Abstract
Light affects almost all aspects of human physiological functioning, including circadian rhythms, sleep–wake regulation, alertness, cognition and mood. We review the existing relevant literature on the effects of various wavelengths of light on these major domains, particularly as they pertain to recovery from mild traumatic brain injuries. Evidence suggests that light, particularly in the blue wavelengths, has powerful alerting, cognitive and circadian phase shifting properties that could be useful for treatment. Other wavelengths, such as red and green may also have important effects that, if targeted appropriately, might also be useful for facilitating recovery. Despite the known effects of light, more research is needed. We recommend a personalized medicine approach to the use of light therapy as an adjunctive treatment for patients recovering from mild traumatic brain injury.
Keywords: : blue light, brain injury, circadian rhythm, concussion, fatigue, mild TBI, phototherapy, sleep–wake disruption
Mild traumatic brain injuries (mTBIs) are currently among the most socially, medically and academically talked-about issues today. The annual mTBI incidence is at least 1.5 million reported injuries in the USA [1–3]. However, this number fails to capture the untold number of such injuries that likely go unreported [4,5]. The long-term consequences – plausibly including neurodegenerative conditions [6–10], impaired cognitive abilities [11–13] and altered psychosocial functioning [14–20] – necessitate the need for efficacious treatments following injury and proactive preventative methods for reducing injury risk and consequence.
Despite significant job-, school- and economic-related burdens associated with the medical management of mTBIs [1], there is no currently accepted gold standard treatment for mTBIs [21,22]. Historically, the treatment of choice was total rest to allow the brain to heal. However, recent research advances are giving way to more active treatments, with an emphasis on early intervention [23–25]. While early reports are promising for short-term management, the long-term impact of these active interventions is not well established. Additionally, there is little information on methods of optimizing these active approaches or complementary treatments that may enhance recovery in both the short and long term.
One complementary treatment method involves the use of light exposure. Light, both visible and invisible, can have powerful effects on numerous neurological and physiological systems [26–29]. Additionally, light is potentially a modifiable aspect of the environment in which one exists to allow for optimal healing, recovery and a return to homeostatic states following mTBI. The purpose of this narrative review is to provide a focused overview of the role and effect of light on neurological processes and to connect these effects with potential areas of intervention with respect to mTBI.
The fate of ambient light: image-forming & nonimage-forming pathways
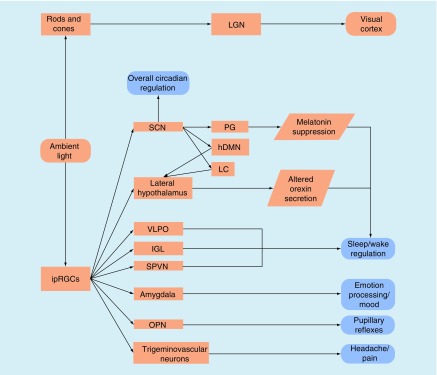
The primary sensory function of the eyes is to translate information contained in light into images [30]. This is accomplished primarily through two classes of retinal photoreceptors. Cones are color-sensitive photoreceptors while rods respond to changes in brightness and are particularly sensitive to dim light. Light information is converted by these photoreceptors, resulting in the stimulation of retinal ganglion cells (RGCs) that project to subcortical nuclei, including the lateral geniculate nucleus of the thalamus, ultimately terminating in the primary visual cortex and visual attention networks (Figure 1). These pathways provide the sensory information for vision and terminate in areas that process and interpret those sensory signals. For a complete review of the transformation from light information to visual interpretation, please see reference [30].
Figure 1. . Schematic representation of the destinations for ambient light entering the eye.
hDMN: Hypothalamic dorsomedial nucleus; IGL: Intergeniculate leaflet; ipRGC: Intrinsically photosensitive retinal ganglion cell; LC: Locus coeruleus; LGN: Lateral geniculate nucleus; OPN: Olivary pretectal nucleus; PG: Pineal gland; SCN: Suprachiasmatic nucleus; SPVN: Supraparaventricular nucleus; VLPO: Ventrolateral preoptic nucleus.
An additional class of photoreceptor was discovered in the early 2000s and is the starting point for the nonimage-forming (NIF) pathway [31–34]. This third type of photoreceptor is expressed directly by a small proportion of RGCs (termed intrinsically photosensitive RGCs; ipRGCs). These ipRGCs are maximally sensitive to blue light (λ = 460–480 nm) and less so to longer wavelengths including green, amber and red [31]. ipRGCs, combined with information regarding illuminance and color from the rods and cones, then directly project to regions involved in the regulation of or influence the actions of (Figure 1):
Circadian rhythms. Projections from the ipRGCs to the suprachiasmatic nucleus (SCN), the primary biological clock for all circadian processes, can directly induce entrainment of either expected or aberrant circadian rhythms [31,35].
Melatonin suppression. Stimulation of ipRGCs results in melatonin suppression via the SCN’s projections to the pineal gland as well as the paraventricular nucleus of hypothalamus and superior cervical ganglion [34,36–38].
Sleep–wake cycle regulation. In conjunction with the above-mentioned melatonin suppression pathways, direct projections from the ipRGCs go to the ventrolateral preoptic nucleus, subparaventricular nucleus and lateral hypothalamus [34]. The SCN may additionally influence the action of the hypothalamus’s dorsomedial nucleus, and locus coeruleus, affecting the lateral hypothalamus secretion of orexin [33,34].
Cognition. The aforementioned pathways regulating circadian rhythms, melatonin suppression and sleep–wake cycles additionally exert both direct and indirect influences on cognition and alertness [26].
Emotional processing and mood. The amygdala, a primary site of emotional processing and integration, receives direct ipRGC projection [34,39].
Intracranial nociception. ipRGCs project to the trigeminovascular neurons of the thalamus that transmit nociceptive information from the dura to the cortex [40].
Pupillary constriction. ipRGCs directly project to the olivary pretectal nucleus [32–34], which in turn project to the Edinger–Westphal nucleus. The cumulative action of this pathway is pupillary constriction. For a complete review of the effects of mTBI on the pupillary light reflex, including NIF pathway contributions, please see [41].
As can be seen, light has the powerful potential to alter numerous biological and cognitive processes through this NIF pathway. Given the complex interactions between circadian timekeepers, hormone and neurotransmitter secretion pathways, cognition, and emotions, light has the potential to positively or negatively influence how individuals function at a very basic level. Consequently, using light as a therapeutic intervention has the potential to directly influence recovery and function following mTBI. In the following sections, we review potential areas of intervention and, where possible, expected outcomes from using light as a therapy.
mTBIs & their consequences
mTBIs are a change or disruption in the normal functioning of the brain subsequent to an external force applied to the head or body [42,43]. Typical guidelines for distinguishing mTBIs from more severe TBIs include a mechanism indicative of mTBI; loss of consciousness <30 min (if at all); post-traumatic amnesia <24 h; Glasgow Coma Scale scores 13–15; and lack of gross abnormalities on traditional neuroimaging [42,44,45]. The effects of a single mTBI are often viewed as transient and may include somatic symptoms, sleep–wake disturbances, and cognitive and behavioral disruptions. However, while these effects are common, the individual manifestations of these are highly individualized and may depend on premorbid functioning and the location and mechanism of injury [46]. Additionally, many individuals experience persistent symptoms associated with an mTBI, and recent findings indicate that the incidence of long-lasting mTBI-related functional decrements may be underestimated [42]. Here we provide an overview of mTBI-related consequences that may be positively affected by light therapy, with an emphasis on sleep and sleep-related consequences given the previously identified NIF-pathway effects.
Sleepiness & fatigue
High-quality sleep is an essential component of all aspects of human performance. Current recommendations for adequate sleep recommend 7–9 h of sleep per night for adults. Despite these recommendations, chronic sleep loss (<5.5 h/night of sleep) in the USA is reaching epidemic levels [47]. For individuals with chronic sleep loss, the consequences are numerous including increased somatization [48,49], poor emotional processing and responsiveness (e.g., increased incidence of depression and anxiety) [50–52], impaired cognition (vigilant attention, executive function, working and long-term memory) [53–56] and poor motor performance [57–59], as well as increased risk for general health issues including diabetes [60–62], cardiovascular disease [60,63], neurodegeneration [64,65] and overall poorer quality of life [66]. While the exact nature of this trend toward chronically undersleeping is not fully understood, work–life stress (e.g., increased expectations for high job-related hours, social stress) as well as the highly prevalent use of fluorescent lighting and blue-shifted light-emitting diode screens at night [67–69] are all implicated.
