Abstract
Background:
Direct oral anticoagulants (DOACs) provide patients with attractive options for anticoagulation in atrial fibrillation (AF). However, dosing these agents in the elderly can be challenging due to factors such as drug interactions, reduced renal function, and less frequent monitoring. This study addressed this challenge by reviewing the dosing of three commonly used DOACs (i.e. apixaban, dabigatran, and rivaroxaban) in elderly patients managed at a pharmacist-run anticoagulation clinic.
Methods:
This was a single-center, retrospective cohort study. A total of 98 cases of DOAC therapy in patients with AF aged 75 years or older receiving care at a large urban healthcare center were identified via chart review. Dosing of each DOAC was assessed at therapy initiation and throughout treatment whenever a serum creatinine was reported, using the Cockcroft–Gault equation to estimate creatinine clearance (CrCl). Dose excursions (defined as instances where patients were exposed to non-Food and Drug Administration (FDA)-approved doses) were documented in each case. Rationales for dose excursions were determined by study investigators via review of progress notes and categorized using clinical judgement.
Results:
Upon therapy initiation apixaban was dosed in accordance with FDA recommendations in 92.9% of patient cases, dabigatran in 91.2% of cases, and rivaroxaban in 86.1% of cases (p = 0.70). FDA-recommended dosing was maintained throughout treatment at the highest rates with dabigatran (88.2% versus 78.6% with apixaban and 58.3% with rivaroxaban; p = 0.01, p = 0.005 for dabigatran versus rivaroxaban). The most common rationales for dose excursion were fluctuation in estimated CrCl near the dosing cutoff, and recommendations from nonpharmacist providers co-managing the patient.
Conclusions:
Prescribing and maintaining FDA-recommended doses of DOAC agents in the elderly is more challenging than initially perceived. Fluctuations in renal function, comorbidities, and concomitant antiplatelet use may necessitate more individualized dosing strategies with these agents.
Keywords: anticoagulant, atrial fibrillation, dosing, elderly
Introduction
The emergence of the direct oral anticoagulant (DOAC) drug class has drastically changed the landscape of anticoagulation in atrial fibrillation (AF). DOACs offer several advantages over warfarin for both clinicians and patients, including more predictable pharmacokinetic properties, fewer drug–drug/food interactions, faster onset of action, and shorter half-lives.1,2 In addition, apixaban and dabigatran have shown superiority compared with warfarin in the prevention of stroke or systemic embolism in two large phase III clinical trials in patients with AF.3,4 Furthermore, four approved DOAC agents (i.e. apixaban, dabigatran, edoxaban, and rivaroxaban) have shown a decreased risk of intracranial hemorrhage in comparison with warfarin in large landmark trials of patients with AF.3–6 As a result, the use of DOAC agents for anticoagulation has increased rapidly in the USA in recent years.7
The attractiveness of DOACs has particular appeal to the geriatric population. In addition to their established safety and efficacy data in clinical trials, DOACs offer a viable option for the many elderly patients who have difficulty with the frequent monitoring requirements of warfarin due to mobility and/or transportation issues. Considering that approximately 70% of those living with AF are between 65 and 85 years of age,8 the recent rise in DOAC usage has major implications in the elderly population with AF.
Several studies have indicated a higher risk for extracranial bleeding events, particularly gastrointestinal bleeding, with certain DOACs in patients above 75 years of age.9–11 A major aspect of this risk may be due to the fact that all DOACs require some form of renal dose adjustment. Elderly patients are generally at higher risk for acute renal failure and chronic kidney disease (CKD) due to a number of factors, including changes in hydration status and comorbid conditions.12–14 Despite this, there is high quality literature that demonstrates no significant differences in major bleeding with DOACs in comparison with warfarin, specifically in patients with CKD.15,16 However, these studies represent only randomized controlled trials, limiting their real-world applicability. In addition, the authors of these studies were unable to perform any subgroup analysis by age, for example, in patients above 75 years of age.
Since DOACs do not require frequent laboratory or point-of-care tests for the monitoring of international normalized ratio (INR), and no gold standard test exists for therapeutic monitoring of DOACs, elderly patients often exercise the option of less frequent provider visits. Though convenient, this could cause short- and/or long-term exposure to DOAC doses much higher (or lower) than recommended based on their renal function, which may increase the risk for bleeding and/or thrombotic adverse events.
This study seeks to determine rates of US Food and Drug Administration (FDA)-recommended dosing with three commonly used DOACs (i.e. apixaban, dabigatran, and rivaroxaban), and rationales for prescribing above or below the recommended range in an elderly ambulatory population with AF.
