Abstract
Metabolism in the liver often determines the overall clearance rates of many pharmaceuticals. Furthermore, induction or inhibition of the liver drug metabolism enzymes by perpetrator drugs can influence the metabolism of victim drugs (drug-drug interactions). Therefore, determining liver-drug interactions is critical during preclinical drug development. Unfortunately, studies in animals are often of limited value because of significant differences in the metabolic pathways of the liver across different species. To mitigate such limitations, the pharmaceutical industry uses a continuum of human liver models, ranging from microsomes to transfected cell lines and cultures of primary human hepatocytes (PHHs). Of these models, PHHs provide a balance of high-throughput testing capabilities together with a physiologically relevant cell type that exhibits all the characteristic enzymes, cofactors, and transporters. However, PHH monocultures display a rapid decline in metabolic capacity. Consequently, bioengineers have developed several tools, such as cellular microarrays, micropatterned cocultures, self-assembled and bioprinted spheroids, and perfusion devices, to enhance and stabilize PHH functions for ≥2 weeks. Many of these platforms have been validated for drug studies, whereas some have been adapted to include liver nonparenchymal cells that can influence hepatic drug metabolism in health and disease. Here, we focus on the design features of such platforms and their representative drug metabolism validation datasets, while discussing emerging trends. Overall, the use of engineered human liver platforms in the pharmaceutical industry has been steadily rising over the last 10 years, and we anticipate that these platforms will become an integral part of drug development with continued commercialization and validation for routine screening use.
Introduction
The pharmaceutical industry uses a variety of human liver models to predict the clearance of novel compounds, identify their major metabolites, and appraise the potential for drug-drug interactions (DDIs) due to multidrug therapy prior to the initiation of human clinical trials. The prediction of drug clearance during preclinical development is important for the selection of the proper drug dose in animal studies and then in human clinical trials, whereas the detection of all major drug metabolites (greater than or equal to 10% of the drug-related material) in vitro allows for the determination of potential metabolite efficacy and/or toxicity in animal studies. Furthermore, determining the potential of DDIs during lead optimization can aid in the design of the appropriate multidrug therapy for a disease indication and/or help to set use directions for which other available drugs should be avoided for coadministration. In addition, as part of preclinical drug development, live-animal tests are required by the Food and Drug Administration to mitigate the safety risk to humans. However, because of species-specific drug metabolism characteristics and the inability of animal models to fully recapitulate human genetics and disease phenotypes (Shih et al., 1999; Olson et al., 2000), it is now clear that animal studies cannot entirely predict human-specific liver-drug interactions. As a result, in recent years a greater emphasis has been placed on the advancement of complementary in vitro human liver cell culture platforms (Godoy et al., 2013). In particular, to promote sufficient stability and reproducibility of liver cell functions in vitro, the field has turned to the implementation of a number of bioengineering-based culture strategies that enable precise control of culture conditions. In this review, we discuss the key design parameters and overall strategies for applying bioengineered liver models for drug metabolism and disposition studies, as well as highlight pending issues and emerging trends in the field of engineered human liver platforms. Representative drug metabolism/disposition datasets from both academic and industrial laboratories are presented with the intention of demonstrating a balanced spectrum of model development and commercial potential. Although toxicity resulting from the metabolism and disposition of drugs is critical to evaluate during preclinical drug development and thus represents an important use of human liver culture platforms, we refer the reader to other reviews that detail validation datasets in this area (Atienzar et al., 2016; Lin and Khetani, 2016; Funk and Roth, 2017); nonetheless, most of the platforms we discuss in this review have been additionally used for drug toxicity detection.
High-Throughput Cellular Microarrays
During the early stages of the drug development pipeline, the metabolism of thousands of compounds are often evaluated as part of screening efforts and requisite follow-up studies. Accordingly, this process necessitates human liver platforms that are high throughput, provide actionable data quickly (within 24–48 hours), are relatively low cost, and can be miniaturized since the amount of novel compound is often rate limiting. In this section, we discuss the utility of high-throughput microplatforms for drug metabolism studies. Notably, these microwell- or microarray-based systems exhibit the dual advantage of supporting efforts aimed at investigating microenvironmental signals that stabilize and/or further mature hepatic functions, such as metabolic capacity, while also enabling high-throughput drug development studies.
One entry point for the high-throughput examination of drug metabolism is through the use of acellular preparations of metabolic enzymes. For example, microsomes are vesicles formed from the endoplasmic reticulum when cells are lysed; these microsomes contain phase I enzymes [e.g., cytochrome P450 (P450)] that enable the determination of which phase I enzymes are involved in the metabolism of a particular drug candidate. More recent research (Lee et al., 2005) has focused on creating miniaturized arrays of spotted enzymes in synthetic or natural hydrogels to increase the throughput of this approach. However, microsomes and related purified enzyme platforms lack the complete repertoire of drug metabolism enzymes and transporters required for the accurate prediction of drug metabolism and disposition across all drug classes. Accordingly, cell-based models are more appropriate for such applications, although cell sourcing and the method of culture are critical factors for evaluation.
As a means to reduce cost compared with primary cell sources, cancerous and immortalized hepatic cell lines can be used. However, for physiologically relevant metabolism, the use of primary human hepatocytes (PHHs) becomes important since cell lines display low levels of drug metabolism enzymes (Wilkening et al., 2003; Gerets et al., 2012). In a key example, a microchip platform was developed that incorporates the parallel transduction of three-dimensional (3D) liver cell cultures with genes encoding drug metabolism enzymes (Kwon et al., 2014). The platform features 532 distinct reaction vessels within a 75 × 25 mm footprint. Cells were suspended within a droplet of protein-rich Matrigel (∼60 nl), which is then spotted onto a micropillar substrate. The micropillar was then placed into a corresponding microwell that contained recombinant adenoviruses. In this initial series of studies, THLE-2 human liver cells (immortalized via simian virus 40 large T antigen) were used, and, through the adenoviral transduction of human drug metabolism genes, the effects of individual enzymes and combinations of enzymes (84 distinct combinations in this work) on cellular phenotypic responses were explored. In other similar efforts, a 3D Hep3B (human hepatoma cell line) microarray was coupled with an adjacent microarray containing defined combinations of recombinant drug metabolism enzymes to perform a high-throughput examination of the metabolism-mediated effects of drugs on cells (Lee et al., 2014). 3D microarrays of Hep3B cells have also been integrated with arrays of purified human P450 isoforms using an approach based on two complementary fabricated systems (DataChip and MetaChip), for studies investigating potential adverse drug reactions (Yu et al., 2018).
In contrast to using cell lines, other studies have used a microfabricated substrate that allowed the immobilization of rat hepatic spheroids (∼100 µm) in microwells and the subsequent analysis of P450 activities after drug treatment (Fukuda and Nakazawa, 2011). In this platform, it was further demonstrated that a row of microwells could be partitioned with a microchannel for the simultaneous detection of the activities of different P450 enzymes. In general, microwell-based platforms can provide the capability of generating 3D aggregates of controlled size, and the effects of 3D culture on hepatocytic functions are discussed in more detail below. Building on recent studies using microwell arrays to generate aggregates of rat hepatocyte or human hepatoma cell lines (Lee et al., 2016, 2017), current efforts in the field are aimed toward the integration of primary human hepatic cell types.
Another method for the high-throughput investigation of the effects of microenvironmental signals on cells is the fabrication of cellular microarrays, in which cells are seeded onto printed spots of materials or biomolecules (Fig. 1A). In these platforms, the printed spots commonly include adhesive components to promote localized cell adhesion, in addition to combinations of other factors to stimulate or measure cellular processes (Flaim et al., 2005; Soen et al., 2006; Brafman et al., 2009a,b; LaBarge et al., 2009). Microarrays of extracellular matrix (ECM) proteins have been used extensively for cell fate and functional studies and, in the liver field, have revealed notable effects of distinct ECM protein combinations on hepatic functions. In particular, a number of initial studies focused on the influence of ECM protein combinations on the early differentiation of embryonic stem cells and the adhesion and survival of primary rat hepatocytes, while highlighting unexpected synergistic or antagonistic effects of ECM components revealed by these high-throughput experimental designs (Flaim et al., 2005, 2008). These experiments, as well as many other studies in both two-dimensional (2D) and 3D contexts (Bhatia et al., 2014), suggest that ECM can influence hepatocyte functions. In more recent studies, an ECM microarray approach was used to demonstrate that ECM composition regulates the adhesion and differentiation of liver progenitor cells toward hepatocytic and biliary epithelial cell fates (Kaylan et al., 2016). Furthermore, cell microarrays can be fabricated on substrates exhibiting a range of stiffness (i.e., elastic modulus). Such platforms can be used to examine the potential cooperative effects of substrate stiffness, ECM protein composition, cell-cell contact, and soluble factors, and can further support traction force microscopy-based analysis of cell traction stresses within the context of distinct microenvironments (Kourouklis et al., 2016). Overall, these high-throughput microarray/microwell approaches can enable the design of experiments aimed at determining combinatorial effects that are difficult to predict or evaluate in standard culture settings. As microarray/microwell platforms are adapted to primary human liver cells, we anticipate that findings from these systems will be critical for the ongoing improvement of lower-throughout culture platforms (as discussed in subsequent sections) toward sustaining physiologically relevant functions in multiple liver cell types for several weeks to months in vitro.