Compounding the endemic social issue of chronic sleep loss, detrimentally altered sleep is among the most common short- and long-term consequences of mTBI [70–74]. Indeed subjectively perceived traumatic brain injury (TBI)-related sleep–wake disruption is reported by plausibly as many as 70% of all individuals who sustain a TBI (regardless of severity) [70,74,75]. Individuals with mTBI commonly self report insomnia [74–79] and hypersomnolence (excessive sleepiness) [71,75,80–86], though hypersomnia [80,85,87,88] and circadian rhythm sleep disorders [82,89] are also reported. Objectively, these reports are often corroborated by poor sleep efficiency, higher than usual wake after sleep onset and sleep latency, as well as more fragmented sleep and changes in sleep architecture [87,90–95]. Clinically, it is important to recognize that post-mTBI insomnia may be misdiagnosed as a circadian phase issue, specifically delayed sleep phase syndrome [89]. Consequently, individuals sustaining an mTBI may be at an increased risk for all of the aforementioned sleep-related health and performance outcomes without treatment for mTBI-related alterations.
The mechanisms by which mTBI induces altered sleep are not fully understood. However, there are implications from both human studies and animal models that suggest any combination of possible mechanisms including altered circadian hormone regulation (e.g., melatonin release) [96–98] and reductions in neurotransmitter function (e.g., loss of or damage to wake-promoting, orexin-secreting neurons in the hypothalamus) [99–101] among others may be responsible.
In addition to these possible mechanisms of post-mTBI sleep changes, sleep loss or low-quality sleep may impede and impair healing following mTBI. There is considerable evidence indicating that decreases in sleep quantity and quality, both in humans and animals as well as apart from and in relation to mTBI, may impair hippocampal neurogenesis, disrupt ATP production thereby extending the mTBI-initiated neurometabolic cascade [102,103], prolong neuroinflammation [104], impede metabolic waste removal in the brain [105], alter cerebrovascular responsiveness and compromise glymphatic removal of phosphorylated tau [105–107]. Collectively, these effects of sleep disruption may contribute to the short- and long-term clinical presentation of mTBI as well as precipitate the neurodegenerative conditions, particularly tau-related pathologies (e.g., chronic traumatic encephalopathy), commonly thought to be associated with repetitive head trauma.
Alertness
As noted, an mTBI may induce a sequela whereby disrupted circadian rhythms lead to sleep dysfunction, culminating in daytime sleepiness or fatigue. Broadly, daytime fatigue is associated with decreased alertness and vigilant attention capabilities. Indeed, a recent study demonstrates that evidence of increased fatigue and decreased alertness in an mTBI sample are closely related concepts that are difficult to disentangle [86]. Furthermore, mTBI is associated with degraded alertness and vigilance in both the short and long term [86,108,109]. With regards to daytime alertness, phototherapy may provide a nonpharmacological route for improving daytime functioning in post-mTBI individuals.
Cognition
mTBIs additionally exert a substantial, negative impact on various cognitive functions, including working memory, attention, executive function and visuospatial processing [20,110–117]. While deficits in these cognitive domains are generally resolved soon after injury (e.g., most within a month, many within 3 months postinjury), there is evidence to suggest subtle, persistent deficits that linger well beyond this clinically accepted time course. Additionally, there are individuals in whom the full impact of these deficits does not resolve quickly.
Apart from mTBI, increasing sleep need as well as sleep deprivation conditions induce marked deterioration in the cognitive capabilities of individuals [53,118–120]. Given the impact of mTBI on sleep, daytime sleepiness and fatigue, it is reasonable to posit that many of the observed cognitive deficits, particularly those that linger beyond the general clinical time course, may be mediated by sleep-related changes.
Depression
Depression and increased reporting of depressive symptoms are common following mTBI. The incidence of post-mTBI depression may be as high as 42% in adults and 22% in children and adolescents [121–124]. Premorbid depression is a risk factor for prolonged recovery from mTBI and may be associated with postconcussion symptoms, as well as sleep disruption, impaired cognition and other post-mTBI psychiatric symptoms (e.g., anxiety) [17,20,125–129]. Therefore, ameliorating post-mTBI depression may improve overall symptom presentation and be associated with improvements in sleep and cognitive function.
Post-traumatic headache & pain
Post-traumatic headaches (PTH) and chronic pain are among the most common symptoms experienced by individuals recovering from mTBI. The incidence of PTH likely may be as high 90% [130–135], and the incidence of chronic pain may be as high as 75% [136]. Additionally, both PTH and chronic pain may mediate, or be mediated by, post-mTBI poor sleep, daytime sleepiness, cognitive deficits and depression [122,137–142]. Consequently, the aforementioned benefits of bright or blue light therapy for sleep, cognitive performance and depression may have positive effects on PTH and pain.
The effects of different types of light & applications to mTBI
Given the range of deficits and changes observed following mTBIs, as well as the known NIF pathways for light, light therapy has the potential to positively influence a wide range of cognitive, emotional and physiological functions. Below, we discuss the known effects of various aspects (colors, intensities) of light across the range of human performance. These findings are additionally summarized in Table 1.
Polychromatic white light
Polychromatic white light is essentially a broad-spectrum light. Because white light includes nearly all wavelengths, it also encompasses the blue light portion of the spectrum (∼460–448 nm) that selectively activates ipRGCs and therefore has important circadian and hormone-secreting properties [31]. Consequently, it could be expected that white or bright light therapy would induce changes in post-mTBI circadian rhythms, sleep and alertness.
A recent meta-analysis indicates that light therapy in general is effective in the treatment of sleep disorders that include circadian rhythm sleep disorders, insomnia, and sleep problems associated with Alzheimer’s disease and dementia [27]. This meta-analysis included randomized controlled trials and within-subject design studies utilizing polychromatic white light, and blue-enriched white light, as well as several studies utilizing monochromic blue light. Overall, positive effects for light therapy were observed for circadian shifts, bed and wake times, sleep onset latency, total sleep time, wake after sleep onset, sleep efficiency, sleepiness and alertness, sleep quality, insomnia symptoms and fatigue [27]. The authors additionally report that light intensity (ranging from 2000 to 10,000 lux in the majority of included studies) had positive effects on individuals with insomnia, with greater intensity increasing the beneficial effects of light therapy [27]. In estimating effect sizes, the authors did not distinguish the effects of bright light from those of blue light; however, only 9% of the included studies specifically examined blue light.
An additional systematic review by Souman et al. examined the effects of light therapy on alertness. Across the reviewed literature, there is the indication that increasing the intensity of polychromatic white light significantly increases subjective alertness [28]. However, this does not appear to translate into improved vigilance or reaction. Additionally, there is limited evidence for significant improvements in subjective or performance-based measures of alertness with blue-shifted polychromatic white light [28]. Cumulatively, these findings suggest that the intensity of polychromatic white light may have positive effects on subjective alertness.
Furthermore, polychromatic white light therapy has been shown to be effective in reducing depressive symptoms in individuals with diagnosed depression, including major depressive disorder. Recent meta-analyses indicate that light therapy, especially polychromatic white light, reduces depressive symptoms at post treatment compared with control participants and this effect is more pronounced for standalone light therapy than for studies using light as an adjunctive therapy [29,143]. The effect is additionally stronger when light therapy is used in the morning than any other time [29]. These effects were observed over studies including polychromatic white, green and pale blue light [29,143].
Monochromatic blue & blue-shifted white light
As previously noted with polychromatic white light, blue light therapy – including monochromatic blue and blue-enriched white light – alters circadian rhythms, particularly the timing of melatonin release, in individuals with a variety of sleep-disrupted conditions [27,144]. These studies demonstrate that short amounts (30 min or more) of focused and intentional daily light therapy in the morning effectively advances individuals’ circadian rhythms, evidenced by the timing of melatonin secretion. In general, the effects of phototherapy are condition dependent, but may include changes in circadian rhythm and improvements in sleep duration, self-reported sleep quality, insomnia symptoms and fatigue [27].
In addition to the direct effects of monochromatic blue light therapy on sleep quantity and quality, the appropriate timing of melatonin secretion is essential for maintaining normal daytime arousal and minimizing fatigue. There is robust evidence that blue-wavelength light is effective in acutely decreasing sleepiness and fatigue [145–152] as well as increasing concentrations of arousal-promoting hormones (e.g., cortisol) [153]. Furthermore, blue light therapy has positive effects on increasing alertness [145,147,149,152,154–169]. This phenomenon is present both during the day (e.g., morning blue light exposure) and night.
Prior work has additionally demonstrated that blue light therapy increases activation in cognition-related, task-specific brain regions [157,159,170–176]. However, while light affects brain activation, the actual behavioral effects of blue light exposure are not quite as clear. Individual studies have demonstrated improvements in cognitive performance on working memory, digit recall, sustained attention and arithmetic tasks while others have shown no improvements or even reduced performance in response to light exposure [145,158,169,176–184]. It is thus unclear the extent to which blue light therapy may directly affect cognitive performance beyond those conferred by improvements or alterations in sleep, fatigue or overall alertness.
