Abstract
Palmar–plantar erythrodysesthesia, also known as hand–foot syndrome (HFS), is a well-known dermatologic adverse event that can occur with a variety of cytotoxic chemotherapies including fluoropyrimidines, cytarabine, liposomal doxorubicin, and taxanes. HFS often presents as painful erythemas and desquamation of the skin involving the palms of the hands and the soles of the feet. Three cases are presented of patients with breast cancer who received multiagent chemotherapy regimens containing docetaxel that developed an atypical presentation of HFS on the dorsal aspect of the hands and feet. All patients received supportive care to manage the symptoms of their dermatologic toxicity. Dorsal HFS improved with supportive care or dose reduction and resolved following completion of the docetaxel-based chemotherapy. Based on the temporal relationship of the event and previous reports, we found that docetaxel was the probable offending agent.
Keywords: adverse event, chemotherapy, dermatology, oncology
Introduction
Dermatologic toxicity is a well-known adverse effect of different cytotoxic and targeted antineoplastic drugs.1 Cutaneous surfaces such as hair, skin, mucous membranes, and nails can experience an array of reactions including, but not limited, to immune-mediated hypersensitivity, hyperpigmentation, nail changes, erythema, and various forms of dermatitis.1,2 Palmar–plantar erythrodysesthesia, also known as hand–foot syndrome (HFS), is a dermatologic condition which often presents as painful erythemas involving the palms and soles of the feet.3 With cutaneous reactions often being inconsistently documented, there exists a wide variety of proposed incidence rates. Conventional chemotherapeutic agents most frequently associated with HFS include capecitabine, cytarabine, fluorouracil, liposomal doxorubicin, and taxanes. The incidence and severity of HFS varies between chemotherapeutic agents with some drugs, such as capecitabine, exhibiting rates as high as 64%.4 Incidence of taxane-induced HFS is thought to be in the range of 5–10%, with docetaxel being a more common offender than paclitaxel.5
While there are many proposed mechanisms and predispositions to chemotherapy-induced HFS, the true pathogenesis is not currently known. A common theory linked to histological findings is a direct cytotoxic effect exerted on the basal keratinocytes by the chemotherapeutic agent.3 This aligns with the clinical presentation of cutaneous involvement of the palms and sole of the feet, which possess highly keratinized epithelial tissue.6 Taxane-induced HFS can be distinct from other classes with occasional erythematous plaques and further severity on the dorsal side of the hand.7 We observed a series of patients experiencing dermatologic reactions secondary to administration of docetaxel in combination with carboplatin, trastuzumab, and pertuzumab (TCHP) for the treatment of breast cancer. The patients experienced bilateral erythematous plaques, hyperpigmentation, and desquamation of the dorsal surface of the hands and feet, with no involvement of the palmar–plantar surfaces. To our knowledge this presents an atypical presentation to a classical dermatologic reaction in the setting of docetaxel therapy. This case series was reviewed and approved by our health-system’s institutional review board.
Case 1
A 58-year-old woman was diagnosed with T1cN0M0 ER(–), PR(–), HER2/neu overexpressing right breast invasive mammary carcinoma. She was initiated on neoadjuvant TCHP for curative intent with a goal of completing six cycles followed by completing 1 year of trastuzumab therapy. On cycle 3 day 7, the patient called into the oncology triage line to report a blotchy, itchy rash that appeared on her hands, neck, and stomach. The patient was advised to use topical diphenhydramine and an over-the-counter hydrocortisone cream for relief. Upon presentation for cycle 4, the patient’s rash persisted and was painful with erythema, hyperpigmentation, and desquamation of the dorsal aspects of the hands and feet; the rash was considered grade 2. This rash appeared bilateral and symmetrical in nature. The palms and soles of her feet were unaffected and normal in appearance. A dermatology consultation was placed. She was advised to undergo supportive care measures by utilizing cool water soaks for her hands and feet as well as applying thick emollient creams. Despite supportive care, the rash persisted. There was no documentation of the patient following up with dermatology. Docetaxel was thought to be the most likely offending agent; a 20% dose reduction was applied to docetaxel for the final two cycles of TCHP. The rash continued to persist, but decreased in severity to grade 1 upon dose reduction. Following the completion of the TCHP portion of her therapy, the rash did not recur and she is currently undergoing maintenance trastuzumab therapy without any dermatologic issues.