Fig. 1.
Static medium platforms for liver cultures/cocultures. (A) Cell microarray platform for the high-throughput investigation of hepatocellular differentiation and functions (Kaylan et al., 2016). Left to right: Schematic representation of a typical cell microarray experiment for studying differentiation processes. Biomolecules and ECM proteins are spotted onto a dehydrated polyacrylamide hydrogel substrate using contact-based microprinting. After hydration and cell seeding, cells are adherent only on the arrayed protein domains, allowing for systematic analysis of the effects of the arrayed protein microenvironments on cellular responses. Single-cell quantification of staining-based readouts (e.g., immunofluorescence) can be obtained for each microenvironmental condition. (B) MPCCs of PHHs and 3T3-J2 murine embryonic fibroblasts (Khetani and Bhatia, 2008; Ware et al., 2015; Lin et al., 2016). Left to right: Relatively uniform PHH islands are stained with a mitochondrial dye in an industry-standard 96-well plate (127.6 mm length × 85.1 mm width). Phase contrast image of an individual PHH island in MPCCs surrounded by 3T3-J2 fibroblasts is shown. CYP3A4 activity, as assessed by the conversion of testosterone into 6β-OH-testosterone, and CYP2D6 activity, as assessed by the conversion of dextromethorphan into dextrorphan, are maintained for several weeks in MPCCs. Scale bar, 250 µm. (C) Self-assembled spheroids of PHHs created on a nonadhesive plate stained with either hemotoxylin and eosin (top image) or the hepatocyte cell junction marker E-cadherin (bottom image: red, E-cadherin; blue, nuclear counterstain) (Bell et al., 2016). Images from day 35 of culture are shown, but spheroids formed after 5–7 days in culture. Scale bars, 100 µm. (D) Liver coculture spheroids/organoids created using 3D bioprinting (Nguyen et al., 2016; Norona et al., 2016). Left to right: Schematic depicting the placement of the 3D bioprinted tissue (created using the proprietary process and instrumentation developed by Organovo, Inc., San Diego, CA) within a transwell insert in a standard 24-well plate format. The image above the schematic is a low-magnification view of a single bioprinted liver coculture spheroid/organoid containing PHHs, HSCs, and endothelial cells (diameter, 2.5 mm; thickness, 0.5 mm). Comparison of hemotoxylin and eosin–stained bioprinted hepatic spheroid and native human liver. CYP3A4 activity, as assessed by the conversion of midazolam to 4-hydroxymidazolam, is detected in the bioprinted liver cocultures over 28 days. Additionally, CYP3A4 activity is induced up to 4-fold via treatment of the cultures with 10 µm rifampicin for 3 days prior to the enzyme activity measurement.
Micropatterned Cocultures
Heterotypic contact/interactions with both liver-derived and non–liver-derived nonparenchymal cells (NPCs) can induce functions in hepatocytes from animals and humans in vitro (Guillouzo, 1998; Bhatia et al., 1999); such an effect likely mimics the physiology of the native liver, which contains three different liver NPCs known to affect hepatic functions in physiologic and disease states, namely liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs), and Kupffer cells/macrophages (KCs). However, randomly distributed liver cocultures typically show unstable and lower liver functions due to suboptimal homotypic and heterotypic cell-cell interactions (Khetani and Bhatia, 2008); indeed, such interactions are critical in the native liver. In contrast, micropatterned cocultures (MPCCs) of hepatocytes and NPCs, created using semiconductor-based lithographic techniques, allow optimization of cell-cell interactions to enhance and stabilize liver functions for ≥4 weeks (Fig. 1B) (Khetani and Bhatia, 2008). MPCCs have been used with primary rat hepatocytes (Ukairo et al., 2013), primary mouse hepatocytes (unpublished data), PHHs (Lin et al., 2016), primary monkey (cynomolgus) hepatocytes, and primary dog (beagle) hepatocytes (Ballard et al., 2016). In contrast to liver-derived NPCs, 3T3-J2 murine embryonic fibroblasts can stabilize functions of all the above-mentioned hepatocyte cell sources, presumably due to the secretion of molecules such as T-cadherin (Khetani et al., 2008) and decorin (Khetani et al., 2004), which mimic common elements of liver physiology across multiple species.
Human MPCCs miniaturized into industry-standard 24-, 96-, and 384-well plates have shown robust utility for drug clearance predictions (Chan et al., 2013; Lin et al., 2016; Kratochwil et al., 2017), DDIs (Khetani and Bhatia, 2008; Dixit et al., 2016; Lin et al., 2016; Moore et al., 2016), drug metabolite profiling (Wang et al., 2010; Ramsden et al., 2014b; Ballard et al., 2016), drug-transporter interactions (Ramsden et al., 2014a; Moore et al., 2016), drug-induced liver injury prediction (Khetani et al., 2013; Trask et al., 2014), gluconeogenesis inhibition (for type 2 diabetes therapies) (Davidson et al., 2015a), and infection with hepatitis B/C viruses (Ploss et al., 2010; Shlomai et al., 2014) and malaria (March et al., 2013; Ng et al., 2014; Gural et al., 2018). Within the context of drug metabolism, when MPCCs, created using three PHH (pooled) donors, were incubated for up to 7 days without a medium change with 27 compounds, they generated 82% of the excretory major metabolites that exceed 10% of dose and 75% of the circulating metabolites that exceed 10% of total circulating drug-related material (Wang et al., 2010); such results exceeded the performance of PHH suspensions and microsomes (<50% metabolite generation). Phase 1 and phase 2 metabolites, as well as metabolites that arise via two or more sequential reactions (secondary metabolites), were generated in MPCCs. In a follow-up study, MPCCs created using primary human, monkey, dog, or rat hepatocytes were used to study the metabolism of drugs that are known to produce human-specific metabolites (Ballard et al., 2016). The metabolite profiles of the compounds in PHH-MPCCs effectively captured the qualitative in vivo metabolite profile, and metabolites unique to humans were not detected in MPCCs created using the animal species (typically dog and rat). These results showed the utility of multispecies MPCCs for the assessment of preclinical species metabolism, which is a critical consideration prior to further investment in specific in vivo animal models and related studies.
Since PHH metabolism is the rate-limiting step in the overall clearance of approximately 70% of drugs on the market, the accurate prediction of human hepatic clearance is crucial during preclinical development toward guiding clinical drug dose selection. Suspension PHH cultures from single cryopreserved vials do not allow drug incubations beyond ∼4–6 hours because of a rapid loss in cell viability, whereas adherent PHH monocultures display a rapid decline in P450 enzyme activities to <10% of those measured in fresh PHHs. The use of multiple thaws of PHH vials to create a “relay” of drug incubations from one set of declining PHH cultures onto newly prepared cultures can extend the drug incubation time in both suspensions (Di et al., 2013) and adherent cultures (Peng et al., 2016); however, PHHs typically do not polarize adequately with a stable localization of apical and basolateral transporters, which limits the prediction of clearance of drugs that, in addition to being metabolized by phase I and/or II enzymes, are also substrates for transporters. Additionally, relays are technically and logistically challenging to execute in a rapid drug-screening campaign. Thus, the above-mentioned PHH culture models are severely restricted for accurately and rapidly predicting the clearance rates of compounds of a wide range of in vivo turnover rates, especially low-turnover compounds that are being developed for desirable one-pill-a-day dosing regimens.
In contrast to the limitations of the above-mentioned platforms for drug clearance prediction, MPCCs created using cryopreserved PHHs display high levels of P450 and phase II conjugation activities for ∼4 weeks (Lin et al., 2016). Additionally, several basolateral and canalicular drug transporters, such as multidrug resistance–associated protein 2, organic anion transporting polypeptide 1B1, and sodium/taurocholate cotransporting polypeptide exhibit active substrate transport within MPCCs, at levels significantly higher than for standard hepatocyte monocultures (Khetani and Bhatia, 2008; Ukairo et al., 2013; Ramsden et al., 2014a; Moore et al., 2016). Such enzyme and transporter activities coupled with the ability to incubate with drugs for up to 7 days without a culture medium exchange allowed for a better overall prediction of drug clearance rates for high-, medium-, and low-turnover compounds than suspension cultures (Chan et al., 2013). Additionally, the long-term drug incubation capability of MPCCs allowed for the formation of major hydroxylated metabolites of a hepatitis C virus drug, faldaprevir; the extent of metabolite formation was comparable to what was observed in vivo. On the other hand, glucuronidation of faldaprevir was a minor pathway of metabolism (Ramsden et al., 2014a). Further studies with MPCCs demonstrated that faldaprevir interacts with multiple uptake transporters, such as organic anion transporting polypeptide 1B1 and Na(+)-dependent transporters, to become concentrated in the liver; thus, metabolism and transport in the hepatocyte interact to determine the overall clearance of this drug.