With respect to mood and affect, blue or blue-shifted light can variously cause [143,185] or improve [29,186] depressive symptoms depending on the timing of therapy (e.g., when timing coincides with or in opposition to naturally expected patterns). Mood disorders, including depression, are associated with the homeostatic maintenance of circulating stress hormones. Among these, glucocorticoids like cortisol exhibit circadian rhythmicity, with a night-time accumulation period and clearance during the day [187,188]. Thus, blue light that influences circadian rhythms, as previously described for sleep and melatonin, may impart a beneficial effect on glucocorticoid expression when utilized in circadian-optimal timings or may induce or worsen mood disorders when mistimed (e.g., night-time use of light-emitting diode screens).
However, one potential pitfall in the application of blue light therapy as described to this point is the exacerbation of PTH. The blue light-sensitive ipRGCs directly project to the trigeminovascular neurons in the thalamus [40]. Prior research related to migraine indicates that these neurons transmit nociceptive signals originating in the dura to cortex, thereby contributing to the perception of intracranial pain during a migraine [40]. Furthermore ipRGC inputs onto the trigeminovascular neurons may modulate the response to light by migraineurs. This neural mechanism may explain why individuals feel worse when exposed to light and preferentially seek dark rooms for relief (photophobia) when experiencing a migraine. While the overarching neural mechanisms of mTBI-related PTH resemble, but may not be exactly the same as those for migraine [140], light-based exacerbation of PTH and/or photophobic responses by individuals post-mTBI may likely have the same neural underpinning. Thus it is plausible that, despite the numerous potential benefits of blue light on circadian rhythms, fatigue, alertness and cognition following mTBI, blue-light or blue-shifted white light treatments may be poorly tolerated and may indeed worsen PTH in some individuals. At present, this specific possibility has not been directly explored in treatment studies using blue light for treating symptoms of mTBI, but research on this topic would be a welcome addition to the literature.
Monochromatic red light
For individuals seeking to enhance alertness without modifying their circadian rhythm (e.g., increasing daytime alertness in the presence of a normal circadian rhythm), utilizing blue light therapy may have unintended and unwanted effects, primarily on melatonin secretion. Interestingly, prior work has shown that longer wavelength light (e.g., red light) may have equally powerful alerting effects [157,160–164]. Red light is detected by L-cones in the retina, and the ipRGCs that are sensitive to blue light are not sensitive to the longer wavelengths (∼630 nm) of red light [31,33]. In some preliminary work, the alerting effects of red light were present both in the late afternoon and at night, and were comparable to the effects of blue light. While the mechanisms by which red light has an alerting effect are not fully understood, a plausible explanation is that it may influence the actions of subcortical regions apart from SCN resulting in alerting effects unrelated to melatonin secretion or suppression [159,170].
Monochromatic green light
While the use of blue light may exert its most profound effects on circadian phase advancement or resetting circadian rhythms that mediate sleep, blue light specifically suppresses melatonin secretion thereby inhibiting or delaying the actual onset of sleep. Though possibly beneficial for altering post-mTBI sleep timing or reducing daytime sleepiness and fatigue, this effect on melatonin does nothing for actually promoting night-time sleep. On the other hand, preliminary evidence from animal models suggests that green light (∼530 nm) indeed has a sleep-promoting function [189]. This has not yet been confirmed in human studies and the specific mechanisms are not described as yet, though multisynaptic M-cone projections to the ventrolateral preoptic area (involved in sleep promotion) and lateral hypothalamus (where wake-promoting orexin is secreted) may plausibly create this relationship [189,190].
As previously noted, blue light may also have the unintended consequence of aggravating PTHs. However, further research with migraineurs demonstrates that the use of green light has a positive effect on migraine symptoms, including at a minimum no exacerbation of the headache and at best a decrease in the intensity of symptoms [191]. This effect is observed relative to the use of white, blue, amber and red light. Additionally, animal models have demonstrated that green light confers antinociceptive benefits, both at the sensory threshold and with neuropathic pain [192]. Therefore, individuals with mTBI-related PTH or pain may benefit either from environments bathed in green light or from glasses, which preferentially filter the spectra of incoming light to preferentially include green light.
Light therapy following mTBI
To date, two published studies have specifically examined the effects of light therapy following mTBI. Sinclair et al. exposed participants to 45 min of morning blue or yellow light for 4 weeks [193]. They demonstrated that individuals receiving blue light, as opposed to yellow light or no treatment, reported less daytime fatigue and sleepiness, faster response times on a sustained psychomotor vigilance task, less self-reported sleep disruption and lower self reports of depression symptoms at 2 and 4 weeks than at baseline. These findings suggest that, for post-mTBI individuals who do self-report fatigue, daytime sleepiness or sleep disruption, daily blue light therapy may be an effective nonpharmacological method for improving function in these areas. It is unclear, however, whether these effects persist after treatment cessation.
Additionally, a study by Bajaj et al. had mTBI participants use blue-wavelength light therapy or an amber-wavelength placebo light for 30 min every morning for 6 weeks and found that it was associated with significant changes in white matter integrity (as measured by water diffusion along axonal tracts) within the corpus collosum, corona radiata and thalamus [194]. Moreover, for those receiving blue-light treatment, the magnitude of white matter changes was associated with greater sleep latency on the multiple sleep latency test, which is an objective measure of biological sleepiness. Additionally, they found that the increases in white matter integrity were associated with an improvement in delayed memory performance, but only among those receiving the blue light treatment. These associations were not significant among those receiving the amber light placebo condition. In other words, 6 weeks of blue light therapy appeared to increase the integrity of axonal white matter, and this change was associated with decreased tendency to fall asleep during the day as well as improved delayed memory performance.
Furthermore there is some evidence suggesting that there may be positive effects of blue light exposure on anxiety following mTBI [195,196]. In fact, even a single 30-min exposure to blue-wavelength light appears to increase activation of the anterior cingulate cortex when anticipating positive stimuli compared with an amber placebo light, which might help explain the mood and anxiety improvements that follow blue light exposure [196]. Thus, limited evidence suggests that blue-wavelength light may be effective for some aspects of recovery from mTBI, but more research is needed before the benefits of this approach are fully understood.
Extrapolating the findings from both healthy individuals and those with other neurological conditions as well as animal studies, there may be additional unidentified benefits of light therapy for mTBI beyond those that have been specifically identified. Both polychromatic white and blue lights may be useful for resetting aberrant circadian rhythms, improving sleep, decreasing daytime sleepiness and fatigue, increasing alertness, and decreasing depressive symptoms. Red light may be beneficial for improving alertness without inducing circadian shifts, but more work is needed. Green light may help to promote night-time sleep, minimize PTH and reduce pain. However, given the paucity of studies on post-mTBI light therapy, these applications are speculative at best. Though there is the indication of positive effects on both neural and behavioral outcomes, these findings require further corroboration with larger studies and diverse mTBI populations. Future research objectives are presented in Box 1.
Box 1. . Future research objectives in the development of light therapy for mild traumatic brain injury recovery.
In light of the potential benefits of light therapy in mild traumatic brain injury recovery as well as the physiological nonimage-forming light pathways, the following research objectives are reasonable targets for exploration:
Sleep-related research
Expanding current efforts to identify the effects of monochromatic blue light to correct aberrant circadian rhythms, improve sleep, reduce daytime fatigue and improve white matter integrity
Identifying the optimal dosage and timing of blue light
Identifying positive effects and differences between polychromatic white light and monochromatic blue light as pertains to circadian rhythms and sleep metrics
Identifying the optimal dosage and timing for the use of green light to improve night-time sleep onset
Identifying the optimal dosage and timing for the use of red light to improve alertness without circadian shifting
Cognition
Identifying the optimal color, timing and dosage of light to improve short-term activation related to cognitive function
Somatic
Identifying whether green light reduces the presentation of post-traumatic headache
Identifying whether green light reduces comorbid pain
Precision medicine
Identifying the personal characteristics that will lead to responsiveness to light treatment (e.g., PER3 polymorphisms, degree of circadian dysrhythmia, level of daytime sleepiness/fatigue)
Development and refinement of treatment parameters throughout the day (i.e., timing of blue vs red vs green light therapy based on current symptoms and needs)
Additional uses of light
In addition to the potential benefits of using visible light as a treatment method, low-level laser therapy (LLLT) has noted wound healing and anti-inflammatory properties, particularly in the near-infrared spectral range. While LLLT is beyond the scope of the present discussion, we encourage readers to see [197] for a recent review on the use of LLLT for TBI.
Current limitations to using phototherapy for mTBI
As has been indicated in the preceding sections, there are numerous potential benefits to using light as an adjunctive therapy in the management of mTBIs. However, there are some limitations that currently limit the scale and scope of the inference that can be made regarding the effects of phototherapy in the recovery from mTBI. Notably, there is significant heterogeneity in light characteristics, timing, duration and illuminance of light therapy between studies that may all influence the outcomes of these studies [26,27,29,198]. Thus considerably more work is required, particularly for mTBI-related applications, to identify the optimal parameters that maximize the benefit to the individual.