Methods
Study design and approval
This study was a noninterventional, single-center, retrospective chart review conducted at the John D. Dingell Veterans Affairs Medical Center (VAMC) in Detroit, MI, USA. The study protocol was approved by the Institutional Review Boards of both the Detroit VAMC and Wayne State University, also located in Detroit.
Subjects were identified if they had an active prescription documented between 1 January 2011 and 31 August 2014 for apixaban, dabigatran, or rivaroxaban. Patients were included in the study if they were taking one of these agents for the indication of anticoagulation in nonvalvular AF, and were at least 75 years of age. Patients were excluded if they were prescribed the drug for any indication other than nonvalvular AF, or if the drug was discontinued within 7 days of initiation.
Data collection
The Detroit VAMC Computerized Patient Record System (CPRS) was used to collect data on subjects meeting the inclusion criteria. Several baseline characteristics were collected, including age, gender, race, weight, comorbidities, DOAC dosage, and presence of clinically significant drug interactions (e.g. concomitant antiplatelet drug[s], cytochrome P450 3A4 [CYP3A4], and/or P-glycoprotein 1-ATP-binding cassette subfamily B member 1 [P-gp/ABCB1] inhibitor[s], CYP3A4 and/or P-gp/ABCB1 inducer[s], omega-3 fatty acids, and/or vitamin E). Comorbidities were identified by documentation either in the subjects’ Problems List or in Progress Notes within the CPRS. Baseline risk for stroke/transient ischemic attack (TIA) was calculated using the CHADS2 score; CHADS2 score: 1 point: presence of congestive heart failure, hypertension, age > 75 years, or diabetes; 2 points: history of stroke/TIA. Baseline risk for major bleeding was calculated using the HAS-BLED score: HAS-BLED score: 1 point for any of the following: presence of hypertension (systolic blood pressure > 160 mmHg), renal disease (dialysis, renal transplant, or serum creatinine [SCr] > 2.26 mg/dL), liver disease (cirrhosis or bilirubin > 2 × upper limit of normal coupled with aspartate transaminase/alanine transaminase/alkaline phosphatase > 3 × upper limit of normal), stroke history, prior major bleeding or predisposition to bleeding, labile INR (time in therapeutic range < 60%), age ≥ 65 years, use of medications predisposed to bleeding (nonsteroidal anti-inflammatory drugs, antiplatelet agents, etc.), alcohol (≥ 8 drinks/week), or drug use. Both of these scores have been well validated to predict stroke and major bleeding risk in patients with AF requiring anticoagulation.17,18
FDA-recommended dosing was defined as the FDA-approved DOAC dose based on estimated creatinine clearance (CrCl) utilizing the Cockcroft–Gault (CG) equation, as displayed in Table 1.
Table 1.
US Food and Drug Administration-approved dosing of apixaban, dabigatran, and rivaroxaban for reduction of risk of stroke and systemic embolism in nonvalvular atrial fibrillation.
| Standard dose | Renal adjustment | Severe chronic kidney disease or end stage renal disease | |
|---|---|---|---|
| Apixaban | 5 mg orally twice daily | If two or more of the following
characteristics: • age ≥ 80 years • body weight ≤ 60 kg • serum creatinine ≥ 1.5 mg/dL Recommended dose is 2.5 mg orally twice daily |
No clinical safety/efficacy data for patients with end stage renal disease on dialysis or CrCl < 15 ml/min |
| Dabigatran | 150 mg orally twice daily | If CrCl 15–30 ml/min: 75 mg orally twice daily | CrCl < 15 ml/min or on dialysis: dosing recommendations cannot be provided |
| Rivaroxaban | 20 mg orally once daily (with the evening meal) | If CrCl 15–50 ml/min: 15 mg orally once daily (with the evening meal) | Use in patients with CrCl < 30 ml/min should be avoided |
Estimated CrCl was calculated by using subjects’ actual SCr and total body weight (TBW): . Pharmacists at our facility commonly use ideal body weight (IBW), no body weight (NBW) , or adjusted body weight (ABW) in addition to TBW to estimate renal function.Calculation via TBW represents the manufacturer-recommended renal dosing,19,20 whereas calculation via NBW represents a conservative approach used by some clinical pharmacists at the outpatient anticoagulation clinic (AC) at the Detroit VAMC. If a patient’s actual body weight is less than IBW, TBW is always used. Our electronic medical record calculates CrCl using the CG equation with ABW if the patient’s actual body weight is more than 130% of IBW. CrCl estimation using the CG equation with TBW was performed during data collection anytime SCr was drawn to determine whether patients were initiated and maintained on the FDA-recommended DOAC dosage throughout the data-collection period. If at any point during treatment a patient was found to be on a dose other than the FDA-recommended dose, irrespective of CrCl calculation method, that case was designated as a ‘dose excursion’ (under- or over-dosed) at the time of SCr measurement. Concomitant drug interactions impacting dosing were also taken into consideration and dose appropriateness was determined by study investigators using FDA-approved and manufacturer-recommended dosing information.