The pioneering results described above have been confirmed in several follow-up studies by academic and industrial groups. For instance, PHH-MPCCs predicted 77%, 92%, and 96% of drug clearance values for all 26 tested drugs within 2-fold, 3-fold, and 4-fold of in vivo values (0.05–19.5 ml/min per kilogram in vivo clearance rates reported in the literature), respectively (Lin et al., 2016). There was a good correlation (R2 = 0.94; slope = 1.05) of predictions between two PHH donors. Furthermore, the induction and inhibition of CYP3A4 and the inhibition of CYP2D6 by perpetrator drugs in MPCCs for up to a 7 days of incubation led to changes in the predicted clearance of victim drugs that were highly correlated with clinical outcomes. Another study (Kratochwil et al., 2017) showed that the determination of intrinsic clearance by nonlinear mixed-effects modeling in PHH-MPCCs significantly increased the confidence in the estimation of in vitro pharmacokinetic parameters and extended the sensitive range toward 3% of liver blood flow (>10-fold lower than suspension PHHs). Additionally, the presence of more albumin molecules per hepatocyte (physiologic) in PHH-MPCCs relative to PHH suspensions and monocultures can potentially facilitate the hepatic uptake of several bound drugs because their intrinsic clearance was increased instead of being decreased by the albumin-binding effect (Da-Silva et al., 2018). The in vitro-to-in vivo extrapolation (IVIVE) method based on the unbound fraction in plasma value adjusted for the albumin-facilitated uptake gave the lowest prediction bias from the statistical analyses. Overall, PHH-MPCCs combined with the adjusted values of the unbound fraction in plasma were found to be the most reliable IVIVE method relative to PHH suspension and monocultures.
The activities of multiple uptake transporters were characterized in MPCCs and PHH monocultures using the same cryopreserved PHH donors (Moore et al., 2016). The higher activities of uptake transporters in MPCCs relative to the monocultures led to significantly higher intracellular concentrations of rifampicin and bosentan, substrates for uptake transporters, in MPCCs. The higher intracellular drug concentrations paralleled lower CYP3A4 EC50 values (mRNA and activity) in MPCCs compared with monocultures. Importantly, the EC50 values determined from MPCC experiments correlated better with clinical findings. On the other hand, the EC50 values for carbamazepine and phenytoin—which are not known to be substrates for uptake transporters—were similar across the two culture platforms. These datasets suggest that MPCCs are useful for the quantitative prediction of DDIs when there is an interplay of uptake transport activity and nuclear receptor–mediated induction of drug metabolism enzymes.
The MPCC platform has similarly been applied toward the investigation of induced pluripotent stem cell (iPSC)–derived human hepatocyte-like (iHep) cell function and maturation (Berger et al., 2015). In general, the incorporation of iHep cells can specifically enable studies aimed at determining hepatocellular responses to drug across distinct genetic backgrounds (Davidson et al., 2015b). In a series of experiments using this model, it was demonstrated that MPCCs containing iHep cells exhibited increased levels of adult-like hepatic functions and a reduction in fetal-like markers (e.g., alpha fetoprotein) compared with iHep cell monocultures over 4 weeks. (Berger et al., 2015). Furthermore, the activities of CYP1A2, CYP2C9, and CYP3A4 enzymes were inducible via prototypical drugs (e.g., omeprazole for CYP1A2, and rifampin and phenobarbital for CYP2C9 and CYP3A4) in iHep-MPCCs, thus suggesting the functionality of nuclear receptors such as aryl hydrocarbon receptor and pregnane X receptor; however, drug-mediated induction of CYP2B6 via constitutive androstane receptor is pending evaluation in this model.
The integration of liver NPCs into micropatterned systems has also been explored. In particular, PHH-MPCCs have been augmented with primary human KCs (Nguyen et al., 2015), primary human HSCs (Davidson et al., 2017), or primary human LSECs (Ware et al., 2017) to study how interactions with PHHs affect drug responses. For the studies incorporating liver KCs, it has been shown that KC stimulation with the bacterial-derived endotoxin lipopolysaccharide can induce the downregulation of P450 activity in cocultured PHHs in a cytokine-mediated manner (Nguyen et al., 2015). This alteration in P450 activity profile can influence drug metabolism and toxicity responses. Furthermore, the inclusion of activated (myofibroblastic) HSCs caused a downregulation of CYP2A6 and CYP3A4 enzymes and canalicular transporter activities (Davidson et al., 2017), which can have deleterious implications for drug metabolism and disposition in patients experiencing liver fibrosis. The MPCC platform is being further developed toward the inclusion of all the liver NPCs into a single configuration, which should aid in the determination of how the cross talk between liver cell types affects drug outcomes in health and disease. Overall, precisely controlling cell-cell interactions between hepatocytes, and between hepatocytes and NPCs has proven to be highly useful for stabilizing liver functions for several weeks in vitro; indeed, such control over cell-cell interactions is now being exercised in other culture configurations as we detail in subsequent sections.
Three-Dimensional Liver Spheroids
Another culture format that has been demonstrated to stabilize hepatocyte functions in vitro is 3D spheroid culture. Within this culture configuration, hepatocyte cell-cell interactions are highly prominent together with cell-secreted ECM proteins that accumulate within and outside the spheroidal aggregates (Godoy et al., 2013). Hepatocyte functions can be further modulated through the introduction of NPCs and the establishment of 3D cocultures. The simplest approach for generating hepatocellular spheroids is the plating of cells onto nontreated (i.e., less adhesive) polystyrene plates or those coated with polymers that resist protein adsorption and cell adhesion, which subsequently leads to the spontaneous formation of 3D cell aggregates (Acikgöz et al., 2013; Bell et al., 2016). Such spheroidal aggregates have been shown to display high viability and some functions (Fig. 1C) (Bell et al., 2016). However, in standard nonadhesive plates, it is difficult to maintain uniform spheroid size, and clusters of spheroids can merge to form larger aggregates that exhibit limitations in nutrient and oxygen diffusion leading to heterogeneous decreases in viability.
To address the limitations correlated with spheroid size variability, specialized culture substrates and hydrogel scaffolds have been used toward directing the assembly of spatially separated controlled-size spheroids that can then be used in various drug treatment or other assays. For example, a platform has been developed for the reproducible generation of hanging liquid drops, which can be used to facilitate the assembly of spheroids of defined diameters (Messner et al., 2013), including the generation of hepatocyte-endothelial-KC spheroids that maintain cellular viability and the secretion of albumin for approximately 1 month in this culture format. In other studies, a nanopillar plate–based method was used to create iHep or HepG2 spheroids (Takayama et al., 2013). However, despite the effective cellular aggregation, iHep spheroids showed reduced sensitivity in drug responses compared with conventional monocultures of primary hepatocytes, suggesting that additional iHep cell maturation is likely required in this culture platform. Last, microwells can now be seamlessly created inside the wells of standard tissue culture plates using a nonadhesive hydrogel such as polyethylene glycol (PEG) and a stamp around which the hydrogel polymerizes. Seeding cells (one or more cell types) into these microwells allows the spontaneous generation of spheroids that can be retained in the microwells over time (Song et al., 2015).
Spheroidal culture systems have been explored as potential platforms to examine the genetic and other possible predisposing factors that cause interpatient variability in drug metabolism and toxicity. For example, donor-specific characteristics have been demonstrated to be retained in long-term serum-free PHH spheroids (Bell et al., 2016). In particular, cellular processes including both endogenous and xenobiotic metabolism, which can vary between donors, have been shown to be maintained in 3D spheroid cultures (Vorrink et al., 2017). Broadly, P450 enzyme activity, as well as the expression of proteins involved in drug absorption and excretion, were found to be significantly higher in 3D spheroid culture compared with 2D monocultures (Bell et al., 2018).
The spheroid culture systems described above do not incorporate any exogenous matrix proteins and rely only on cell-secreted ECMs. Although the importance of cell-secreted ECMs should not be undervalued, such systems do not allow for the controlled modulation of the insoluble microenvironment around the cells. To better manipulate the biochemical and biomechanical microenvironment of cells within 3D spheroid cultures, various biomaterials have been used to generate an engineered polymer matrix for cell encapsulation (Godoy et al., 2013). These include both naturally derived (e.g., alginate, chitosan) and synthetic (e.g., PEG) biomaterials. In particular, biocompatible PEG hydrogels can provide substantial control of mechanical properties through the optimization of chain length and polymer weight percentage factors (Lutolf and Hubbell, 2005). Further, these hydrogel networks can facilitate the controlled modulation of biochemical properties based on the incorporation of biologic moieties including cell adhesion peptides and growth factors. Accordingly, a number of studies have used hydrogel scaffolds for generating hepatocellular culture models. For instance, the cocultivation of PHHs, 3T3-J2 cells, and LSECs (immortalized line) in PEG hydrogels modified with cell adhesion ligands (i.e., arginylglycylaspartic acid) has been demonstrated to support relatively stable hepatic functions for ≥8 days in vitro (Chen et al., 2011). In other work using a naturally derived material (Tasnim et al., 2016), iHep cells were encapsulated in galactosylated cellulose sponges, and this approach was demonstrated to promote the formation and stability of cellular spheroids. In another study (Larkin et al., 2013), a mechanically tunable biomaterial overlay containing polyelectrolyte multilayers of chitosan and hyaluronic acid was used as a space of Disse mimic to separate rat hepatocytes from a coculture of LSECs and KCs. Higher albumin secretion and CYP1A activity were detected in the hepatocytes when the biomaterial was tuned to the stiffness of the liver in this platform.