Additionally, there is evidence that polymorphisms in the PER3 clock gene may additionally explain interindividual differences in the relationship between light exposure and cognition [175]. Consequently, future studies and clinical applications of bright or blue light therapy, particularly for improving cognitive performance, should take genetic variations into account.
Furthermore, many studies employ some form of light box to deliver the phototherapy. These boxes are portable and allow individuals to be treated at home, which is a tremendous benefit. However, an unavoidable drawback to at-home treatments of this nature is compliance and adherence. Additionally, these boxes have limited spatial effectiveness and require the user to be within a certain distance from the light source in order to be effective. Consequently, treatment may be challenging or even impossible in individuals who are unable to remain in front of the light box for the treatment duration. Alternative light presentation methods, such as goggle-mounted light systems are currently being tested, and may afford greater ambulation and flexibility of use. At present, these devices have only been tested with a limited range of wavelengths and will require further research to determine their effectiveness.
Finally, safety concerns are always critical in deciding whether to use a particular treatment, and light therapy is no different. Some safety concerns have been raised for blue light, in particular. There is some evidence to suggest that retinal damage is possible with prolonged exposure to short-wavelength light. Though the optimal wavelength to stimulate ipRGCs (∼480 nm) is considerably greater than violet and ultraviolet wavelengths where damage is more certain, it is a plausible concern [199]. However, there is no evidence in the published literature of such damage from the types of therapy described here [200]. Some companies who manufacture blue-wavelength light-box devices have had their products independently evaluated for blue-light retinal hazard and have reported the exposure hazard to be minimal at the distance, intensity and duration of light emitted for those devices. Another consideration is that the amount of blue-wavelength light emitted by most light-box devices is considerably less than that obtained by an equal duration of exposure to midday outdoor sunlight. Additionally, as noted with photophobia and PTH, many individuals do report headaches and other somatic symptoms with the use of light boxes [200]. Although there is no compelling evidence at this time that standard light therapy devices pose a significant optical hazard when used according to manufacturer’s instructions, further research into the long-term safety and side effects of light exposure treatment is warranted. As with any treatment, decisions to engage in light therapy should involve judicious evaluation of the benefits and potential risks involved.
Conclusions
Light is all around us and is an ingrained natural part of our biological rhythms and daily functioning. It is essential for image-forming vision and our ability to interact with the environment. The NIF impacts of light on physiology make it a powerful tool as well as a potent inhibitor of function. While considerable in-roads are being made to understand how early and active intervention may improve the outcomes from mTBIs, the individual’s environment is an oft-ignored but important consideration. Light is a critical feature of our environment that has a powerful influence on our biology. There are numerous, interconnected systems that are impaired or altered by mTBIs and whose function can be influenced by exposure to light. Outcomes from two small-scale trials indicate that daily blue-wavelength light therapy may be effective for reducing daytime fatigue and improving sleep and efforts are underway to corroborate these findings. Further evidence from other populations indicates that other wavelengths may confer additional therapeutic benefits (e.g., green light for improving post-traumatic headache) and such findings require future research specific to mTBI.
Future perspective
We believe that significant advances in the use of light therapy can and will be made in the decade ahead to leverage these advantages and minimize the drawbacks. It is our position, though, that in order for light therapies to be effective for mTBI, or any condition, they must be specifically tailored to the individual (i.e., personalized medicine) and account for the uncontrollable aspects of the environment (e.g., sunlight during commuting, work environments with limited capacity to be modified). Accordingly, this requires a complete and ongoing needs assessment of the individual as well as an understanding of those aspects of the environment that can be adapted or modified to meet these needs.
For example, technological control of circadian lighting will require smart lights that shift dominant or active wavelengths throughout the day to mimic lighting patterns that more closely reflect sunlight while indoors (e.g., more blue light in the morning that gives way to more amber wavelengths in the late afternoon). These types of lights do not require a light box, but instead would completely replace existing ambient lighting methods. This technology could be leveraged at the home, or potentially the workplace, to ensure that ambient light maximizes the circadian benefit for the individual, and indeed entrains appropriate rhythms given individual needs. Furthermore, many of these circadian lighting systems could be internet connected. Therefore, tuning of ambient colors in response to an individual’s daily needs could be accomplished through needs and symptom reporting via an internet-ready device (e.g., tablet, cellular phone).
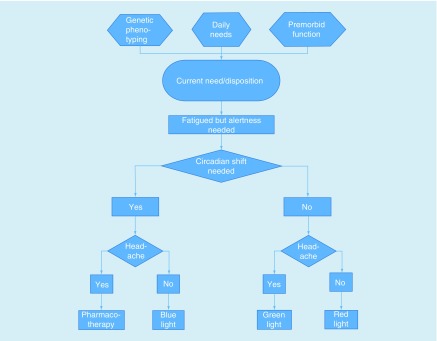
Such a scenario would enable fine tuning the environment to meet daily, and even moment by moment, presentation of individualized mTBI-related symptoms. This may mean more blue in the morning for a person on a normal sleep–wake schedule, but for someone regularly engaged in shiftwork, this may mean more blue is presented in the evening as they prepare for work. Likewise, lighting could be manipulated to address symptom expression and behavioral needs, such as shifting to a more green-lit room if the individual reports pain or a headache on a given day or when falling asleep is a goal (Figure 2). Additionally, individuals could conceivably maximize cognitive performance and alertness by shifting to red light during the afternoon, evening or night for improved alertness without inducing an unwanted or unnecessary circadian shift that is associated with blue light.
Figure 2. . Example decision tree for a precision medicine, needs-based approach to light therapy.
In these scenarios, lighting patterns could be altered on a daily basis to reflect not only the individual’s unchangeable physiology (e.g., PER3 phenotype, which may limit the effect of blue light on sleep and cognition) and overarching needs (e.g., necessary wake times), but also day-to-day changes in mTBI-related symptoms, fatigue and cognitive demands. In so doing, and in conjunction with other medical management, we can leverage the environment to maximize recovery following mTBI and return biological systems to homeostatic states rapidly to facilitate full returns to work, school, sport and life.
Executive summary.
Mild traumatic brain injury, sleepiness & fatigue
Blue light is selectively absorbed by intrinsically photosensitive retinal ganglion cells. These cells project directly the suprachiasmatic nucleus and influence circadian rhythms and melatonin secretion.
There is some evidence suggesting that mild traumatic brain injuries (mTBIs) induced circadian dysrhythmias.
Targeted use of blue light can be used to shift circadian rhythms and improve nighttime sleeping and daytime fatigue.
Emerging evidence suggests that green light may be an effective sleep promoter.
mTBI & alertness
Blue light improves daytime fatigue and sleepiness, leading to greater alertness.
Red light also improves alertness and may be useful when affecting circadian rhythms is undesired.
mTBI & cognition
Blue light potentiates activation in areas associated with multidomain cognition. Further work is needed to more completely understand these mechanisms.
mTBI & depression
Depression is a common post-mTBI complaint. Prior work demonstrates that blue light is effective in improving both seasonal and nonseasonal depression.
mTBI, post-traumatic headache & pain
Despite the positive effects of blue light, the intrinsically photosensitive retinal ganglion cells also project to thalamic neurons that receive input from dural nociceptors that are active during migraine headaches. Blue light therapy may exacerbate post-traumatic headaches.
Green light does not exacerbate migraine headaches and in some cases improves symptom presentation.
Future perspective
Phototherapy has the potential to modify the brain’s functioning across a wide range of affected systems following mTBI.
Technology exists that enables ambient lighting to be modified on demand to change the visible spectrum to meet needs.
A personalized medicine approach – combining genetic phenotyping, injury characteristics, current symptoms and current needs to create an optimal ambient light profile – could make phototherapy a potent, ever-present aspect of mTBI management and recovery.