Study population and data analysis
The vast majority of subjects (94%) in this study received anticoagulation therapy and monitoring by the AC, which currently manages over 900 active patients and is staffed by two full-time clinical pharmacy specialists 5 days per week. In addition to INR point-of-care testing for the prescribing and management of warfarin therapy, the AC also offers protocolized care for patients receiving DOAC agents, including laboratory monitoring, assessment for adverse events, and adherence monitoring. DOAC patients are typically seen for an initial visit to determine which agent is most suitable for them based on individual patient factors. This visit is followed by a 1-month follow-up appointment, and then every 3–6 months thereafter based on patients’ individual risk factors for bleeding and/or thrombosis.
A total of 114 patients were identified who were at least 75 years of age and had an active prescription for DOAC within the study time period. A total of 87 patients met the inclusion criteria and 27 patients were excluded due to the following rationales: indication other than nonvalvular AF (13), lack of required documentation (8), less than 7 days of therapy (4), or prescribed one of the DOAC agents but never actually took the drug (2). Of the 87 patients included, we derived 98 total patient cases. To explain this concept: if a patient was switched from one DOAC agent to another during the study period, as long as they received at least 7 days of therapy on each drug they were considered two separate patient cases. We encountered 11 of these patients during the study period. This ultimately resulted in 28 apixaban, 34 dabigatran, and 36 rivaroxaban patient cases (see Figure 1). Data were collected retrospectively on each subject from the date of first fill of the DOAC prescription through 31 December 2014. Baseline characteristics of the study population as well as rates of FDA-recommended dosing were compared using the chi-square test, Fisher’s exact test, or Kruskal–Wallis one-way analysis of variance, where appropriate. A p value of < 0.05 for each was considered statistically significant. Data analysis was completed utilizing Statistical Analysis System V9.4.
Figure 1.
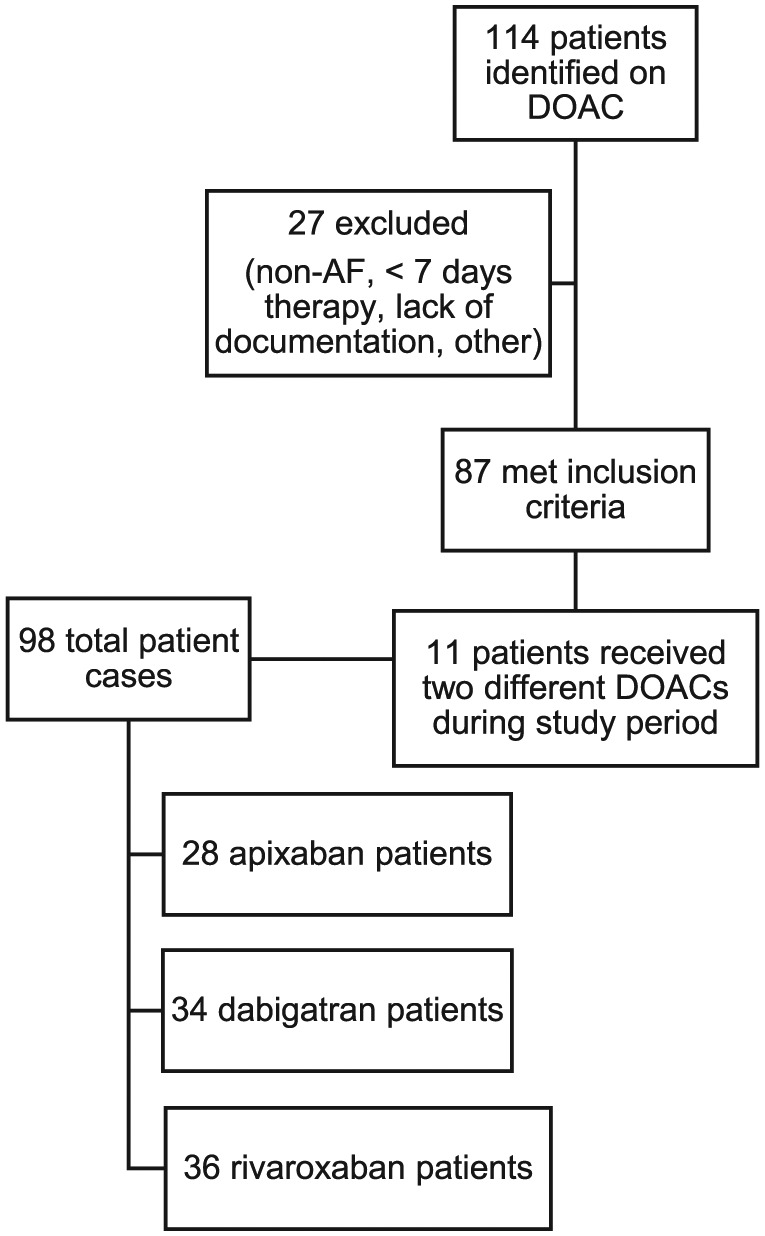
Selection of study population. AF, atrial fibrillation; DOAC, direct oral anticoagulant.