More recently, microfluidic droplet generators have been used to very rapidly create ECM microgels containing cells with the intention of using fewer cells and increasing the throughput for downstream screening compared with macroscale bulk gels (Li et al., 2014; Brett et al., 2016). These droplet generators typically use fluorocarbon oil with surfactant to create aqueous emulsions containing unpolymerized ECM mixed with cells. The polymerization strategy of the aqueous emulsions is then dependent on the chemistry being used. For application in liver, PEG/arginylglycylaspartic acid–based microgels have been created containing PHHs and 3T3-J2 fibroblasts (Li et al., 2014). Albumin secretion was higher if the two cell types were first organized in MPCCs in circular colonies of empirically optimized dimensions, the colonies were released using collagenase, and then these colonies were incorporated into the microgels using the microfluidic droplet generator. Microfluidic droplet generators can also be used to encapsulate cells in a natural ECM such as a collagen (Brett et al., 2016). The use of a natural ECM (as opposed to synthetic PEG) can allow the cells to interact with in vivo–like biochemical and biomechanical cues. We anticipate that the use of such technologies for creating precisely engineered human liver cultures/cocultures will continue to grow in the coming years.
One potential limitation of approaches based on randomly distributed cell spheroids is the inherent difficulty in defining the spatial arrangement of distinct cell types. In contrast, 3D bioprinting methods can enable the positioning of different cell populations in spheroids to more closely mimic liver lobule architecture; bioprinting also has the advantage of creating varying architectures on demand as opposed to the expensive photomasks that are required for lithographic techniques discussed above. For example, a bioprinted human liver spheroid (centimeter scale) has been developed that contains a PHH compartment printed adjacent to an NPC (HSCs and endothelial cells) compartment; the printed tissue is then placed on the membrane of a porous transwell within a 24-well plate format (Fig. 1D) (Norona et al., 2016). These bioprinted spheroids displayed secretion of albumin, basal CYP3A4 activity, and rifampicin-induced CYP3A4 activity for up to 28 days in culture. Using a similar strategy, a 3D bioprinting system has been used to assemble HepG2 spheroids in alginate, which demonstrated improved growth and expression of liver-specific genes relative to 2D monolayers (Jeon et al., 2017). In addition, a 3D bioprinting–based approach has been used to create hexagonal spheroids containing iHep cells, endothelia, and stem cells derived from adipose tissue embedded in a hydrogel matrix (Ma et al., 2016). Hepatic-specific gene expression and functions, including the activity and drug-mediated inducibility of P450 enzymes, were detected at significantly higher levels compared with iHep cell–only spheroids or 2D monocultures for approximately 32 days. Despite the ability to precisely control the architecture of tissues, 3D bioprinting is currently a low-throughput technique and requires a larger number of cells than other methods (e.g., microfluidic droplet generators). However, we anticipate that these limitations will likely be overcome with rapid technological advances in this space, which will provide an unprecedented ability to create 3D culture models on demand for academic and industrial investigations alike.
Perfused Liver Devices
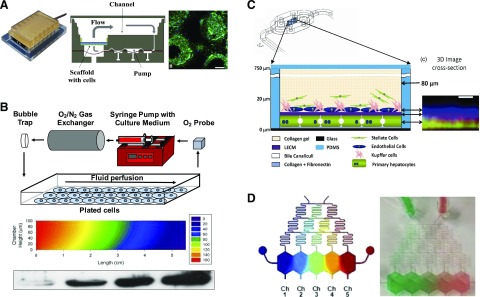
Perfusion systems have been explored to facilitate automated control of culture conditions including culture medium pH, temperature, nutrient supply, waste removal, as well as fluid pressures and shear stress. In one platform, termed the “LiverChip,” hepatocyte aggregates within collagen-coated microchannels (fabricated using silicon or polycarbonate with a multiwell footprint) were perfused using an optimized range of flow rates that provided sufficient nutrient and oxygen transport while minimizing shear stress exposure (Fig. 2A) (Powers et al., 2002; Domansky et al., 2010). The hepatocyte aggregates maintained functions under perfusion that were an order of magnitude increased relative to static controls. In addition, PHH-KC aggregates in the LiverChip responded appropriately to lipopolysaccharide stimulation by increasing the secretion of 11 different proinflammatory cytokines (e.g., interleukin-6, tumor necrosis factor-α, and chemokine (C-C motif) ligand 5) (Sarkar et al., 2015; Tsamandouras et al., 2017). Stimulation of PHH-KC aggregates with interleukin-6 resulted in a dose-dependent decrease in hepatocyte CYP3A4 activity (Long et al., 2016), similar to the decreases observed in vivo. Last, in subsequent studies (Sarkar et al., 2017), it was demonstrated that PHH-KC aggregates produced major phase I and II metabolites of diclofenac that were similar to those previously observed in humans.
Fig. 2.
Perfused medium platforms for liver cultures/cocultures. (A) Perfused spheroidal liver cocultures (Long et al., 2016). Left to right: A plate with six cell culture chambers is connected to a pneumatic plate to enable independent perfusion across each chamber. The schematic shown is that of a single perfused chamber with its own pump and polycarbonate filter in which the cell spheroids attach to the walls of the pores of the filter. Fluorescent image illustrating the morphology of PHH spheroids (day 7) adhered to the pores of a filter within a single fluidic chamber (green, f-actin; blue, nuclei). Scale bar, 100 μm. (B) Parallel-plate bioreactor for subjecting liver cultures/cocultures to oxygen concentration gradients to induce zonated hepatic functions (Allen and Bhatia, 2003; Allen et al., 2005). Top to bottom: Design schematic for the bioreactor. A 2D plot of computationally predicted oxygen concentrations that the cell monolayer is subjected to across the cross-sectional length of the bioreactor. The oxygen concentrations are high at the bioreactor inlet compared with the outlet due to the cell-mediated depletion of the oxygen as medium flows from left to right in the bioreactor. The reducing oxygen concentrations from the bioreactor inlet to the outlet lead to higher CYP2B protein expression in the cells as measured by Western blotting; this finding is consistent with known zonated outcomes in the liver. (C) Layered liver cocultures housed in a commercially available microfluidic device (top drawing) (Vernetti et al., 2016; Lee-Montiel et al., 2017). Schematic (bottom) depicts the layering of the cells and materials, whereas the fluorescent image (right) represents an X–Z projection created from stacked images acquired using a confocal microscope. Hepatocytes were labeled with a cytochrome c biosensor; the liver ECM (LECM) was labeled with a rhodamine-labeled fibronectin; endothelial cells were labeled with an antibody against CD31. Scale bar, 10 μm. (D) Microfluidic device with fluidic dilution “tree” to subject hepatocyte cultures in separated chambers to soluble factor gradients (left, simulation of a gradient; right, food dye gradient in the actual device) (Kang et al., 2018).
Polydimethylsiloxane (PDMS)–based microfluidic devices have also been used to generate perfused liver cultures/cocultures for drug development applications. PDMS offers the ability to rapidly prototype different device designs, biocompatibility with cell culture, and transparency (optical clarity) for light microscopy. For instance, one early design was based on the development of an 8 × 8 element nonaddressable (i.e., not individually accessible) array of microfluidic wells containing micropatterned rat hepatocytes surrounded by 3T3-J2 fibroblasts (Kane et al., 2006). These cocultures were perfused independently with both culture medium and oxygen. Notably, based on the use of microfluidic systems, it has been observed that perfused hepatocyte-endothelial cocultures exhibit a greater rate of production of drug metabolites relative to static controls (Novik et al., 2010). In addition, increased albumin and urea secretions have been measured in perfused cocultures of PHHs and a mixture of liver NPCs (KCs, HSCs, and fibroblasts) compared with static conditions (Esch et al., 2015). In other similar work, human liver cocultures were generated in a single chamber using a commercially available microfluidic device (Vernetti et al., 2016; Lee-Montiel et al., 2017). Specifically, PHHs were seeded and allowed to adhere overnight. This was followed by the seeding of a mixture of monocytes and endothelial cells directly on top of the adherent PHHs, and then this coculture was overlaid with immortalized HSCs encapsulated within a collagen gel (Vernetti et al., 2016) or subsequently within a hydrogel composed of a porcine-derived whole-liver ECM (Lee-Montiel et al., 2017). In this platform, CYP2C9, CYP3A4, and glucuronidation activities were detected via the use of prototypical substrates and metabolites. Despite its advantages for cell culture noted above, PDMS is a porous and hydrophobic material that can absorb lipophilic drugs. Although this issue can be mitigated to some extent by presoaking the PDMS devices with albumin and/or serum, alternative materials, such as micromachined plastics and glass, are beginning to be used for creating microfluidic devices that use minimal PDMS and/or entirely eliminate it altogether (Lenguito et al., 2017; Li et al., 2018).
