (2008) Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci USA 105:11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) (2012) Guideline on the Investigation of Drug Interactions Products CfHM European Medicines Agency Science Medicines Health, London. [Google Scholar]
- Food and Drug Administration (FDA) (2016) Safety Testing for Drug Metabolites: Guidance for Industry, US Department of Health and Human Services FDA, Silver Spring, MD. [Google Scholar]
- Food and Drug Administration (FDA) (2017a) Draft Guidance for Industry: Clinical Drug Interaction Studies- Study Design, Data Analysis, and Clinical Implications, US Department of Health and Human Services FDA, Silver Spring, MD. [Google Scholar]
- Food and Drug Administration (FDA) (2017b) Draft Guidance for Industry: In Vitro Metabolism and Transporter-Mediated Drug-Drug Interaction Studies, US Department of Health and Human Services FDA, Silver Spring, MD. [Google Scholar]
- Fedecostante M, Westphal KGC, Buono MF, Sanchez Romero N, Wilmer MJ, Kerkering J, Baptista PM, Hoenderop JG, Masereeuw R. (2018) Recellularized native kidney scaffolds as a novel tool in nephrotoxicity screening. Drug Metab Disp 46:1338–1350. [DOI] [PubMed] [Google Scholar]
- Feng B, LaPerle JL, Chang G, Varma MV. (2010) Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol 6:939–952. [DOI] [PubMed] [Google Scholar]
- Fisel P, Renner O, Nies AT, Schwab M, Schaeffeler E. (2014) Solute carrier transporter and drug-related nephrotoxicity: the impact of proximal tubule cell models for preclinical research. Expert Opin Drug Metab Toxicol 10:395–408. [DOI] [PubMed] [Google Scholar]
- Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, et al. (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B, You D, Joy MS, Aleksunes LM. (2017) Xenobiotic transporters and kidney injury. Adv Drug Deliv Rev 116:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozalpour E, Fenner KS. (2018) Current state of in vitro cell-based renal models. Curr Drug Metab 19:310–326. [DOI] [PubMed] [Google Scholar]
- Gundert-Remy U, Bernauer U, Blömeke B, Döring B, Fabian E, Goebel C, Hessel S, Jäckh C, Lampen A, Oesch F, et al. (2014) Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev 46:291–324. [DOI] [PubMed] [Google Scholar]
- Gunness P, Aleksa K, Bend J, Koren G. (2011) Acyclovir-induced nephrotoxicity: the role of the acyclovir aldehyde metabolite. Transl Res 158:290–301. [DOI] [PubMed] [Google Scholar]
- Gunness P, Aleksa K, Kosuge K, Ito S, Koren G. (2010) Comparison of the novel HK-2 human renal proximal tubular cell line with the standard LLC-PK1 cell line in studying drug-induced nephrotoxicity. Can J Physiol Pharmacol 88:448–455. [DOI] [PubMed] [Google Scholar]
- Habu Y, Yano I, Takeuchi A, Saito H, Okuda M, Fukatsu A, Inui K. (2003) Decreased activity of basolateral organic ion transports in hyperuricemic rat kidney: roles of organic ion transporters, rOAT1, rOAT3 and rOCT2. Biochem Pharmacol 66:1107–1114. [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell A-L, Karlsson J. (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340. [DOI] [PubMed] [Google Scholar]
- Ho ES, Lin DC, Mendel DB, Cihlar T. (2000) Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol 11:383–393. [DOI] [PubMed] [Google Scholar]
- Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA. (2016) Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6:34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JX, Kaeslin G, Ranall MV, Blaskovich MA, Becker B, Butler MS, Little MH, Lash LH, Cooper MA. (2015) Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: comparison of HK-2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol Res Perspect 3:e00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Shin HJ, Yang KJ, Jung SY, Song HY, Hwang HS, Gil HW. (2017) Cilastatin attenuates vancomycin-induced nephrotoxicity via P-glycoprotein. Toxicol Lett 277:9–17. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Launay-Vacher V, Deray G. (2005) Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 45:804–817. [DOI] [PubMed] [Google Scholar]
- Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE. (2013) Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5:1119–1129. [DOI] [PubMed] [Google Scholar]
- Jen KY, Haragsim L, Laszik ZG. (2011) Kidney microvasculature in health and disease. Contrib Nephrol 169:51–72. [DOI] [PubMed] [Google Scholar]
- Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD. (2012) The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch 464:601–611. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Chuah JKC, Su R, Huang P, Eng KG, Xiong S, Li Y, Chia CS, Loo L-H, Zink D. (2015) Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep 5:12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, et al. (2017) Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells 35:2366–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Higgins JW, Nino CR, Smith TR, Paffenroth EH, Fairbairn CE, Docuyanan A, Shah VD, Chen AE, Presnell SC, et al. (2017) 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights KM, Rowland A, Miners JO. (2013) Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br J Clin Pharmacol 76:587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK. (2001) Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci USA 98:12491–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, Bankir L, The Renal Commission of the International Union of Physiological Sciences (IUPS) (1988) A standard nomenclature for structures of the kidney. Kidney Int 33:1–7. [DOI] [PubMed] [Google Scholar]
- Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. (2014) Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol 25:1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH. (1994) Role of renal metabolism in risk to toxic chemicals. Environ Health Perspect 102 (Suppl 11):75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler RH, West JW, McNULTY PH, Clancy EJ, Murphy RP. (1950) Homotransplantation of the kidney in the human. J Am Med Assoc 144:844–845. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao J, Huang R, Steiner T, Bourner M, Mitchell M, Thompson DC, Zhao B, Xia M. (2017) Development and application of human renal proximal tubule epithelial cells for assessment of compound toxicity. Curr Chem Genomics Transl Med 11:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kandasamy K, Chuah JK, Lam YN, Toh WS, Oo ZY, Zink D. (2014) Identification of nephrotoxic compounds with embryonic stem-cell-derived human renal proximal tubular-like cells. Mol Pharm 11:1982–1990. [DOI] [PubMed] [Google Scholar]
- Li Y, Oo ZY, Chang SY, Huang P, Eng KG, Zeng JL, Kaestli AJ, Gopalan B, Kandasamy K, Tasnim F, et al. (2013) An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol Res 2:352–365. [Google Scholar]
- Lock EA, Reed CJ. (1998) Xenobiotic metabolizing enzymes of the kidney. Toxicol Pathol 26:18–25. [DOI] [PubMed] [Google Scholar]
- Lohr JW, Willsky GR, Acara MA. (1998) Renal drug metabolism. Pharmacol Rev 50:107–141. [PubMed] [Google Scholar]
- Long KR, Shipman KE, Rbaibi Y, Menshikova EV, Ritov VB, Eshbach ML, Jiang Y, Jackson EK, Baty CJ, Weisz OA. (2017) Proximal tubule apical endocytosis is modulated by fluid shear stress via an mTOR-dependent pathway. Mol Biol Cell 28:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, et al. (2015) Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5:8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JO, Yang X, Knights KM, Zhang L. (2017) The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin Pharmacol Ther 102:436–449. [DOI] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. (2013) Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53:503–529. [DOI] [PubMed] [Google Scholar]
- Moss DM, Neary M, Owen A. (2014) The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol 5:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. (2002) Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 13:866–874. [DOI] [PubMed] [Google Scholar]
- Murphy SV, Atala A. (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785. [DOI] [PubMed] [Google Scholar]
- Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, et al. (2017) Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1:0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Christensen EI, Birn H. (2016) Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 89:58–67. [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Damme K, Schwab M. (2011) Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy, in Drug Transporters (Fromm MF, Kim RB. eds), pp 105–167, Springer, Berlin. [DOI] [PubMed] [Google Scholar]
- Nieskens TT, Peters JG, Schreurs MJ, Smits N, Woestenenk R, Jansen K, van der Made TK, Röring M, Hilgendorf C, Wilmer MJ, et al. (2016) A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. AAPS J 18:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. (2015) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee SH. (2015) Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip 15:141–150. [DOI] [PubMed] [Google Scholar]
- Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. (2018) Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360:758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzonia JH, Gesek FA, Kennedy SM, Coutermarsh BA, Bacskai BJ, Friedman PA. (1991) Immunomagnetic separation, primary culture, and characterization of cortical thick ascending limb plus distal convoluted tubule cells from mouse kidney. In Vitro Cell Dev Biol 27A:409–416. [DOI] [PubMed] [Google Scholar]
- Prasad B, Johnson K, Billington S, Lee C, Chung GW, Brown CDA, Kelly EJ, Himmelfarb J, Unadkat JD. (2016) Abundance of drug transporters in the human kidney cortex as quantified by quantitative targeted proteomics. Drug Metab Dispos 44:1920–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Johnson DW, Vesey DA, Pollock CA, Chen X. (2007) Isolation, propagation and characterization of primary tubule cell culture from human kidney. Nephrology (Carlton) 12:155–159. [DOI] [PubMed] [Google Scholar]
- Racine C, Ward D, Anestis DK, Ferguson T, Preston D, Rankin GO. (2014) 3,4,5-Trichloroaniline nephrotoxicity in vitro: potential role of free radicals and renal biotransformation. Int J Mol Sci 15:20900–20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA. (2014) Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci USA 111:8506–8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern W, Ewart L, Hammond T, Bialecki R, Kinter L, Lindgren S. (2010) Impact and frequency of different toxicities throughout the pharmaceutical life cycle. Toxicologist 114:1081–1085. [Google Scholar]
- Rezzani R, Angoscini P, Borsani E, Rodella L, Bianchi R. (2002) Cyclosporine A-induced toxicity in two renal cell culture models (LLC-PK1 and MDCK). Histochem J 34:27–33. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. (1994) HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45:48–57. [DOI] [PubMed] [Google Scholar]
- Secker PF, Luks L, Schlichenmaier N, Dietrich DR. (2017) RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix. ALTEX 35:223–234. [DOI] [PubMed] [Google Scholar]
- Shen H, Lai Y, Rodrigues AD. (2017) Organic anion transporter 2: an enigmatic human solute carrier. Drug Metab Dispos 45:228–236. [DOI] [PubMed] [Google Scholar]
- Simon BR, Wilson MJ, Wickliffe JK. (2014) The RPTEC/TERT1 cell line models key renal cell responses to the environmental toxicants, benzo[a]pyrene and cadmium. Toxicol Rep 1:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Friedt BR, Wilson MJ, Blake DA, Yu H, Eriksson Y, Wickliffe JK. (2015) The RPTEC/TERT1 cell line as an improved tool for in vitro nephrotoxicity assessments. Biol Trace Elem Res 166:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn S-J, Kim SY, Kim HS, Chun Y-J, Han SY, Kim SH, Moon A. (2013) In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett 217:235–242. [DOI] [PubMed] [Google Scholar]
- Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. (2012) The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7:e46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Marchioro TL, Huntley RT, Rifkind D, Rowlands DT, Jr, Dickinson TC, Waddell WR. (1964) Experimental and clinical homotransplantation of the liver. Ann N Y Acad Sci 120:739–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Little MH. (2016) Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11:1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M, Little MH. (2017) Making a kidney organoid using the directed differentiation of human pluripotent stem cells. Methods Mol Biol 1597:195–206. [DOI] [PubMed] [Google Scholar]