Results
Baseline characteristics of the study population are outlined in Table 2. No statistically significant differences were found in baseline characteristics among the three DOAC cohorts. The average duration of DOAC treatment among subjects was 293 days (standard error of mean: 30 days).
Table 2.
Baseline characteristics of the study population.
| Apixaban (n = 28) | Dabigatran (n = 34) | Rivaroxaban (n = 36) | p value | |
|---|---|---|---|---|
| Average age (years; median [IQR]) | 82.5 (79.5–87.5) | 80 (77–84) | 83 (79–86) | 0.06* |
| Male sex | 27 (96%) | 34 (100%) | 36 (100%) | 0.29$ |
| Caucasian race | 19 (67.9%) | 29 (85.3%) | 27 (75%) | 0.26 |
| Body mass index (kg/m2; median [IQR]) | 28.8 (24.2–30.5) | 28.0 (25.1–33.1) | 27.9 (24.6–29.8) | 0.48* |
| CHADS2 score | ||||
| 1–2 | 9 (32.1%) | 14 (41.2%) | 16 (44.4%) | 0.60 |
| > 3 | 19 (67.9%) | 20 (58.8%) | 20 (55.6%) | |
| HAS-BLED score | ||||
| 1–2 | 12 (42.9%) | 20 (58.8%) | 15 (41.7%) | 0.29 |
| > 3 | 16 (57.1%) | 14 (41.2%) | 21 (58.3%) | |
| Baseline comorbidities | ||||
| Tobacco user | 2 (7.1%) | 3 (8.8%) | 2 (5.6%) | 0.89$ |
| Prior stroke/transient ischemic attack | 6 (21.4%) | 9 (26.5%) | 5 (13.9%) | 0.42 |
| Coronary artery disease/Prior myocardial infarction | 19 (67.9%) | 20 (58.8%) | 28 (77.8%) | 0.23 |
| Heart failure | 10 (35.7%) | 6 (17.7%) | 6 (16.7%) | 0.14 |
| Diabetes mellitus | 13 (46.4%) | 17 (50%) | 13 (36.1%) | 0.48 |
| Hypertension | 27 (96.4%) | 31 (91.2%) | 34 (94.4%) | 0.77$ |
| Chronic obstructive pulmonary disease | 7 (25%) | 7 (20.6%) | 8 (22.2%) | 0.91 |
| Peripheral vascular disease | 6 (21.4%) | 8 (23.5%) | 12 (33.3%) | 0.50 |
| Chronic kidney disease | 11 (39.3%) | 7 (20.6%) | 12 (33.3%) | 0.26 |
| Gastrointestinal pathology‡ | 6 (21.4%) | 11 (32.4%) | 15 (41.67%) | 0.23 |
| Presence of potentially interacting drugs | ||||
| Aspirin | 17 (60.7%) | 22 (64.7%) | 26 (72.2%) | 0.61 |
| Any antiplatelet agent§ | 17 (60.7%) | 24 (70.6%) | 29 (80.6%) | 0.22 |
| Cytochrome P450 3A4 or P-glycoprotein 1-ATP-binding cassette subfamily B member 1 inhibitor | 15 (53.6%) | 13 (38.2%) | 14 (38.9%) | 0.40 |
| Cytochrome P450 3A4 or P-glycoprotein 1-ATP-binding cassette subfamily B member 1 inducer | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Other‖ | 3 (10.7%) | 1 (2.9%) | 6 (16.7%) | 0.17$ |
Calculated using the Kruskal–Wallis test.