Building on the previous efforts discussed above, the most recent microfluidic strategies have explored the incorporation of multiple chambers as a means to mimic the sinusoidal architecture present within the liver. For example, in one device configuration, a polyethylene terephthalate membrane was used to separate adjacent cell culture chambers (Prodanov et al., 2016). PHHs were cultured in the bottom chamber together with an overlaid collagen gel containing immortalized HSCs. A mixture of monocytes and immortalized endothelial cells was added to the top chamber. In another device, primary human LSECs and THP-1 cell line–derived macrophages were layered on one side of a porous polyethylene terephthalate membrane, whereas PHHs and an HSC line (LX-2) were layered on the other side of the membrane (Li et al., 2018). The cell-seeded membrane was then sandwiched between two glass layers using polymer gaskets. Both sides of the membrane could be independently perfused with tunable flow rates and ensuing shear stresses. Last, in a cone-and-plate viscometer configuration, PHHs were cultured within a collagen-based gel on a polycarbonate transwell membrane, while a coculture of HSCs and macrophages was cultured on the other surface of the membrane (Feaver et al., 2016). The HSC + macrophage coculture was subjected to liver-like hemodynamic flow via the viscometer. One key advantage of mimicking the sinusoidal (layered) architecture of the liver is the ability to introduce immune cells (e.g., leukocytes) into the endothelial compartment and let them migrate into the hepatic compartment toward modeling inflammation and the ensuing effects on hepatic functions, including drug metabolism capacities. However, the throughput of such layered designs for drug screening with dual microfluidic streams is significantly reduced compared with nonlayered static configurations.
Perfusion culture configurations can additionally provide a strategy for introducing physiologically relevant gradients of oxygen, hormones, and nutrients. Notably, such gradients have been demonstrated to play important roles in the induction of differential functions (including distinct P450 activities) of hepatocytes along the length of the sinusoid, a phenomenon termed “zonation” (Jungermann and Kietzmann, 1996; Kietzmann, 2017). In the initial demonstration of this type of platform, a parallel plate bioreactor with resulting oxygen gradients was used to induce a zonated pattern of P450 enzymes in rat hepatocyte cultures (Allen and Bhatia, 2003) and hepatocyte/fibroblast cocultures (Fig. 2B) (Allen et al., 2005). Subsequently, using an alternative strategy based on variable culture medium perfusion rates in separate devices, human hepatocyte/nonparenchymal cocultures were subjected to characteristic zone 1 oxygen (10%–12%) or zone 3 oxygen (3%–5%) levels (Fig. 2C), and hepatocellular functions were analyzed (Lee-Montiel et al., 2017). Zone 1 cocultures exhibited elevated oxidative phosphorylation, albumin secretion, and urea secretion compared with zone 3 cocultures. In contrast, cocultures in zone 3 demonstrated relative increases in CYP2E1 activity, glycolysis, α1-antitrypsin activity, and steatosis. Taken together, these functional profiles recapitulate aspects of hepatic zonation, as observed in rodent studies in vivo.
In addition to oxygen gradients, parallel efforts in the field have explored the integration of gradients of other soluble factors, such as physiologic hormones or drugs. For example, rat hepatocyte monolayer cultures have been subjected to a microfluidic device gradient of 3-methylcholanthrene (3-MC), which is an inducer of P450 enzymes and glutathione S-transferase (McCarty et al., 2016). In this device, increased hepatotoxicity in response to allyl alcohol was observed in the low 3-MC region, whereas elevated hepatotoxicity of acetaminophen was observed in the high 3-MC region. A more recent configuration of this device uses a fluidic dilution “tree” design to subject primary rat hepatocytes or PHHs in spatially separated chambers (with an ability to sample supernatants from each chamber) to different concentrations of hormones and drugs with the intention of creating liver-like gradually changing metabolic zones (Kang et al., 2018) (Fig. 2D). Indeed, subjecting the cells to 3-MC led to a dose-dependent and expected increase in CYP1A activity. Overall, we anticipate that the next-generation microfluidic devices will combine fluid flow on an LSEC + KC layer while protecting PHHs and HSCs from shear stress and exposure of cells to gradients of oxygen, hormones, and drugs to fully recapitulate the sinusoid-on-a-chip in a parallel multichamber design to improve throughput relative to existing low-throughput microfluidic platforms.
Future Directions and Conclusions
Advances in protein micropatterning (Khetani and Bhatia, 2008), microfluidic chips (Sivaraman et al., 2005; Vernetti et al., 2016), specialized plates for creating spheroids (Messner et al., 2013; Miyamoto et al., 2015), biomaterials scaffolds (Liu Tsang et al., 2007), and 3D bioprinting (Norona et al., 2016) have broadly enabled more precise control of in vitro cell microenvironments, which in turn have led to culture strategies that support stabilized hepatocyte functions for several weeks (Table 1). This stability of hepatocellular functions has proven very useful for long-term treatment with drugs (≥7 days), which can substantially improve the fidelity of drug metabolism outcome studies compared with short-term (<24 hours) treatment of functionally declining hepatocyte monocultures or suspensions (Lin and Khetani, 2016). In particular, the most recent human liver models allow for the detection of a greater number of clinically relevant metabolites in vitro and the prediction of the clearance of low-turnover drugs (i.e., one-pill-a-day dosing regimen) with better IVIVE; such improvements are mediated by the higher (more physiologic) activities of drug metabolism enzymes/transporters and the ability to treat the cultures for a longer timeframe. Additionally, since hepatocytes become properly polarized with the formation of bile canaliculi in advanced human liver platforms, the uptake and efflux of drugs and their metabolites via major transporters can be determined along with the interplay of drug transport with metabolism; indeed, the investigation of such an interplay embodies an important utility of advanced human liver platforms. Furthermore, whereas short-term (<4 days) P450 induction and inhibition via drugs can be carried out using conventional PHH monocultures, the most recent human liver models provide the ability to evaluate long-term (>7 days) P450 induction/inhibition and the coupling of such outcomes with drug clearance, metabolite formation, and toxicity assessments, as occurs in the clinic.
TABLE 1.
Benefits, limitations, and validated utility of engineered primary human liver platforms for drug metabolism studies
| Model | Benefits | Potential Limitations | Drug Metabolism Applications (Primary Human Liver Cells) |
|---|---|---|---|
| Cellular microarrays | Can be used to evaluate the metabolism of many drugs simultaneously in a small footprint | Primarily dependent on imaging-based readouts when using cells | These arrays have so far been adapted to cancerous hepatic cell lines and application to primary liver cells is pending (Lee et al., 2005, 2008; Yu et al., 2018) |
| Low novel drug/chemical usage | Current array configurations are limited in their ability to interrogate responses of adhered cells to molecular gradients | ||
| Dual advantage with the ability to evaluate the effects of combinatorial biochemical and biophysical signals on enhancement of liver cell functions. | |||
| Conventional cocultures (random distribution) | No specialized systems/equipment is needed to create randomly distributed cocultures in standard multiwell culture plates | Function of hepatocytes is highly dependent on the choice of the nonparenchymal support cell type | Measured activities of NAT2, UGT1A1, SULT, AKR, AO, FMO, CYP1A2, CYP2B6, CYP2C9, CYP2D6, and CYP3A4 after 8 days of culture (Kratochwil et al., 2017) |
| Part of the randomly distributed cocultures can display morphologic and functional instability due to suboptimal (random) cell-cell contact/interactions | Measured activities of CYP2D6 and CYP3A4 for 14 days (Novik et al., 2017) | ||
| Typically, have lower activities of some drug metabolism enzymes than micropatterned cocultures | |||
| Micropatterned cocultures (MPCCs) | Controlled homotypic hepatocyte interactions on micropatterned protein domains allow for proper cell polarity and higher/stable liver functions in coculture with fibroblasts for 4–6 weeks | Specialized masks created using lithographic techniques are needed to pattern ECM proteins (e.g., collagen) to enable subsequent clustering of the hepatocytes | Measured activities of NAT2, UGT1A1, SULT, AKR, AO, FMO, CYP1A2, CYP2B6, CYP2C9, CYP2D6, and CYP3A4 after 8 days of culture (Kratochwil et al., 2017) |
| Available in industry standard multiwell formats (up to 384-well plates) | A single configuration containing all major liver cell types is currently lacking | Measured activities of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, CYP3A4, UGT, and SULT for 30 days (Khetani and Bhatia, 2008; Lin et al., 2016) | |
| Diverse types of liver NPCs can be cultured in MPCCs without significantly affecting the homotypic interactions and polarity of hepatocytes on the micropatterned colonies | Use nonliver fibroblasts for inducing optimal functions in hepatocytes | Drug metabolite detection (Wang et al., 2010; Ballard et al., 2016) | |
| Drug clearance prediction (Chan et al., 2013; Lin et al., 2016) | |||
| DDIs including P450 induction and inhibition (Khetani and Bhatia, 2008; Lin et al., 2016; Kratochwil et al., 2018) | |||
| Transporter, metabolism, and/or P450 induction interplay on drug disposition (Ramsden et al., 2014a; Moore et al., 2016) | |||
| Self-assembled Spheroids | Many off-the-shelf plate formats are available for the creation of spheroids | Cellular necrosis can occur in the center of spheroids if their diameter exceeds 250–300 µm | Measured activities of CYP1A2, CYP2C8, CYP2C9, CYP2D6, and CYP3A4 for 35 days (Bell et al., 2016, 2018) |
| Some plate formats can cause large variations in the sizes of the spheroids, which leads to nonuniform viability and function | |||
| Single spheroids may not provide enough material for sensitive drug metabolite identification | |||
| Heterogeneous cell distribution without any defined architecture | |||
| Bioprinted spheroids/organoids | Printing head allows control over the placement of cells in different locations/compartments (e.g., hepatic, vascular, and other nonparenchymal cell compartments) | Microscale printing resolution for control of single cell placement is currently lacking | Measured activity of CYP3A4 for 28 days (Norona et al., 2016) |
| Software programming allows on-demand 3D architectures to the created with same instrumentation configuration (i.e., expensive masks are not needed as for lithographic techniques) | Low-throughput creation of the cultures | ||
| The method requires complex and often expensive instrumentation with the need for well-trained technologists | |||
| The method requires a larger number of cells than microscale patterning methods using lithography | |||
| Printed tissues on the millimeter to centimeter scale can potentially display necrosis in the core of the tissues in the absence of a connected/perfused vasculature | |||
| Perfused liver culture/coculture platforms (some are also known as liver-on-a-chip) | Enable fluid flow to allow automated nutrient and waste exchange as well as the generation of in vivo-like molecular gradients (e.g., oxygen), which can lead to zonated hepatic phenotypes | Perfusion requires specialized fluid pumps and control systems | Measured activities of CYP2D6 and CYP3A4 for 6 days of culture (Novik et al., 2010) |
| Many microfluidic perfusion devices are now commercially available for the creation of perfused liver cultures | Drugs can potentially bind and be sequestered by the tubing and materials used in the perfusion devices | Measured activities of CYP2C9, CYP3A4, and UGT for 14 days (Vernetti et al., 2016) | |
| Due to the need for tubing, perfusion devices have a large dead volume, which can necessitate higher quantities of the novel (and often very limited) compounds for treating cell cultures | Drug clearance prediction (Dash et al., 2009; Novik et al., 2010) | ||
| Most devices are currently low-throughput, allowing up to 12 devices to be perfused at a single time | Drug metabolite identification (Sarkar et al., 2017) |
AKR, aldo-keto reductase; FMO, flavin monooxygenase; NAT2, N-acetyltransferase 2; SULT, sulfotransferase; UGT1A1, UDP glucuronosyltransferase 1A1.