Footnotes
Financial & competing interests disclosure
This work was supported by multiple grants from the US Army Medical Research and Materiel Command (USAMRMC) to W D S Killgore, including W81XWH-14-1-0570 and W81XWH-14-1-0571. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, USA: Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006 [Internet]http://origin.glb.cdc.gov/traumaticbraininjury/pdf/blue_book.docx Available from. [Google Scholar]
- 2.Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin. Sports Med. 2011;30(1):1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kerr ZY, Register-Mihalik JK, Kroshus E, Baugh CM, Marshall SW. Motivations associated with nondisclosure of self-reported concussions in former collegiate athletes. Am. J. Sports Med. 2016;44(1):220–225. doi: 10.1177/0363546515612082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Register-Mihalik JK, Guskiewicz KM, McLeod TCV, Linnan LA, Mueller FO, Marshall SW. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J. Athletic Training. 2013;48(5):645–653. doi: 10.4085/1062-6050-48.3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nature Rev. Neurol. 2013;9(4):211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3, Suppl.):S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 2016;27(4):529–535. doi: 10.1016/j.nec.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris MA, Shen H, Marion SA, Tsui JK, Teschke K. Head injuries and Parkinson’s disease in a case–control study. Occup. environ. Med. 2013;70(12):839–844. doi: 10.1136/oemed-2013-101444. [DOI] [PubMed] [Google Scholar]
- 10.Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(3):472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 11.Martini DN, Broglio SP. Long-term effects of sport concussion on cognitive and motor performance: a review. Int. J. Psychophysiol. 2017 doi: 10.1016/j.ijpsycho.2017.09.019. www.sciencedirect.com/science/article/pii/S0167876017305779 [DOI] [PubMed] [Google Scholar]
- 12.Dalecki M, Albines D, Macpherson A, Sergio LE. Prolonged cognitive-motor impairments in children and adolescents with a history of concussion. Concussion. 2016;1:CNC14. doi: 10.2217/cnc-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaup AR, Peltz C, Kenney K, Kramer JH, Diaz-Arrastia R, Yaffe K. Neuropsychological profile of lifetime traumatic brain injury in older veterans. J. Int. Neuropsychol. Soc. 2017;23(1):56–64. doi: 10.1017/S1355617716000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami R, Dufort P, Tartaglia MC, et al. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct. Funct. 2016;221(4):1911–1925. doi: 10.1007/s00429-015-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esopenko C, Chow TW, Tartaglia MC, et al. Cognitive and psychosocial function in retired professional hockey players. J. Neurol. Neurosurg. Psychiatry. 2017;88(6):512–519. doi: 10.1136/jnnp-2016-315260. [DOI] [PubMed] [Google Scholar]
- 16.Rao V, Syeda A, Roy D, Peters ME, Vaishnavi S. Neuropsychiatric aspects of concussion: acute and chronic sequelae. Concussion. 2017;2(1):CNC29. doi: 10.2217/cnc-2016-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 18.Stein E, Howard W, Rowhani-Rahbar A, Rivara FP, Zatzick D, McCarty CA. Longitudinal trajectories of post-concussive and depressive symptoms in adolescents with prolonged recovery from concussion. Brain Inj. 2017;31(13–14):1736–1744. doi: 10.1080/02699052.2017.1380843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontos AP, Deitrick JM, Reynolds E. Mental health implications and consequences following sport-related concussion. Br. J. Sports Med. 2015;50(3):139–140. doi: 10.1136/bjsports-2015-095564. [DOI] [PubMed] [Google Scholar]
- 20.Kontos AP, Covassin T, Elbin RJ, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch. Phys. Med. Rehabil. 2012;93(10):1751–1756. doi: 10.1016/j.apmr.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Maas AIR, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7(1):115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobney DM, Miller MB, Tufts E. Non-pharmacological rehabilitation interventions for concussion in children: a scoping review. Disabil. Rehabil. 2017:1–13. doi: 10.1080/09638288.2017.1400595. [DOI] [PubMed] [Google Scholar]
- 23.Kontos AP, Collins MW, Holland CL, et al. Preliminary evidence for improvement in symptoms, cognitive, vestibular, and oculomotor outcomes following targeted intervention with chronic mTBI patients. Mil Med. 2018;183(Suppl. 1):333–338. doi: 10.1093/milmed/usx172. [DOI] [PubMed] [Google Scholar]
- 24.Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg. Sports Traumatol. Arthrosc. 2014;22(2):235–246. doi: 10.1007/s00167-013-2791-6. [DOI] [PubMed] [Google Scholar]
- 25.Dobney DM, Grilli L, Kocilowicz H, et al. Evaluation of an active rehabilitation program for concussion management in children and adolescents. Brain Inj. 2017;31(13/14):1753–1759. doi: 10.1080/02699052.2017.1346294. [DOI] [PubMed] [Google Scholar]
- 26.Gaggioni G, Maquet P, Schmidt C, Dijk D-J, Vandewalle G. Neuroimaging, cognition, light and circadian rhythms. Front. Syst. Neurosci. 2014:8. doi: 10.3389/fnsys.2014.00126. www.frontiersin.org/articles/10.3389/fnsys.2014.00126/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med. Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BNS. Acute alerting effects of light: a systematic literature review. Behav. Brain Res. 2018;337:228–239. doi: 10.1016/j.bbr.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Perera S, Eisen R, Bhatt M, et al. Light therapy for non-seasonal depression: systematic review and meta-analysis. BJPsych. Open. 2016;2(2):116–126. doi: 10.1192/bjpo.bp.115.001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10(5):360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 32.Dacey DM, Liao H-W, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 33.Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34(5):593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein DC, Smoot R, Weller JL, et al. Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic→ spinal cord circuit in the melatonin rhythm generating system. Brain Res. Bull. 1983;10(5):647–652. doi: 10.1016/0361-9230(83)90033-3. [DOI] [PubMed] [Google Scholar]
- 37.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 38.Pickard GE, Silverman A-J. Direct retinal projections to the hypothalamus, piriform cortex, and accessory optic nuclei in the golden hamster as demonstrated by a sensitive anterograde horseradish peroxidase technique. J. Comp. Neurol. 1981;196(1):155–172. doi: 10.1002/cne.901960111. [DOI] [PubMed] [Google Scholar]
- 39.Vandewalle G, Schwartz S, Grandjean D, et al. Spectral quality of light modulates emotional brain responses in humans. PNAS. 2010;107(45):19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciuffreda KJ, Joshi NR, Truong JQ. Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion. 2017;2(3):CNC36. doi: 10.2217/cnc-2016-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport – The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 43.Basic information about traumatic brain injury and concussion | Concussion | Traumatic brain injury | CDC Injury Center [Internet] www.cdc.gov/traumaticbraininjury/basics.html
- 44.O'Neil ME, Carlson K, Storzbach D, et al. Department of Veterans Affairs (US); Definition of MTBI from the VA/DOD clinical practice guideline for management of concussion/mild traumatic brain injury (2009) [Internet]www.ncbi.nlm.nih.gov/books/NBK189784/ [Google Scholar]
- 45.American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8(3):86. [Google Scholar]
- 46.Makdissi M, Schneider KJ, Feddermann-Demont N, et al. Approach to investigation and treatment of persistent symptoms following sport-related concussion: a systematic review. Br. J. Sports Med. 2017;51(12):958–968. doi: 10.1136/bjsports-2016-097470. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine (US) Committee on Sleep Medicine and Research. National Academies Press; WA (DC), USA: Sleep disorders and sleep deprivation: an unmet public health problem [Internet]www.ncbi.nlm.nih.gov/books/NBK19960/ [PubMed] [Google Scholar]
- 48.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J. Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlarb AA, Claßen M, Hellmann SM, Vögele C, Gulewitsch MD. Sleep and somatic complaints in university students. J. Pain Res. 2017;10:1189–1199. doi: 10.2147/JPR.S125421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prather AA, Vogelzangs N, Penninx BWJH. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands Study of Depression and Anxiety (NESDA) J. Psychiatr. Res. 2015;60:95–102. doi: 10.1016/j.jpsychires.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samaranayake CB, Arroll B, Fernando AT. Sleep disorders, depression, anxiety and satisfaction with life among young adults: a survey of university students in Auckland, New Zealand. N. Z. Med. J. 2014;127(1399):13–22. [PubMed] [Google Scholar]
- 53.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010;136(3):375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renn RP, Cote KA. Performance monitoring following total sleep deprivation: effects of task type and error rate. Int. J. Psychophysiol. 2013;88(1):64–73. doi: 10.1016/j.ijpsycho.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Nankar M, Szturm T, Marotta J, Shay B, Beauchet O, Allali G. The interacting effects of treadmill walking and different types of visuospatial cognitive task: discriminating dual task and age effects. Arch. Gerontol. Geriatr. 2017;73:50–59. doi: 10.1016/j.archger.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Heaton KJ, Maule AL, Maruta J, Kryskow EM, Ghajar J. Attention and visual tracking degradation during acute sleep deprivation in a military sample. Aviat. Space Environ. Med. 2014;85(5):497–503. doi: 10.3357/asem.3882.2014. [DOI] [PubMed] [Google Scholar]