Calculated using the Fisher’s exact test. All other p values were calculated using the chi-square test.
Presence of gastroesophageal reflux disease, gastritis, or peptic ulcer disease.
Any antiplatelet agents: aspirin, aspirin/dipyridamole, cilostazol, clopidogrel, non-acetylsalicylic acid-based nonsteroidal anti-inflammatory drugs, prasugrel, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, ticagrelor, ticlopidine, or any combination thereof.
Omega-3 fatty acids, vitamin E.
IQR, interquartile range; N/A, not available.
Rates of FDA-recommended dosing were compared across individual DOAC cohorts upon therapy initiation and throughout the treatment period based on both TBW and NBW CrCl estimation. Rates of FDA-recommended dosing are shown in Table 3. Rates are broken down by each DOAC cohort in three categories: dose upon initiation based on TBW CrCl estimation, dose throughout treatment based on TBW CrCl estimation, and dose throughout treatment based on NBW CrCl estimation. DOAC dosing throughout treatment based on NBW CrCl was also included to determine if dosing accuracy rates significantly improved with a more conservative CrCl estimation.
Table 3.
Rates of US Food and Drug Administration-recommended dosing across direct oral anticoagulant groups.
| Apixaban (n = 28) | Dabigatran (n = 34) | Rivaroxaban (n = 36) | p value(s) | |
|---|---|---|---|---|
| Upon initiation (TBW CrCl) | 26 (92.9%) | 31 (91.2%) | 31 (86.1%) | 0.70* |
| Throughout treatment (TBW CrCl) | 22 (78.6%) | 30 (88.2%) | 21 (58.3%) | 0.01 0.005D-R 0.30D-A 0.09R-A |
| Throughout treatment (NBW CrCl) | 22 (78.6%) | 31 (91.2%) | 26 (72.2%) | 0.13 |
Calculated using the Fisher’s exact test. All other p values were calculated using the chi-square test.
Chi-square comparison of dabigatran cohort versus rivaroxaban cohort.
Chi-square comparison of dabigatran cohort versus apixaban cohort.
Chi-square comparison of rivaroxaban cohort versus apixaban cohort.
CrCl, creatinine clearance; NBW, no body weight; TBW, total body weight.
As shown in Table 3, upon therapy initiation and based on TBW CrCl, apixaban was dosed according to FDA recommendations in 92.9% of patient cases, dabigatran in 91.2% of cases, and rivaroxaban in 86.1% of cases, but these differences were not found to be statistically significant (p = 0.70). FDA-recommended dosing was maintained throughout treatment at the highest rates with dabigatran (88.2% based on TBW CrCl, 91.2% based on NBW CrCl), compared with both apixaban (78.6% based on both TBW and NBW CrCl) and rivaroxaban (58.3% based on TBW CrCl, 72.2% based on NBW CrCl). However, the only rate difference that was found to be statistically significant was that of dabigatran (throughout treatment based on TBW CrCl) at 88.2%, versus rivaroxaban at 58.3% in the same measurement (p = 0.005).
Dose excursions (represented by patients exposed to a non-FDA approved DOAC dose at any point during therapy) were documented by study investigators with each SCr measurement in all patient cases. A total of 25 patients experienced a dose excursion(s) at one or more instances during therapy, including 10 patients that were actually initiated on a non-FDA approved dose. Rationales for dose excursions in each case are displayed in Table 4.
Table 4.
Assessment of rationales for dose excursions across direct oral anticoagulant groups.
| Number of patients experiencing dose excursions (25) | Rationales for excursion outside US Food and Drug Administration-recommended dosing | |
|---|---|---|
| Apixaban | 6 | • Reduced dose due to: ○ drug interaction with amiodarone (1) ○ outside provider recommendation (1) ○ bilateral knee amputation (1) ○ fluctuation in body weight near the dosing cutoff (1) ○ age nearing 80 years (1) • Maintained reduced dose, despite serum creatinine temporarily < 1.5 mg/dl (1) |
| Dabigatran | 4 | •Reduced dose due to: ○ outside provider recommendation (2) ○ fluctuation in estimated CrCl near the dosing cutoff (1) • Maintained standard dose despite estimated CrCl temporarily < 30 ml/min (1) |
| Rivaroxaban | 15 | • Reduced dose due to: ○ fluctuation in estimated CrCl near the dosing cutoff (7) ○ outside provider recommendation (5) • Maintained reduced dose despite estimated CrCl temporarily > 50 ml/min (3) |
CRCl, creatinine clearance.