Most of the high-functioning liver models, both animal and human derived, cocultivate hepatocytes with NPCs. However, it is important to note that even non–liver-derived cell types (i.e., 3T3-J2 murine embryonic fibroblasts) can induce increased levels of characteristic markers and functions in hepatocytes from multiple species, including humans, suggesting that the underlying molecular mediators are species conserved (Bhatia et al., 1999). Interestingly, many of the culture platforms used in the field, and described here, do not fully recapitulate the in vivo liver architecture, but still act to induce robust levels of hepatocyte function and have broadly enabled the acquisition of reproducible datasets across many experiments. This observation is suggestive of the importance of microenvironmental signals, which may outweigh any requirement for mimicking the macroarchitecture of the liver. Future studies will undoubtedly explore potential strategies for scaling up liver cell systems, particularly in the context of implantable constructs for applications in regenerative medicine.
Despite great and sustained progress in the development of long-term human liver platforms, pending issues need to be addressed. First, it is currently difficult to compare the performance of different model systems because of the use of different cell donors, endpoints, data normalization schemes, and drug sets. Some studies by pharmaceutical companies are beginning to emerge that compare different engineered platforms using similar parameters (i.e., drugs, donors, endpoints) (Kratochwil et al., 2017), but further work is needed in this area to determine the most optimal human liver platforms and clearly define key criteria for the building of next-generation devices. Such unbiased functional comparisons by the end-users will allow the selection of the most appropriate human liver platform in a specific part of the drug development pipeline. For instance, high-throughput multiwell/microwell platforms may be best suited for the early stages of drug development (i.e., drug discovery and lead selection). On the other hand, more complex and physiologically relevant, but low-throughput, platforms may be best used during lead optimization to accurately determine the parameters for drug testing in patients, and toward determining the mechanisms of liver-drug interactions observed in human populations in clinical trials and/or the marketplace.
Second, although some engineered liver platforms have been used to determine the effects of major liver diseases on drug metabolism capacities of hepatocytes (Davidson et al., 2017; Kostrzewski et al., 2017), further research is needed to more faithfully mimic the disease phenotypes over several weeks in vitro and determine the effects of the diseased phenotype on drug metabolism, disposition, and toxicity. Such a direction will allow clear stratification of how specific patient populations will respond to drugs across the different phases of clinical trials. Third, although PHHs are ideal for drug metabolism studies, the number of donors available is limited, which does not allow the determination of how genotype affects drug metabolism outcomes as it does in the wider human population. Hepatocytes derived from iPSCs obtained from many human patients with distinct genetic backgrounds can mitigate the above-mentioned limitation with PHHs. However, two key issues with iPSC technology need to be addressed, including continued optimization of culture protocols to further mature the iHep cells toward a PHH (adult) phenotype, and differentiation of iPSCs into liver NPCs to adequately model interaction of multiple liver cell types with the same donor background (Scott et al., 2013; Davidson et al., 2015b). High-throughput culture platforms, such as the microarrays discussed here, could help in the progress toward these goals given the large space of microenvironmental signals, including combinatorial conditions, that will need to be examined. Finally, although many NPC types, including LSECs, HSCs, and KCs, have been integrated into various liver coculture systems, it remains unclear how to introduce biliary epithelial cells in a functional manner consistent with the role of bile canaliculi and bile ducts in the transport of factors such as drug metabolites.
In conclusion, engineered human liver platforms with varying throughputs, technological complexities, and cell sources (e.g., cell lines and primary animal/human liver cells) are now available to the pharmaceutical industry to investigate drug metabolism/disposition (and toxicity) at different stages of drug development prior to human clinical trials. These tools are rapidly increasing in usage for “live” drug development projects, and we anticipate that their use will rise dramatically with continued participation from the pharmaceutical industry and regulatory agencies in validating and adopting commercially available platforms. Further, parallel efforts will continue to exploit these advances in other industries, including the examination of liver cellular responses to exposures to industrial chemicals, as well as basic science studies focused on better understanding liver functions and diseases. Overall, we anticipate that the increased use of these optimized in vitro platforms should reduce the overall cost of drug development, reduce the reliance on in vivo animal studies, and accelerate the market availability of novel drugs. The ultimate beneficiaries of such advances will be patients, who will have faster access to novel treatment options, and will be subjected to fewer suboptimal drugs with respect to their metabolism and toxicity in the body.
Acknowledgments
We thank Chase Monckton, Christine Lin, and Brenton Ware for helpful discussions.
Abbreviations
- DDI
drug-drug interactions
- ECM
extracellular matrix
- HSC
hepatic stellate cell
- iHep
induced pluripotent stem cell–derived human hepatocyte–like cell
- iPSC
induced pluripotent stem cell
- IVIVE
in vitro-to-in vivo extrapolation
- KC
Kupffer cells/macrophage
- LSEC
liver sinusoidal endothelial cell
- 3-MC
3-methylcholanthrene
- MPCC
micropatterned coculture
- NPC
nonparenchymal cell
- P450
cytochrome P450
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PHH
primary human hepatocyte
- 3D
three dimensional
- 2D
two dimensional
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Underhill and Khetani.
Footnotes
This work was funded by the National Institutes of Health [Grant 1R01-DK-115747-01 to S.R.K. and G.H.U.].