Case 2
A 39-year-old woman was diagnosed with T3N0M0 ER(+), PR(+), HER2/neu overexpressing left breast invasive mammary carcinoma. She was initiated on neoadjuvant TCHP for curative intent with a goal of completing six cycles followed by completing 1 year of trastuzumab therapy. With cycle 3 day 15, she presented with complaints of a newly developed grade 1 rash on the dorsal aspect of both hands. The rash appeared to be erythematous with small red papules and some desquamation (Figure 1). The palms of her hands were unaffected and normal in appearance on physical examination. Her chemotherapy was continued without any delay or dose adjustments; the rash appeared following each subsequent cycle and spontaneously resolved after 1 week with the use of emollient creams. Following the completion of the TCHP portion of her therapy, the rash did not recur and she is currently undergoing maintenance trastuzumab therapy without any dermatologic issues.
Figure 1.
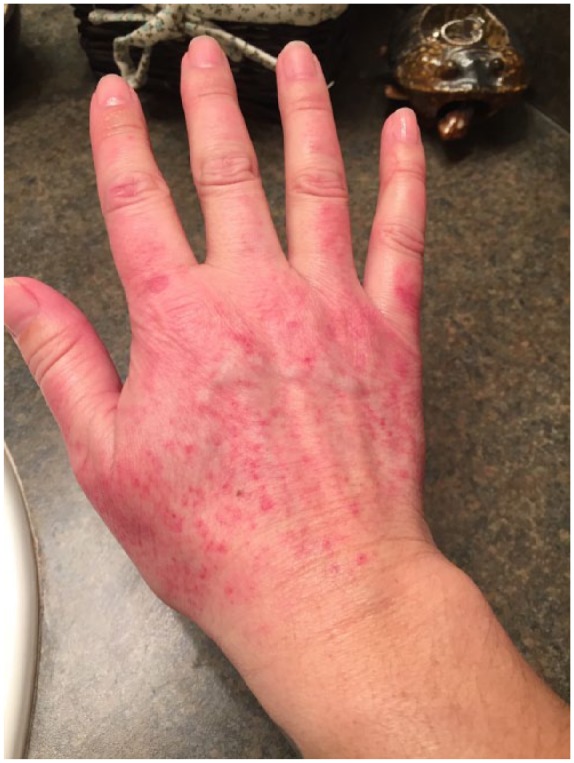
Appearance of dorsal hand–foot syndrome in case 2.
Case 3
A 53-year-old woman was diagnosed with T2N0M0 ER(+), PR(+), HER2/neu overexpressing left breast invasive mammary carcinoma. She was initiated on neoadjuvant TCHP for six cycles followed by trastuzumab to complete 1 year of therapy. On cycle 3 day 10, a grade 2 rash developed on the dorsal surface of her hands that was erythematous with desquamation. She also complained of a painful burning sensation on the affected area. The patient was advised to use bag balm alternating with zinc oxide to alleviate the symptoms. Docetaxel was continued at full dose with cycle 4. Upon consideration of cycle 5, the symptoms worsened. Continued use of supportive care was recommended again and the docetaxel dose was reduced by 25%. The symptoms resolved with this method; there was no recurrence of dermatologic toxicity. She is currently undergoing maintenance trastuzumab therapy.
Discussion
The incidence of dermatologic manifestations with docetaxel is difficult to quantify and predict. Currently, there are case reports available in the literature that provide examples of docetaxel-induced HFS.7,8 Our case series provides an atypical presentation of this toxicity. In the NeoSphere trial, the incidence of rash was reported to be 21–29% when docetaxel was combined with trastuzumab and/or pertuzumab.9 Interestingly, the incidence of rash in the TRYPHAENA study was not profound.10 Both studies did not report a description of the rash that occurred.