The most common rationales for dose excursions were as follows: patient fluctuation in renal function near the dosing cutoff (8 of 25 patients experiencing excursion[s]; 32%) and requests or recommendations from providers outside the pharmacist-led AC who were comanaging the patient’s drug therapies (8 of 25 patients experiencing excursion[s]; 32%). Other rationales for dose excursions are listed in Table 4. Overall, prescription and/or maintenance of a reduced dose (below what is FDA-recommended based on CrCl) was most commonly encountered (24 of 25 [96%] of patients experiencing excursion[s]), versus only one patient who was maintained on the standard dose of dabigatran (150 mg twice daily) despite a one-time CrCl estimation of less than 30 ml/min. Further analysis of patients that experienced a dose excursion(s) revealed that 16 of 25 (64%) of these patients had a HAS-BLED score of 3 or more. Similarly, 16 of 25 (64%) of these patients had a CHADS2 score of 3 or more. This indicates that although the majority of these patients were characterized as having a high risk for both bleeding and/or thrombosis,17,18 most of these patients were dosed at lower than the FDA-recommended dose.
For patients in the study population that switched DOAC therapy during the data-collection period, the most common switch encountered was from dabigatran (3) or rivaroxaban (4) to apixaban (7 of 11; 64% of switches). Rationales for switches were highly variable, though all patients who switched from rivaroxaban to apixaban did so due to minor bleeding events (hematuria [2], excessive bruising [1], and recurrent epistaxis [1]) experienced while taking rivaroxaban.
Discussion
In our study, the use of a DOAC-dosing strategy utilizing multiple estimations for CrCl led to rates of FDA-approved dosing of 86.1–92.3% upon therapy initiation, and 58.3–88.2% throughout treatment, for the three DOACs studied. These findings are relatively consistent with existing real-world data that show off-label dosing of DOACs occurring at rates from 13% to as high as 57%.21–24
We found that the majority (96%) of dose excursions outside the FDA-approved range were representative of under-dosing. Many of these instances occurred due to patient fluctuation in estimated CrCl near the dosing cutoff for the DOAC being used. For example, 10 of 15 (66.7%) patients who experienced dose excursions while on rivaroxaban did so due to fluctuation in CrCl near 50 ml/min, the cutoff for 20 mg daily versus 15 mg daily dosing. These findings reveal that even in an AC setting with diligent laboratory monitoring, maintaining elderly patients on FDA-recommended doses of DOACs can be extremely difficult. Many other factors may have played a role in under-dosing, including the high prevalence of concomitant aspirin and/or other antiplatelet drug(s), and clinician concern for the risk of major bleeding potentially outweighing the risk of thromboembolic events in this population. This risk versus benefit ratio has raised significant debate regarding the safety and efficacy of reduced, off-label DOAC doses. Recent retrospective data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF II) has shown under-dosing of DOACs to be associated with an increase in cardiovascular hospitalization (adjusted hazard ratio 1.26; 95% confidence interval 1.07–1.50; p = 0.007)23 and higher unadjusted rates of thromboembolic events and death (though not statistically different after adjustment).24 In addition, a recent retrospective propensity weighed cohort study of over 55,000 patients with AF and a mean age of 74 years, trialed on a low-dose DOAC (apixaban 2.5 mg twice daily, dabigatran 110 mg twice daily, or rivaroxaban 15 mg daily) or warfarin was performed by Nielsen and colleagues. Patients were restricted to lowered DOAC doses, despite the fact that only 7.8% of the total study population had any form of renal dysfunction.25 Interestingly, no statistically significant differences were found in the rates of ischemic stroke/systemic embolism across the four groups. Dabigatran 110 mg twice daily displayed significantly lower rates of bleeding than warfarin, but no difference in bleeding was found for apixaban or rivaroxaban when compared with warfarin.25 These studies add to the continuous debate in the USA about whether DOAC dosing should be based solely on renal function versus a more individualized approach adopted by other international regulatory agencies.26
We also found that rivaroxaban had the lowest rate of FDA-approved dosing throughout treatment, but this was statistically significant only in comparison with dabigatran. Interestingly, a similar study by Whitworth and colleagues in 120 patients with nonvalvular AF or venous thromboembolism (VTE) found apixaban to be the most commonly misdosed agent.22 However, these patients were significantly younger (average age 66 years), and DOAC dosing for VTE treatment differs significantly to that of dosing for anticoagulation in nonvalvular AF, which may explain our conflicting findings.