References
- Acikgöz A, Giri S, Cho MG, Bader A. (2013) Morphological and functional analysis of hepatocyte spheroids generated on poly-HEMA-treated surfaces under the influence of fetal calf serum and nonparenchymal cells. Biomolecules 3:242–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JW, Bhatia SN. (2003) Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng 82:253–262. [DOI] [PubMed] [Google Scholar]
- Allen JW, Khetani SR, Bhatia SN. (2005) In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol Sci 84:110–119. [DOI] [PubMed] [Google Scholar]
- Atienzar FA, Blomme EA, Chen M, Hewitt P, Kenna JG, Labbe G, Moulin F, Pognan F, Roth AB, Suter-Dick L, et al. (2016) Key challenges and opportunities associated with the use of in vitro models to detect human DILI: integrated risk assessment and mitigation plans. BioMed Res Int 2016:9737920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TE, Wang S, Cox LM, Moen MA, Krzyzewski S, Ukairo O, Obach RS. (2016) Application of a micropatterned cocultured hepatocyte system to predict preclinical and human-specific drug metabolism. Drug Metab Dispos 44:172–179. [DOI] [PubMed] [Google Scholar]
- Bell CC, Dankers ACA, Lauschke VM, Sison-Young R, Jenkins R, Rowe C, Goldring CE, Park K, Regan SL, Walker T, et al. (2018) Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: a multicenter study. Toxicol Sci 162:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Hendriks DFG, Moro SML, Ellis E, Walsh J, Renblom A, Fredriksson Puigvert L, Dankers ACA, Jacobs F, Snoeys J, et al. (2016) Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep 6:25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. (2015) Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61:1370–1381. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. (1999) Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 13:1883–1900. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Underhill GH, Zaret KS, Fox IJ. (2014) Cell and tissue engineering for liver disease. Sci Transl Med 6:245sr2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brafman DA, de Minicis S, Seki E, Shah KD, Teng D, Brenner D, Willert K, Chien S. (2009a) Investigating the role of the extracellular environment in modulating hepatic stellate cell biology with arrayed combinatorial microenvironments. Integr Biol 1:513–524. [DOI] [PubMed] [Google Scholar]
- Brafman DA, Shah KD, Fellner T, Chien S, Willert K. (2009b) Defining long-term maintenance conditions of human embryonic stem cells with arrayed cellular microenvironment technology. Stem Cells Dev 18:1141–1154. [DOI] [PubMed] [Google Scholar]
- Brett M-E, Crampton AL, Wood DK. (2016) Rapid generation of collagen-based microtissues to study cell–matrix interactions. Technology 04:80–87. [Google Scholar]
- Chan TS, Yu H, Moore A, Khetani SR, Tweedie D. (2013) Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac [published correction appears in Drug Metab Dispos (2014) 42:200]. Drug Metab Dispos 41:2024–2032. [DOI] [PubMed] [Google Scholar]
- Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. (2011) Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci USA 108:11842–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash A, Inman W, Hoffmaster K, Sevidal S, Kelly J, Obach RS, Griffith LG, Tannenbaum SR. (2009) Liver tissue engineering in the evaluation of drug safety. Expert Opin Drug Metab Toxicol 5:1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Silva F, Boulenc X, Vermet H, Compigne P, Gerbal-Chaloin S, Daujat-Chavanieu M, Klieber S, Poulin P. (2018) Improving prediction of metabolic clearance using quantitative extrapolation of results obtained from human hepatic micropatterned cocultures model and by considering the impact of albumin binding. J Pharm Sci 107:1957–1972. [DOI] [PubMed] [Google Scholar]
- Davidson MD, Kukla DA, Khetani SR. (2017) Microengineered cultures containing human hepatic stellate cells and hepatocytes for drug development. Integr Biol 9:662–677. [DOI] [PubMed] [Google Scholar]
- Davidson MD, Lehrer M, Khetani SR. (2015a) Hormone and drug-mediated modulation of glucose metabolism in a microscale model of the human liver. Tissue Eng Part C Methods 21:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MD, Ware BR, Khetani SR. (2015b) Stem cell-derived liver cells for drug testing and disease modeling. Discov Med 19:349–358. [PMC free article] [PubMed] [Google Scholar]
- Di L, Atkinson K, Orozco CC, Funk C, Zhang H, McDonald TS, Tan B, Lin J, Chang C, Obach RS. (2013) In vitro-in vivo correlation for low-clearance compounds using hepatocyte relay method. Drug Metab Dispos 41:2018–2023. [DOI] [PubMed] [Google Scholar]
- Dixit V, Moore A, Tsao H, Hariparsad N. (2016) Application of micropatterned cocultured hepatocytes to evaluate the inductive potential and degradation rate of major xenobiotic metabolizing enzymes. Drug Metab Dispos 44:250–261. [DOI] [PubMed] [Google Scholar]
- Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch MB, Prot JM, Wang YI, Miller P, Llamas-Vidales JR, Naughton BA, Applegate DR, Shuler ML. (2015) Multi-cellular 3D human primary liver cell culture elevates metabolic activity under fluidic flow. Lab Chip 15:2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver RE, Cole BK, Lawson MJ, Hoang SA, Marukian S, Blackman BR, Figler RA, Sanyal AJ, Wamhoff BR, Dash A. (2016) Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight 1:e90954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim CJ, Chien S, Bhatia SN. (2005) An extracellular matrix microarray for probing cellular differentiation. Nat Methods 2:119–125. [DOI] [PubMed] [Google Scholar]
- Flaim CJ, Teng D, Chien S, Bhatia SN. (2008) Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev 17:29–39. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Nakazawa K. (2011) Hepatocyte spheroid arrays inside microwells connected with microchannels. Biomicrofluidics 5:22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Roth A. (2017) Current limitations and future opportunities for prediction of DILI from in vitro. Arch Toxicol 91:131–142. [DOI] [PubMed] [Google Scholar]
- Gerets HHJ, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. (2012) Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 28:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, et al. (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87:1315–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouzo A. (1998) Liver cell models in in vitro toxicology. Environ Health Perspect 106 (Suppl 2):511–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gural N, Mancio-Silva L, Miller AB, Galstian A, Butty VL, Levine SS, Patrapuvich R, Desai SP, Mikolajczak SA, Kappe SHI, et al. (2018) In vitro culture, drug sensitivity, and transcriptome of plasmodium vivax hypnozoites. Cell Host Microbe 23:395–406.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H, Kang K, Park SA, Kim WD, Paik SS, Lee SH, Jeong J, Choi D. (2017) Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver 11:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K, Kietzmann T. (1996) Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr 16:179–203. [DOI] [PubMed] [Google Scholar]
- Kane BJ, Zinner MJ, Yarmush ML, Toner M. (2006) Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem 78:4291–4298. [DOI] [PubMed] [Google Scholar]
- Kang YBA, Eo J, Mert S, Yarmush ML, Usta OB. (2018) Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci Rep 8:8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylan KB, Ermilova V, Yada RC, Underhill GH. (2016) Combinatorial microenvironmental regulation of liver progenitor differentiation by Notch ligands, TGFβ, and extracellular matrix. Sci Rep 6:23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani SR, Bhatia SN. (2008) Microscale culture of human liver cells for drug development. Nat Biotechnol 26:120–126. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Chen AA, Ranscht B, Bhatia SN. (2008) T-cadherin modulates hepatocyte functions in vitro. FASEB J 22:3768–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani SR, Kanchagar C, Ukairo O, Krzyzewski S, Moore A, Shi J, Aoyama S, Aleo M, Will Y. (2013) Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci 132:107–117. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. (2004) Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology 40:545–554. [DOI] [PubMed] [Google Scholar]
- Kietzmann T. (2017) Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol 11:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzewski T, Cornforth T, Snow SA, Ouro-Gnao L, Rowe C, Large EM, Hughes DJ. (2017) Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J Gastroenterol 23:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourouklis AP, Kaylan KB, Underhill GH. (2016) Substrate stiffness and matrix composition coordinately control the differentiation of liver progenitor cells. Biomaterials 99:82–94. [DOI] [PubMed] [Google Scholar]
- Kratochwil NA, Meille C, Fowler S, Klammers F, Ekiciler A, Molitor B, Simon S, Walter I, McGinnis C, Walther J, et al. (2017) Metabolic profiling of human long-term liver models and hepatic clearance predictions from in vitro data using nonlinear mixed-effects modeling. AAPS J 19:534–550. [DOI] [PubMed] [Google Scholar]
- Kratochwil NA, Triyatni M, Mueller MB, Klammers F, Leonard B, Turley D, Schmaler J, Ekiciler A, Molitor B, Walter I, et al. (2018) Simultaneous assessment of clearance, metabolism, induction, and drug-drug interaction potential using a long-term in vitro liver model for a novel hepatitis B virus inhibitor. J Pharmacol Exp Ther 365:237–248. [DOI] [PubMed] [Google Scholar]
- Kwon SJ, Lee DW, Shah DA, Ku B, Jeon SY, Solanki K, Ryan JD, Clark DS, Dordick JS, Lee M-Y. (2014) High-throughput and combinatorial gene expression on a chip for metabolism-induced toxicology screening. Nat Commun 5:3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, Petersen OW, Bissell MJ. (2009) Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr Biol 1:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin AL, Rodrigues RR, Murali TM, Rajagopalan P. (2013) Designing a multicellular organotypic 3D liver model with a detachable, nanoscale polymeric Space of Disse. Tissue Eng Part C Methods 19:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Lee M-Y, Ku B, Yi SH, Ryu J-H, Jeon R, Yang M. (2014) Application of the DataChip/MetaChip technology for the evaluation of ajoene toxicity in vitro. Arch Toxicol 88:283–290. [DOI] [PubMed] [Google Scholar]