- Tchernitchko D, Tavernier Q, Lamoril J, Schmitt C, Talbi N, Lyoumi S, Robreau AM, Karim Z, Gouya L, Thervet E, et al. (2017) A variant of peptide transporter 2 predicts the severity of porphyria-associated kidney disease. J Am Soc Nephrol 28:1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D. (2014) Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm 11:1933–1948. [DOI] [PubMed] [Google Scholar]
- Tramonti G, Romiti N, Norpoth M, Chieli E. (2001) P-glycoprotein in HK-2 proximal tubule cell line. Ren Fail 23:331–337. [DOI] [PubMed] [Google Scholar]
- Tune BM, Fravert D. (1980) Mechanisms of cephalosporin nephrotoxicity: a comparison of cephaloridine and cephaloglycin. Kidney Int 18:591–600. [DOI] [PubMed] [Google Scholar]
- Van der Hauwaert C, Savary G, Buob D, Leroy X, Aubert S, Flamand V, Hennino M-F, Perrais M, Lo-Guidice J-M, Broly F, et al. (2014) Expression profiles of genes involved in xenobiotic metabolism and disposition in human renal tissues and renal cell models. Toxicol Appl Pharmacol 279:409–418. [DOI] [PubMed] [Google Scholar]
- van Midwoud PM, Janse A, Merema MT, Groothuis GM, Verpoorte E. (2012) Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal Chem 84:3938–3944. [DOI] [PubMed] [Google Scholar]
- Varma MV, Lai Y, El-Kattan AF. (2017) Molecular properties associated with transporter-mediated drug disposition. Adv Drug Deliv Rev 116:92–99. [DOI] [PubMed] [Google Scholar]
- Vulto P, Podszun S, Meyer P, Hermann C, Manz A, Urban GA. (2011) Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab Chip 11:1596–1602. [DOI] [PubMed] [Google Scholar]
- Wainford RD, Weaver RJ, Stewart KN, Brown P, Hawksworth GM. (2008) Cisplatin nephrotoxicity is mediated by gamma glutamyltranspeptidase, not via a C-S lyase governed biotransformation pathway. Toxicology 249:184–193. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu Y, Yuan M, Darling IM, Repasky EA, Morris ME. (2006) Characterization of monocarboxylate transport in human kidney HK-2 cells. Mol Pharm 3:675–685. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Dark JH. (2012) Organ transplantation: historical perspective and current practice. Br J Anaesth 108 (Suppl 1):i29–i42. [DOI] [PubMed] [Google Scholar]
- Weber EJ, Chapron A, Chapron BD, Voellinger JL, Lidberg KA, Yeung CK, Wang Z, Yamaura Y, Hailey DW, Neumann T, et al. (2016) Development of a microphysiological model of human kidney proximal tubule function. Kidney Int 90:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber EJ, Himmelfarb J, Kelly EJ. (2017) Concise review: current and emerging biomarkers of nephrotoxicity. Curr Opin Toxicol 4:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R. (2008) hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295:F1365–F1375. [DOI] [PubMed] [Google Scholar]
- Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, Mathieson PW, Monnens LA, van den Heuvel LP, Levtchenko EN. (2010) Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res 339:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windass AS, Lowes S, Wang Y, Brown CDA. (2007) The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther 322:1221–1227. [DOI] [PubMed] [Google Scholar]
- Wu Y, Connors D, Barber L, Jayachandra S, Hanumegowda UM, Adams SP. (2009) Multiplexed assay panel of cytotoxicity in HK-2 cells for detection of renal proximal tubule injury potential of compounds. Toxicol In Vitro 23:1170–1178. [DOI] [PubMed] [Google Scholar]
- Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. (2007) Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74:477–487. [DOI] [PubMed] [Google Scholar]
- Zou L, Stecula A, Gupta A, Prasad B, Chien HC, Yee SW, Wang L, Unadkat JD, Stahl SH, Fenner KS, et al. (2018) Molecular mechanisms for species differences in organic anion transporter 1, OAT1: implications for renal drug toxicity. Mol Pharmacol 94:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]