The most evident challenge experienced by our AC clinic and likely many others is accurately estimating patients’ renal function, which can vary greatly depending on choice of patient weight used (e.g. TBW, IBW, ABW, etc.) in the numerator of the CG equation. The Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) and Rivaroxaban Once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial investigators used TBW to calculate estimated CrCl in dabigatran and rivaroxaban patients, respectively.27 Therefore it would appear most appropriate to use TBW in CrCl estimation to dose these two agents. However, a large meta-analysis performed by Wilhelm and Kale-Pradhan in 2011 compared 24 hour measured CrCl with CG estimated CrCl in over 1100 patients using various body weights to determine the most accurate predictor of measured CrCl. The authors found that using the CG equation with NBW and actual SCr most closely predicted measured CrCl, and provided a better estimate than using TBW (which overestimated measured CrCl) or IBW (which underestimated measured CrCl).28 This study justifies the dosing approach used at our AC, as described above. It is important to note that other renal function estimations, such as the use of serum cystatin C (versus SCr), may provide more conservative estimations, especially in frail elderly patients.29 Though not routinely used in the USA, calculation with serum cystatin C may be useful in DOAC dosing, especially in frail patients with mild or moderately reduced kidney function and other risk factors for bleeding.
DOAC selection based on other factors (besides renal function) may alleviate some concern over fluctuating or difficult-to-estimate renal function commonly seen in elderly patients. For example, dabigatran was recently placed on the Beers List by the American Geriatrics Society as potentially inappropriate for use in older adults.30 As seen in our study, rivaroxaban may present the most difficulty in accurately dosing due to its higher CrCl dosing cutoff of 50 ml/min. With that said, an agent such as apixaban, with the lowest amount of renal excretion (27%) of any DOAC,31 and dosing based on weight, age, and actual SCr32 (rather than estimated CrCl) may be a favorable choice, particularly for those with documented fluctuations in renal function. Of note, measures of therapeutic drug monitoring are available for DOACs (dilute thrombin time and/or ecarin clotting time for direct thrombin inhibitors, and anti-activated factor X assay for factor Xa inhibitors), but are not currently recommended for routine use due to a number of factors, including cost and drug-specific calibration requirements.33
Several limitations apply to our study. Most notably, our study population was much smaller in comparison with most other studies that evaluate DOACs in AF. However, our study was designed to assess retrospectively DOAC doses prescribed, for which a small study population with descriptive outcomes can suffice. This being a retrospective chart review study at a single center, we were limited to clinical documentation found only in the Detroit VAMC CPRS. It is important to note that all but one patient were men, which is to be expected in a cohort of patients within a VA health system. However, evidence to this point has shown no significant differences in DOAC safety or efficacy with respect to gender.34,35 Lastly, the most recently approved DOAC agent, edoxaban, was not included in our study since it was not approved for use in the USA until January 2015, after the end of the data-collection period.
This study was conducted to provide anticoagulation providers with an example of how often patients can be exposed to non-FDA recommended doses of DOACs, even in a setting with stringent laboratory monitoring and more frequent patient visits. Our study gives a real-world perspective of the challenge faced by clinicians in dosing, monitoring, and the overall management of anticoagulation with DOAC therapy in geriatric patients. We hope that this study supports further research, through comparison and/or compilation of data from existing AF registries and other anticoagulation settings, to help us establish the most accurate and effective dosing strategies for DOACs in the elderly.
Acknowledgments
The authors would like to thank Cindy Mansfield, CPhT for her assistance in data extraction and organization during this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Joseph P. Fava  https://orcid.org/0000-0002-8631-4670
https://orcid.org/0000-0002-8631-4670
Contributor Information
Joseph P. Fava, Clinical Assistant Professor, Department of Pharmacy Practice, Wayne State University Eugene Applebaum College of Pharmacy and Health Sciences, 259 Mack Ave, Suite 2128, Detroit, MI 48201, USA.
Katelyn M. Starr, Department of Pharmacy, John D. Dingell Veterans Affairs Medical Center, Detroit, MI, USA
David Ratz, Veterans Affairs Center for Clinical Management Research, Ann Arbor, MI, USA.