- 57.Furtado F, Gonçalves B da SB, Abranches ILL, Abrantes AF, Forner-Cordero A. Chronic low quality sleep impairs postural control in healthy adults. PLoS ONE. 2016;11(10):e0163310. doi: 10.1371/journal.pone.0163310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin T, Gauthier A, Ying Z, et al. Effect of sleep deprivation on diurnal variation of vertical perception and postural control. J. Appl. Physiol. 2018 doi: 10.1152/japplphysiol.00595.2017. www.physiology.org/doi/abs/10.1152/japplphysiol.00595.2017 [DOI] [PubMed] [Google Scholar]
- 59.Watson AM. Sleep and athletic performance. Curr. Sports Med. Rep. 2017;16(6):413. doi: 10.1249/JSR.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 60.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261–1270. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr. Diab. Rep. 2010;10(1):43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens. Res. 2013;36(11):985–995. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. SpringerPlus. 2015;4:25. doi: 10.1186/s40064-014-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan SL, Storm V, Reinwand DA, Wienert J, de Vries H, Lippke S. Understanding the positive associations of sleep, physical activity, fruit and vegetable intake as predictors of quality of life and subjective health across age groups: a theory based, cross-sectional web-based study. Front. Psychol. 2018:9. doi: 10.3389/fpsyg.2018.00977. www.frontiersin.org/articles/10.3389/fpsyg.2018.00977/full Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figueiro M, Overington D. Self-luminous devices and melatonin suppression in adolescents. Lighting Res. Technol. 2016;48(8):966–975. [Google Scholar]
- 68.Gooley JJ. Biological Timekeeping: Clocks, Rhythms and Behaviour. Springer; New Delhi, India: 2017. Light resetting and entrainment of human circadian rhythms [Internet] pp. 297–313.https://link.springer.com/chapter/10.1007/978-81-322-3688-7_14 [Google Scholar]
- 69.Zietzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 2004;526(3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickwire EM, Williams SG, Roth T, et al. Sleep, sleep disorders, and mild traumatic brain injury. what we know and what we need to know: findings from a National Working Group. Neurotherapeutics. 2016;13(2):403–417. doi: 10.1007/s13311-016-0429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J. Head Trauma Rehabil. 2006;21(3):199–212. doi: 10.1097/00001199-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Sleep–wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14(7):746–757. doi: 10.1016/S1474-4422(15)00068-X. [DOI] [PubMed] [Google Scholar]
- 73.Wiseman-Hakes C, Colantonio A, Gargaro J. Sleep and wake disorders following traumatic brain injury: a systematic review of the literature. Crit. Rev. Phys. Rehabil. Med. 2009;21(3–4):317–374. [Google Scholar]
- 74.Gosselin N, Lassonde M, Petit D, et al. Sleep following sport-related concussions. Sleep Med. 2009;10(1):35–46. doi: 10.1016/j.sleep.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 75.Theadom A, Cropley M, Parmar P, et al. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015;16(8):926–932. doi: 10.1016/j.sleep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Sullivan KA, Edmed SL, Allan AC, Karlsson LJE, Smith SS. Characterizing self-reported sleep disturbance after mild traumatic brain injury. J. Neurotrauma. 2014;32(7):474–486. doi: 10.1089/neu.2013.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinds A, Jungquist CR, Leddy JJ, Seemant F, Baker JG, Willer B. Sleep disturbance in patients with chronic concussive effects. Concussion. 2016;1(3):CNC15. doi: 10.2217/cnc-2016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fichtenberg NL, Zafonte RD, Putnam S, Mann NR, Millard AE. Insomnia in a post-acute brain injury sample. Brain Inj. 2002;16(3):197–206. doi: 10.1080/02699050110103940. [DOI] [PubMed] [Google Scholar]
- 79.Fichtenberg NL, Putnam SH, Mann NR, Zafonte RD, Millard AE. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am. J. Phys. Med. Rehabil. 2001;80(5):339–345. doi: 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep–wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130(7):1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 81.Theadom A, Rowland V, Levack W, et al. Exploring the experience of sleep and fatigue in male and female adults over the 2 years following traumatic brain injury: a qualitative descriptive study. BMJ Open. 2016;6(4):e010453. doi: 10.1136/bmjopen-2015-010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2010;81(12):1402–1405. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- 83.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med. 2007;3(4):349–356. [PMC free article] [PubMed] [Google Scholar]
- 84.Imbach LL, Büchele F, Valko PO, et al. Sleep–wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology. 2016;86(21):1945–1949. doi: 10.1212/WNL.0000000000002697. [DOI] [PubMed] [Google Scholar]
- 85.Imbach LL, Valko PO, Li T, et al. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2015;138(3):726–735. doi: 10.1093/brain/awu391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mollayeva T, Shapiro CM, Cassidy JD, Mollayeva S, Colantonio A. Assessment of concussion/mild traumatic brain injury-related fatigue, alertness, and daytime sleepiness: a diagnostic modelling study. Neuropsychiatry. 2017;7(2):86–104. [Google Scholar]
- 87.Sommerauer M, Valko PO, Werth E, Baumann CR. Excessive sleep need following traumatic brain injury: a case–control study of 36 patients. J. Sleep Res. 2013;22(6):634–639. doi: 10.1111/jsr.12068. [DOI] [PubMed] [Google Scholar]
- 88.Watson NF, Dikmen S, Machamer J, Doherty M, Temkin N. Hypersomnia following traumatic brain injury. J. Clin. Sleep Med. 2007;3(4):363–368. [PMC free article] [PubMed] [Google Scholar]
- 89.Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68(14):1136–1140. doi: 10.1212/01.wnl.0000258672.52836.30. [DOI] [PubMed] [Google Scholar]
- 90.Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 2007;3(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- 91.Schreiber S, Barkai G, Gur-Hartman T, et al. Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 2008;9(5):481–487. doi: 10.1016/j.sleep.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Williams BR, Lazic SE, Ogilvie RD. Polysomnographic and quantitative EEG analysis of subjects with long-term insomnia complaints associated with mild traumatic brain injury. Clin. Neurophysiol. 2008;119(2):429–438. doi: 10.1016/j.clinph.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 93.Khoury S, Chouchou F, Amzica F, et al. Rapid EEG activity during sleep dominates in mild traumatic brain injury patients with acute pain. J. Neurotrauma. 2013;30(8):633–641. doi: 10.1089/neu.2012.2519. [DOI] [PubMed] [Google Scholar]
- 94.Lu W, Cantor JB, Aurora RN, et al. The relationship between self-reported sleep disturbance and polysomnography in individuals with traumatic brain injury. Brain Inj. 2015;29(11):1342–1350. doi: 10.3109/02699052.2015.1043947. [DOI] [PubMed] [Google Scholar]
- 95.Mollayeva T, Colantonio A, Cassidy JD, Vernich L, Moineddin R, Shapiro CM. Sleep stage distribution in persons with mild traumatic brain injury: a polysomnographic study according to American Academy of Sleep Medicine standards. Sleep Med. 2017;34:179–192. doi: 10.1016/j.sleep.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 96.Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SMW. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74(21):1732. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paparrigopoulos T, Melissaki A, Tsekou H, et al. Melatonin secretion after head injury: a pilot study. Brain Inj. 2006;20(8):873–878. doi: 10.1080/02699050600832114. [DOI] [PubMed] [Google Scholar]
- 98.Seifman MA, Gomes K, Nguyen PN, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front. Neurol. 2014:5. doi: 10.3389/fneur.2014.00237. http://journal.frontiersin.org/article/10.3389/fneur.2014.00237/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann. Neurol. 2015;77(1):177–182. doi: 10.1002/ana.24298. [DOI] [PubMed] [Google Scholar]
- 100.Baumann CR, Stocker R, Imhof H, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65(1):147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 101.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 2009;66(4):555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 103.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(4):S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-α and lipopolysaccharide in mice. Sleep. 2013;36(8):1227–1238. doi: 10.5665/sleep.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucke-Wold BP, Smith KE, Nguyen L, et al. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci. Biobehav. Rev. 2015;55:68–77. doi: 10.1016/j.neubiorev.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beaulieu C, Turcotte-Giroux A, Carrier-Toutant F, Brisson B, Jolicoeur P, De Beaumont L. Long-term effects of concussions on psychomotor speed and cognitive control processes during motor sequence learning. J. Psychophysiol. 2018:1–13. [Google Scholar]