- Lee G, Lee J, Oh H, Lee S. (2016) Reproducible construction of surface tension-mediated honeycomb concave microwell arrays for engineering of 3D microtissues with minimal cell loss. PLoS One 11:e0161026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Lee JS, Lee GH, Joung WY, Kim SH, Lee SH, Park JY, Kim DH. (2017) Networked concave microwell arrays for constructing 3D cell spheroids. Biofabrication 10:015001. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kumar RA, Sukumaran SM, Hogg MG, Clark DS, Dordick JS. (2008) Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl Acad Sci USA 105:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Park CB, Dordick JS, Clark DS. (2005) Metabolizing enzyme toxicology assay chip (MetaChip) for high-throughput microscale toxicity analyses. Proc Natl Acad Sci USA 102:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Montiel FT, George SM, Gough AH, Sharma AD, Wu J, DeBiasio R, Vernetti LA, Taylor DL. (2017) Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp Biol Med (Maywood) 242:1617–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenguito G, Chaimov D, Weitz JR, Rodriguez-Diaz R, Rawal SA, Tamayo-Garcia A, Caicedo A, Stabler CL, Buchwald P, Agarwal A. (2017) Resealable, optically accessible, PDMS-free fluidic platform for ex vivo interrogation of pancreatic islets. Lab Chip 17:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Stevens KR, Schwartz RE, Alejandro BS, Huang JH, Bhatia SN. (2014) Micropatterned cell-cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Eng Part A 20:2200–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, George SM, Vernetti L, Gough AH, Taylor DL. (2018) A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip 18:2614–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Khetani SR. (2016) Advances in engineered liver models for investigating drug-induced liver injury. BioMed Res Int 2016:1829148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Shi J, Moore A, Khetani SR. (2016) Prediction of drug clearance and drug-drug interactions in microscale cultures of human hepatocytes. Drug Metab Dispos 44:127–136. [DOI] [PubMed] [Google Scholar]
- Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. (2007) Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J 21:790–801. [DOI] [PubMed] [Google Scholar]
- Long TJ, Cosgrove PA, Dunn RT, II, Stolz DB, Hamadeh H, Afshari C, McBride H, Griffith LG. (2016) Modeling therapeutic antibody-small molecule drug-drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab Dispos 44:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23:47–55. [DOI] [PubMed] [Google Scholar]
- Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, et al. (2016) Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA 113:2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, Carpenter AE, Thomas D, Sim BKL, Mota MM, et al. (2013) A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty WJ, Usta OB, Yarmush ML. (2016) A microfabricated platform for generating physiologically-relevant hepatocyte zonation. Sci Rep 6:26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner S, Agarkova I, Moritz W, Kelm JM. (2013) Multi-cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol 87:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S. (2015) Spheroid formation and evaluation of hepatic cells in a three-dimensional culture device. Cell Med 8:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, Chothe PP, Tsao H, Hariparsad N. (2016) Evaluation of the interplay between uptake transport and CYP3A4 induction in micropatterned cocultured hepatocytes. Drug Metab Dispos 44:1910–1919. [DOI] [PubMed] [Google Scholar]
- Ng S, March S, Galstian A, Hanson K, Carvalho T, Mota MM, Bhatia SN. (2014) Hypoxia promotes liver stage malaria infection in primary human hepatocytes in vitro. Dis Model Mech 7:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB. (2016) Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One 11:e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Ukairo O, Khetani SR, McVay M, Kanchagar C, Seghezzi W, Ayanoglu G, Irrechukwu O, Evers R. (2015) Establishment of a hepatocyte-Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos 43:774–785. [DOI] [PubMed] [Google Scholar]
- Norona LM, Nguyen DG, Gerber DA, Presnell SC, LeCluyse EL. (2016) Editor’s highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci 154:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. (2010) A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol 79:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novik EI, Dwyer J, Morelli JK, Parekh A, Cho C, Pludwinski E, Shrirao A, Freedman RM, MacDonald JS, Jayyosi Z. (2017) Long-enduring primary hepatocyte-based co-cultures improve prediction of hepatotoxicity. Toxicol Appl Pharmacol 336:20–30. [DOI] [PubMed] [Google Scholar]
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, et al. (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32:56–67. [DOI] [PubMed] [Google Scholar]
- Peng CC, Doshi U, Prakash C, Li AP. (2016) A novel plated hepatocyte relay assay (PHRA) for in vitro evaluation of hepatic metabolic clearance of slowly metabolized compounds. Drug Metab Lett 10:3–15. [DOI] [PubMed] [Google Scholar]
- Ploss A, Khetani SR, Jones CT, Syder AJ, Trehan K, Gaysinskaya VA, Mu K, Ritola K, Rice CM, Bhatia SN. (2010) Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci USA 107:3141–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, et al. (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng 78:257–269. [DOI] [PubMed] [Google Scholar]
- Prodanov L, Jindal R, Bale SS, Hegde M, McCarty WJ, Golberg I, Bhushan A, Yarmush ML, Usta OB. (2016) Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol Bioeng 113:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden D, Tweedie DJ, Chan TS, Taub ME, Li Y. (2014a) Bridging in vitro and in vivo metabolism and transport of faldaprevir in human using a novel cocultured human hepatocyte system, HepatoPac. Drug Metab Dispos 42:394–406. [DOI] [PubMed] [Google Scholar]
- Ramsden D, Tweedie DJ, St George R, Chen LZ, Li Y. (2014b) Generating an in vitro-in vivo correlation for metabolism and liver enrichment of a hepatitis C virus drug, faldaprevir, using a rat hepatocyte model (HepatoPac). Drug Metab Dispos 42:407–414. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Ravindra KC, Large E, Young CL, Rivera-Burgos D, Yu J, Cirit M, Hughes DJ, Wishnok JS, Lauffenburger DA, et al. (2017) Integrated assessment of diclofenac biotransformation, pharmacokinetics, and omics-based toxicity in a three-dimensional human liver-immunocompetent coculture system. Drug Metab Dispos 45:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar U, Rivera-Burgos D, Large EM, Hughes DJ, Ravindra KC, Dyer RL, Ebrahimkhani MR, Wishnok JS, Griffith LG, Tannenbaum SR. (2015) Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab Dispos 43:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CW, Peters MF, Dragan YP. (2013) Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett 219:49–58. [DOI] [PubMed] [Google Scholar]
- Shih H, Pickwell GV, Guenette DK, Bilir B, Quattrochi LC. (1999) Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum Exp Toxicol 18:95–105. [DOI] [PubMed] [Google Scholar]
- Shlomai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN, Rice CM. (2014) Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci USA 111:12193–12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman A, Leach JK, Townsend S, Iida T, Hogan BJ, Stolz DB, Fry R, Samson LD, Tannenbaum SR, Griffith LG. (2005) A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab 6:569–591. [DOI] [PubMed] [Google Scholar]
- Soen Y, Mori A, Palmer TD, Brown PO. (2006) Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol Syst Biol 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Lu YC, Frankel AS, An D, Schwartz RE, Ma M. (2015) Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep 5:16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, et al. (2013) 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 34:1781–1789. [DOI] [PubMed] [Google Scholar]
- Tasnim F, Toh Y-C, Qu Y, Li H, Phan D, Narmada BC, Ananthanarayanan A, Mittal N, Meng RQ, Yu H. (2016) Functionally enhanced human stem cell derived hepatocytes in galactosylated cellulosic sponges for hepatotoxicity testing. Mol Pharm 13:1947–1957. [DOI] [PubMed] [Google Scholar]
- Trask OJ, Jr, Moore A, LeCluyse EL. (2014) A micropatterned hepatocyte coculture model for assessment of liver toxicity using high-content imaging analysis. Assay Drug Dev Technol 12:16–27. [DOI] [PubMed] [Google Scholar]
- Tsamandouras N, Kostrzewski T, Stokes CL, Griffith LG, Hughes DJ, Cirit M. (2017) Quantitative assessment of population variability in hepatic drug metabolism using a perfused three-dimensional human liver microphysiological system. J Pharmacol Exp Ther 360:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukairo O, Kanchagar C, Moore A, Shi J, Gaffney J, Aoyama S, Rose K, Krzyzewski S, McGeehan J, Andersen ME, et al. (2013) Long-term stability of primary rat hepatocytes in micropatterned cocultures. J Biochem Mol Toxicol 27:204–212. [DOI] [PubMed] [Google Scholar]
- Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Shun TY, Gough A, Taylor DL. (2016) A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood) 241:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorrink SU, Ullah S, Schmidt S, Nandania J, Velagapudi V, Beck O, Ingelman-Sundberg M, Lauschke VM. (2017) Endogenous and xenobiotic metabolic stability of primary human hepatocytes in long-term 3D spheroid cultures revealed by a combination of targeted and untargeted metabolomics. FASEB J 31:2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WW, Khetani SR, Krzyzewski S, Duignan DB, Obach RS. (2010) Assessment of a micropatterned hepatocyte coculture system to generate major human excretory and circulating drug metabolites. Drug Metab Dispos 38:1900–1905. [DOI] [PubMed] [Google Scholar]
- Ware BR, Berger DR, Khetani SR. (2015) Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci 145:252–262. [DOI] [PubMed] [Google Scholar]
- Ware BR, Durham MJ, Monckton CP, Khetani SR. (2017) A cell culture platform to maintain long-term phenotype of Primary human hepatocytes and endothelial cells. Cell Mol Gastroenterol Hepatol 5:187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S, Stahl F, Bader A. (2003) Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos 31:1035–1042. [DOI] [PubMed] [Google Scholar]
- Yu KN, Nadanaciva S, Rana P, Lee DW, Ku B, Roth AD, Dordick JS, Will Y, Lee MY. (2018) Prediction of metabolism-induced hepatotoxicity on three-dimensional hepatic cell culture and enzyme microarrays. Arch Toxicol 92:1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]