Jennifer L. Clemente, Department of Pharmacy, John D. Dingell Veterans Affairs Medical Center, Detroit, MI, USA
References
- 1. Yang E. A clinician’s perspective: novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation –full speed ahead or proceed with caution? Vasc Health Risk Manag 2014; 10: 507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 4. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 7. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med 2015; 128: 1300–1305. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laish-Farkash A, Khalameizer V, Katz A. Atrial fibrillation in the elderly: to ablate or not to ablate. J Cardiovasc Electrophysiol 2013; 24: 739–741. [DOI] [PubMed] [Google Scholar]
- 9. Pfeilschifter W, Luger S, Brunkhorst R, et al. The gap between trial data and clinical practice – an analysis of case reports on bleeding complications occurring under dabigatran and rivaroxaban anticoagulation. Cerebrovasc Dis 2013; 36: 115–119. [DOI] [PubMed] [Google Scholar]
- 10. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 11. Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). Circulation 2014; 130: 138–146. [DOI] [PubMed] [Google Scholar]
- 12. Hodgkinson B, Evans D, Wood J. Maintaining oral hydration in older adults: a systematic review. Int J Nurs Pract 2003; 9: S19–S28. [DOI] [PubMed] [Google Scholar]
- 13. Fliser D. Assessment of renal function in elderly patients. Curr Opin Nephrol Hypertens 2008; 17: 604–608. [DOI] [PubMed] [Google Scholar]
- 14. Abdel-Kader K, Palevsky P. Acute kidney injury in the elderly. Clin Geriatr Med 2009; 25: 331–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimachi M, Furukawa TA, Kimachi K, et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 2017; 11: CD011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harel Z, Sholzberg M, Shah PS, et al. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol 2014; 25: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 18. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 2012; 126: 860–865. [DOI] [PubMed] [Google Scholar]
- 19. Boehringer Ingelheim Pharmaceuticals, Inc. Pradaxa(R) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc, 2015. [Google Scholar]
- 20. Janssen Pharmaceuticals, Inc. Xarelto(R) package insert. Titusville, NJ; Janssen Pharmaceuticals, Inc, 2016, https://www.xareltohcp.com/shared/product/xarelto/prescribing-information.pdf (accessed 20 February 2018). [Google Scholar]
- 21. Larock AS, Mullier F, Sennesael AL, et al. Appropriateness of prescribing dabigatran etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective study. Ann Pharmacother 2014; 48: 1258–1268. [DOI] [PubMed] [Google Scholar]
- 22. Whitworth MM, Haase KK, Fike DS, et al. Utilization and prescribing patterns of direct oral anticoagulants. Int J Gen Med 2017; 10: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol 2016; 68: 2597–2604. [DOI] [PubMed] [Google Scholar]
- 24. Steinberg BA, Shrader P, Pieper K, et al. Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc 2018; 7: pii: e007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nielsen PB, Skjoth F, Sogaard M, et al. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2017; 356: j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez I. Time to reconsider dabigatran 110 mg in the USA. Am J Cardiovasc Drugs 2015; 15: 307–309. [DOI] [PubMed] [Google Scholar]
- 27. Longo L. Getting the most from our safety surveillance – TSOACs and Renal Function: information on dosing and calculating creatinine clearance from the pivotal trials. Hines, IL: VA Pharmacy Benefits Management Services & Center for Medication Safety, 2014, pp.2–3. [Google Scholar]
- 28. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy 2011; 31: 658–664. [DOI] [PubMed] [Google Scholar]
- 29. Ballew SH, Chen Y, Daya NR, et al. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017; 69: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 31. Lutz J, Jurk K, Schinzel H. Direct oral anticoagulants in patients with chronic kidney disease: patient selection and special considerations. Int J Nephrol Renovasc Dis 2017; 10: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bristol-Myers Squibb Company. Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Company, 2016. [Google Scholar]
- 33. Lippi G, Favaloro EJ. Laboratory monitoring of direct oral anticoagulants (DOACs) – the perfect storm? Ann Transl Med 2017; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dentali F, Sironi AP, Gianni M, et al. Gender difference in efficacy and safety of nonvitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation or venous thromboembolism: a systematic review and a meta-analysis of the literature. Semin Thromb Hemost 2015; 41: 774–787. [DOI] [PubMed] [Google Scholar]
- 35. Mehta N, Ragupathi L. No difference with NOACs in women with nonvalvular atrial fibrillation: one less gender gap! J Womens Health (Larchmt) 2017; 26: 197–198. [DOI] [PubMed] [Google Scholar]