- 109.Beaulieu-Bonneau S, Fortier-Brochu É, Ivers H, Morin CM. Attention following traumatic brain injury: neuropsychological and driving simulator data, and association with sleep, sleepiness, and fatigue. Neuropsychol. Rehabil. 2017;27(2):216–238. doi: 10.1080/09602011.2015.1077145. [DOI] [PubMed] [Google Scholar]
- 110.Collie A, Makdissi M, Maruff P, Bennell K, McCrory P. Cognition in the days following concussion: comparison of symptomatic versus asymptomatic athletes. J. Neurol. Neurosurg. Psychiatry. 2006;77(2):241–245. doi: 10.1136/jnnp.2005.073155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McCrea M. Mild traumatic brain injury and postconcussion syndrome: the new evidence base for diagnosis and treatment [Internet] Oxford University Press; NY, USA: ***https://books.google.com/books?hl=en&lr=&id=Y0M9LOL40EMC&oi=fnd&pg=PR5&dq=(%E2%80%9Cconcuss*%E2%80%9D+OR+%E2%80%9CmTBI%E2%80%9D+OR+%E2%80%9Cmild+traumatic+brain+injury%E2%80%9D+OR+%E2%80%9Cclosed+head+injury%E2%80%9D)+*cognitive+OR+%22*cognitive+test%22&ots=O-7Hb_xFz3&sig=5bYbyWPNwzegDkNwGnYlSloUQ5M [Google Scholar]
- 112.McCrea M, Guskiewicz K, Doncevic S, et al. Day of injury cognitive performance on the Military Acute Concussion Evaluation (MACE) by US military service members in OEF/OIF. Mil. Med. 2014;179(9):990–997. doi: 10.7205/MILMED-D-13-00349. [DOI] [PubMed] [Google Scholar]
- 113.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. JAMA. 2003;290(19):2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 114.Gaines KD, Soper HV, Berenji GR. Executive functioning of combat mild traumatic brain injury. Appl. Neuropsychol. Adult. 2016;23(2):115–124. doi: 10.1080/23279095.2015.1012762. [DOI] [PubMed] [Google Scholar]
- 115.Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J. Neurosurg. 2008;108(3):511–516. doi: 10.3171/JNS/2008/108/3/0511. [DOI] [PubMed] [Google Scholar]
- 116.Covassin T, Elbin RJ, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys. Sportsmed. 2010;38(4):87–93. doi: 10.3810/psm.2010.12.1830. [DOI] [PubMed] [Google Scholar]
- 117.Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am. J. Sports Med. 2012;40(6):1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 118.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 2008;1129(1):305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 119.Killgore WDS. Effects of sleep deprivation on cognition [Internet] In: Kerkhof GA, van Dongen HPA, editors. Progress in Brain Research. Elsevier; CambridgeMA: 2010. pp. 105–129.www.sciencedirect.com/science/article/pii/B9780444537027000075 [DOI] [PubMed] [Google Scholar]
- 120.Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: a comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009;32(2):205–216. doi: 10.1093/sleep/32.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yrondi A, Brauge D, LeMen J, Arbus C, Pariente J. Depression and sports-related concussion: a systematic review. La Presse Médicale. 2017;46(10):890–902. doi: 10.1016/j.lpm.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 122.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 123.Decq P, Gault N, Blandeau M, et al. Long-term consequences of recurrent sports concussion. Acta Neurochir. 2016;158(2):289–300. doi: 10.1007/s00701-015-2681-4. [DOI] [PubMed] [Google Scholar]
- 124.Stazyk K, DeMatteo C, Moll S, Missiuna C. Depression in youth recovering from concussion: correlates and predictors. Brain Inj. 2017;31(5):631–638. doi: 10.1080/02699052.2017.1283533. [DOI] [PubMed] [Google Scholar]
- 125.Vargas G, Rabinowitz A, Meyer J, Arnett PA. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J. Athl. Train. 2015;50(3):250–255. doi: 10.4085/1062-6050-50.3.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang J, Peek-Asa C, Covassin T, Torner JC. Post-concussion symptoms of depression and anxiety in division I collegiate athletes. Dev. Neuropsychol. 2015;40(1):18–23. doi: 10.1080/87565641.2014.973499. [DOI] [PubMed] [Google Scholar]
- 127.Theadom A, Parag V, Dowell T, et al. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br. J. Gen. Pract. 2016;66(642):e16–e23. doi: 10.3399/bjgp16X683161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chaput G, Giguère J-F, Chauny J-M, Denis R, Lavigne G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009;10(7):713–716. doi: 10.1016/j.sleep.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 129.Kerr ZY, Marshall SW, Harding HP, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am. J. Sports Med. 2012;40(10):2206–2212. doi: 10.1177/0363546512456193. [DOI] [PubMed] [Google Scholar]
- 130.Packard RC. Chronic post-traumatic headache: associations with mild traumatic brain injury, concussion, and post-concussive disorder. Curr. Pain Headache Rep. 2008;12(1):67–73. doi: 10.1007/s11916-008-0013-6. [DOI] [PubMed] [Google Scholar]
- 131.Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J. Head Trauma Rehabil. 1999;14(1):9. doi: 10.1097/00001199-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 132.Faux S, Sheedy J. A prospective controlled study in the prevalence of posttraumatic headache following mild traumatic brain injury. Pain Med. 2008;9(8):1001–1011. doi: 10.1111/j.1526-4637.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 133.Stovner LJ, Schrader H, Mickevičiene D, Surkiene D, Sand T. Headache after concussion. Eur. J. Neurol. 2009;16(1):112–120. doi: 10.1111/j.1468-1331.2008.02363.x. [DOI] [PubMed] [Google Scholar]
- 134.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 135.Theeler BJ, Flynn FG, Erickson JC. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache. 2010;50(8):1262–1272. doi: 10.1111/j.1526-4610.2010.01700.x. [DOI] [PubMed] [Google Scholar]
- 136.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711–719. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- 137.Mollayeva T, Cassidy JD, Shapiro CM, Mollayeva S, Colantonio A. Concussion/mild traumatic brain injury-related chronic pain in males and females: a diagnostic modelling study. Medicine. 2017;96(7):e5917. doi: 10.1097/MD.0000000000005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kjeldgaard D, Forchhammer H, Teasdale T, Jensen RH. Chronic post-traumatic headache after mild head injury: a descriptive study. Cephalalgia. 2014;34(3):191–200. doi: 10.1177/0333102413505236. [DOI] [PubMed] [Google Scholar]
- 139.Grandhi R, Tavakoli S, Ortega C, Simmonds MJ. A review of chronic pain and cognitive, mood, and motor dysfunction following mild traumatic brain injury: complex, comorbid, and/or overlapping conditions? Brain Sci. 2017;7(12):1–8. doi: 10.3390/brainsci7120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruff RL, Blake K. Pathophysiological links between traumatic brain injury and post-traumatic headaches. F1000Res. 2016:5. doi: 10.12688/f1000research.9017.1. www.ncbi.nlm.nih.gov/pmc/articles/PMC5007746/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ruff RL, Ruff SS, Wang X-F. Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. J. Rehabil. Res. Dev. 2009;46(9):1071–1084. doi: 10.1682/jrrd.2009.05.0062. [DOI] [PubMed] [Google Scholar]
- 142.Ruff RL, Riechers RG, II, Wang X-F, Piero T, Ruff SS. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J. Rehabil. Res. Dev. 2012;49(9):1305. doi: 10.1682/jrrd.2011.12.0251. [DOI] [PubMed] [Google Scholar]
- 143.Mårtensson B, Pettersson A, Berglund L, Ekselius L. Bright white light therapy in depression: a critical review of the evidence. J. Affect. Disord. 2015;182:1–7. doi: 10.1016/j.jad.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 144.Najjar RP, Wolf L, Taillard J, et al. Chronic artificial blue-enriched white light is an effective countermeasure to delayed circadian phase and neurobehavioral decrements. PLoS One. 2014;9(7):e102827. doi: 10.1371/journal.pone.0102827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Phipps-Nelson J, Redman JR, Dijk D-J, Rajaratnam SMW. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26(6):695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 146.Phipps-Nelson J, Redman JR, Schlangen LJM, Rajaratnam SMW. Blue light exposure reduces objective measures of sleepiness during prolonged nighttime performance testing. Chronobiol. Int. 2009;26(5):891–912. doi: 10.1080/07420520903044364. [DOI] [PubMed] [Google Scholar]
- 147.Viola AU, James LM, Schlangen LJ, Dijk D-J. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand. J. Work Environ. Health. 2008;34(4):297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 148.Mills PR, Tomkins SC, Schlangen LJ. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J. Circadian Rhythms. 2007;5:2. doi: 10.1186/1740-3391-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS ONE. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Münch M, Linhart F, Borisuit A, Jaeggi SM, Scartezzini J-L. Effects of prior light exposure on early evening performance, subjective sleepiness, and hormonal secretion. Behav. Neurosci. 2012;126(1):196–203. doi: 10.1037/a0026702. [DOI] [PubMed] [Google Scholar]
- 151.Gordijn MC, Beerma DGM, Rüger M, Daan S. The effects of blue light on sleepiness. Annu. Proc. Dutch Sleep-Wake Soc. 2005;16:67–70. [Google Scholar]