While palmar–plantar erythrodysesthesia is a classical adverse event of docetaxel, our case series is particularly atypical as the HFS was dorsal in nature and not palmar–plantar. While a previous case report has described dorsal desquamation of the hands, the patient presented also with palmar–plantar desquamation.7 Although a common reaction, the particular presentation of this toxicity in our patients is not well represented in the literature. Periarticular thenar erythema and onycholysis (PATEO) syndrome is a dermatologic reaction that can occur with docetaxel and often affects the dorsum of the hands.5,8 PATEO was considered in the differential diagnosis of the treatment-related toxicities observed in each of our patients. The dorsal nature of the dermatologic toxicity in our patients could represent a mild or early case of PATEO, however this reaction was considered less likely due to the lack of rash on the area of the Achilles tendon and lack of onycholysis in all three patients. According to the Naranjo adverse drug reaction probability scale, docetaxel was considered the probable offending agent in each case.11 Considering the atypical nature of this adverse event occurring in several patients, we investigated if there was any issue with the particular lot number of docetaxel that was being administered. However, each patient received different lot numbers and other patients in our clinic (including those diagnosed with a different primary malignancy) also received the drug without developing this particular issue.
Optimal management of HFS has yet to be defined.6 Treatment depends on the severity of HFS and includes supportive care using emollients and creams to pacify the symptoms. Topical corticosteroids can also be considered as part of management, however there has been variable efficacy described in the literature with this approach to treating HFS. Dose reductions may also be required in severe cases.12,13 In our case series, two out of three patients required dose reduction in addition to using topical therapy; dose reductions appeared to be effective in decreasing the severity of HFS.
In conclusion, docetaxel-induced HFS can be a challenging classic taxane reaction for both patients and clinicians and can present in a unique and atypical manner.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jolly Patel, Levine Cancer Institute, Atrium Health, Department of Pharmacy, Charlotte, NC, USA.
J. Tanner Ringley, Levine Cancer Institute, Atrium Health, Department of Pharmacy, Charlotte, NC, USA.
Donald C. Moore, Pharmacist Clinical Coordinator – Oncology, Levine Cancer Institute, Atrium Health, Department of Pharmacy, 1021 Morehead Medical Drive, Charlotte, NC 28204, USA.
References
- 1. Payne AS, James WD, Weiss RB. Dermatologic toxicity of chemotherapeutic agents. Semin Oncol 2006; 33: 86–97. [DOI] [PubMed] [Google Scholar]
- 2. Remlinger KA. Cutaneous reactions to chemotherapy drugs: the art of consultation. Arch Dermatol 2003; 139: 77–81. [DOI] [PubMed] [Google Scholar]
- 3. Janusch M, Fischer M, Marsch WCh, et al. The hand foot syndrome: a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol 2006; 16: 494–499. [PubMed] [Google Scholar]
- 4. Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 2007; 25: 5210–5217. [DOI] [PubMed] [Google Scholar]
- 5. Sibaud V, Lebœuf NR, Roche H, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol 2016; 26: 427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagore E, Insa A, Sanmartín O. Anti-neoplastic therapy-induced palmar plantar erythrodysthesia (‘hand-foot’) syndrome. Am J Clin Dermatol 2000; 1: 225–234. [DOI] [PubMed] [Google Scholar]
- 7. Jain A, Dubashi B. Docetaxel-induced hand foot syndrome: “no dose is a safe dose.” J Pharmacol Pharmacother 2012; 3: 200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Childress J, Lokich J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol 2003; 26: 435–436. [DOI] [PubMed] [Google Scholar]
- 9. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory or early HER-2 positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 25–32. [DOI] [PubMed] [Google Scholar]
- 10. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimen in patients with HER-2 positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013; 24: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 11. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 12. Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 2006; 12: 131–141. [DOI] [PubMed] [Google Scholar]
- 13. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer 2008; 44: 781–790. [DOI] [PubMed] [Google Scholar]