- 152.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 153.Figueiro MG, Rea MS. Short-wavelength light enhances cortisol awakening response in sleep-restricted adolescents. Int. J. Endocrinol. 2012:7. doi: 10.1155/2012/301935. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beaven CM, Ekström J. A comparison of blue light and caffeine effects on cognitive function and alertness in humans. PLoS One. 2013;8(10):e76707. doi: 10.1371/journal.pone.0076707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodríguez-Morilla B, Madrid JA, Molina E, Correa A. Blue-enriched white light enhances physiological arousal but not behavioral performance during simulated driving at early night. Front. Psychol. 2017:8. doi: 10.3389/fpsyg.2017.00997. www.frontiersin.org/articles/10.3389/fpsyg.2017.00997/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodríguez-Morilla B, Madrid JA, Molina E, Pérez-Navarro J, Correa Á. Blue-enriched light enhances alertness but impairs accurate performance in evening chronotypes driving in the morning. Front. Psychol. 2018:9. doi: 10.3389/fpsyg.2018.00688. www.frontiersin.org/articles/10.3389/fpsyg.2018.00688/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Łaszewska K, Goroncy A, Weber P, et al. Daytime acute non-visual alerting response in brain activity occurs as a result of short- and long-wavelengths of light. J. Psychophysiol. 2017:1–25. [Google Scholar]
- 158.Smolders KCHJ, de Kort YAW, Cluitmans PJM. A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol. Behav. 2012;107(1):7–16. doi: 10.1016/j.physbeh.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 159.Vandewalle G, Balteau E, Phillips C, et al. Daytime light exposure dynamically enhances brain responses. Curr. Biol. 2006;16(16):1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 160.Figueiro MG, Bierman A, Plitnick B, Rea MS. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci. 2009;10:105. doi: 10.1186/1471-2202-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Figueiro MG, Sahin L, Wood B, Plitnick B. Light at night and measures of alertness and performance: implications for shift workers. Biol. Res. Nurs. 2016;18(1):90–100. doi: 10.1177/1099800415572873. [DOI] [PubMed] [Google Scholar]
- 162.Plitnick B, Figueiro M, Wood B, Rea M. The effects of red and blue light on alertness and mood at night. Lighting Res. Technol. 2010;42(4):449–458. [Google Scholar]
- 163.Sahin L, Figueiro MG. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol. Behav. 2013;116–117:1–7. doi: 10.1016/j.physbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 164.Sahin L, Wood BM, Plitnick B, Figueiro MG. Daytime light exposure: effects on biomarkers, measures of alertness, and performance. Behav. Brain Res. 2014;274:176–185. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 165.Rüger M, Gordijn MCM, Beersma DGM, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(5):R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 166.Myers BL, Badia P. Immediate effects of different light intensities on body temperature and alertness. Physiol. Behav. 1993;54(1):199–202. doi: 10.1016/0031-9384(93)90067-p. [DOI] [PubMed] [Google Scholar]
- 167.Chang A-M, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J. Physiol. 2012;590(13):3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cajochen C, Zeitzer JM, Czeisler CA, Dijk D-J. Dose–response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 2000;115(1):75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 169.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol. Behav. 1991;50(3):583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 170.Vandewalle G, Schmidt C, Albouy G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS ONE. 2007;2(11):e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alkozei A, Smith R, Pisner DA, et al. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep. 2016;39(9):1671–1680. doi: 10.5665/sleep.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perrin F, Peigneux P, Fuchs S, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Current Biol. 2004;14(20):1842–1846. doi: 10.1016/j.cub.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 173.Vandewalle G, Gais S, Schabus M, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb. Cortex. 2007;17(12):2788–2795. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 174.Okamoto Y, Nakagawa S. Effects of daytime light exposure on cognitive brain activity as measured by the ERP P300. Physiol. Behav. 2015;138:313–318. doi: 10.1016/j.physbeh.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 175.Vandewalle G, Archer SN, Wuillaume C, et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J. Biol. Rhythms. 2011;26(3):249–259. doi: 10.1177/0748730411401736. [DOI] [PubMed] [Google Scholar]
- 176.Killgore WDS, Kent HC, Knight SA, Alkozei A. Changes in morning salivary melatonin correlate with prefrontal responses during working memory performance. Neuroreport. 2018;29(6):488–494. doi: 10.1097/WNR.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 177.Kretschmer V, Schmidt K-H, Griefahn B. Bright light effects on working memory, sustained attention and concentration of elderly night shift workers. Lighting Res. Technol. 2012;44(3):316–333. [Google Scholar]
- 178.Boyce PR, Beckstead JW, Eklund NH, Strobel RW, Rea MS. Lighting the graveyard shift: the influence of a daylight-simulating skylight on the task performance and mood of night-shift workerst. Int. J. Lighting Res. Technol. 1997;29(3):105–134. [Google Scholar]
- 179.Gabel V, Maire M, Reichert CF, et al. Effects of artificial dawn and morning blue light on daytime cognitive performance, well-being, cortisol and melatonin levels. Chronobiol. Int. 2013;30(8):988–997. doi: 10.3109/07420528.2013.793196. [DOI] [PubMed] [Google Scholar]
- 180.Gabel V, Maire M, Reichert CF, et al. Dawn simulation light impacts on different cognitive domains under sleep restriction. Behav. Brain Res. 2015;281:258–266. doi: 10.1016/j.bbr.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 181.Santhi N, Groeger JA, Archer SN, Gimenez M, Schlangen LJM, Dijk D-J. Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PLoS ONE. 2013;8(11):e79688. doi: 10.1371/journal.pone.0079688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smolders KCHJ, de Kort YAW. Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J. Environ. Psychol. 2017;50:80–93. [Google Scholar]
- 183.Smolders KCHJ, de Kort YAW. Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J. Environ. Psychol. 2014;39:77–91. [Google Scholar]
- 184.Alkozei A, Smith R, Dailey NS, Bajaj S, Killgore WDS. Acute exposure to blue wavelength light during memory consolidation improves verbal memory performance. PLoS ONE. 2017;12(9):e0184884. doi: 10.1371/journal.pone.0184884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry. 2017;7(1):e1017. doi: 10.1038/tp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. AJP. 2005;162(4):656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 187.Wolkowitz Owen M, Burke Heather, Epel Elissa S, Reus Victor I. Glucocorticoids. Mood, memory, and mechanisms. Ann. N. Y. Acad. Sci. 2009;1179(1):19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- 188.Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front. Neuroendocrinol. 2011;32(4):451–465. doi: 10.1016/j.yfrne.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 189.Pilorz V, Tam SKE, Hughes S, et al. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14(6):e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cajochen C, Chellappa SL. Commentary: melanopsin regulates both sleep-promoting and arousal-promoting responses to light. Front. Neural Circuits. 2016:10. doi: 10.3389/fncir.2016.00094. www.frontiersin.org/articles/10.3389/fncir.2016.00094/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Noseda R, Bernstein CA, Nir R-R, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139(7):1971–1986. doi: 10.1093/brain/aww119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ibrahim MM, Patwardhan A, Gilbraith KB, et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 2017;158(2):347. doi: 10.1097/j.pain.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sinclair KL, Ponsford JL, Taffe J, Lockley SW, Rajaratnam SMW. Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabil. Neural Repair. 2014;28(4):303–313. doi: 10.1177/1545968313508472. [DOI] [PubMed] [Google Scholar]
- 194.Bajaj S, Vanuk JR, Smith R, Dailey NS, Killgore WDS. Blue-light therapy following mild traumatic brain injury: effects on white matter water diffusion in the brain. Front. Neurol. 2017:8. doi: 10.3389/fneur.2017.00616. www.frontiersin.org/articles/10.3389/fneur.2017.00616/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vanuk J, Shane B, Bajaj S, Millan M, Killgore WDS. 538. Blue light therapy following a mild traumatic brain injury improves MPFC-amygdala functional connectivity and mood. Biol. Psychiatry. 2017;81(10):S218. [Google Scholar]
- 196.Alkozei A, Smith R, Killgore WDS. Exposure to blue wavelength light modulates anterior cingulate cortex activation in response to ‘uncertain’ versus ‘certain’ anticipation of positive stimuli. Neurosci. Lett. 2016;616:5–10. doi: 10.1016/j.neulet.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 197.Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 2017;96(4):731–743. doi: 10.1002/jnr.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu Q, Lang CP. Revisiting the alerting effect of light: a systematic review. Sleep Med. Rev. 2017 doi: 10.1016/j.smrv.2017.12.001. www.sciencedirect.com/science/article/pii/S1087079217300989 [DOI] [PubMed] [Google Scholar]
- 199.International Commission on Non-Ionizing Radiation Protection. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Physics. 2013;105(1):74. doi: 10.1097/HP.0b013e318289a611. [DOI] [PubMed] [Google Scholar]
- 200.Brouwer A, Nguyen H-T, Snoek FJ, et al. Light therapy: is it safe for the eyes? Acta Psychiatrica Scandinavica. 2017;136(6):534–548. doi: 10.1111/acps.12785. [DOI] [PubMed] [Google Scholar]