Abstract
Background
Bariatric surgery is a widely adopted treatment for obesity and its secondary complications. In the past decade, microvesicles (MVs) and CD36 have increasingly been considered as possible biomarkers for obesity, the metabolic syndrome (MetSy), type 2 diabetes mellitus (T2DM). Thus, the purpose of this study was to investigate how weight loss resulting from bariatric surgery affects levels of specific MV phenotypes and their expression of CD36 scavenger receptor. Additionally, we hypothesised that subjects with MetSy had higher baseline concentrations of investigated MV phenotypes.
Methods
Twenty individuals undergoing Roux-en-Y gastric bypass surgery were evaluated before and 3 months after surgery. MVs were characterised by flow cytometry at both time points and defined as lactadherin-binding particles within a 100-1000 nm size gate. MVs of monocyte (CD14) and endothelial (CD62E) origin were defined by cell-specific markers, and their expression of CD36 was investigated.
Results
Following bariatric surgery, subjects incurred an average BMI reduction (delta) of − 8.4 ± 1.4 (p < 0.0001). Significant reductions were observed for the total MVs (− 66.55%, p = 0.0017) and MVs of monocyte (− 36.11%, p = 0.0056) and endothelial (− 40.10%, p = 0.0007) origins. Although the bulk of CD36-bearing MVs were unaltered, significant reductions were observed for CD36-bearing MVs of monocyte (− 60.04%, p = 0.0192) and endothelial (− 54.93%, p = 0.04) origin. No differences in levels of MVs were identified between subjects who presented with MetSy at baseline (n = 13) and those that did not (n = 7).
Conclusion
Bariatric surgery resulted in significantly altered levels of CD36-bearing MVs of monocyte and endothelial origin. This likely reflects improvements in ectopic fat distribution, plasma lipid profile, low-grade inflammation, and oxidative stress following weight loss. Conversely, however, the presence of MetSy at baseline had no impact on MV phenotypes.
Electronic supplementary material
The online version of this article (10.1186/s12986-018-0309-4) contains supplementary material, which is available to authorized users.
Keywords: Extracellular vesicles, CD36, Obesity, Ectopic fat deposition, Metabolic syndrome, Bariatric surgery
Background
Decades of research indicate that lifestyle interventions and pharmacotherapy of obesity often fail to result in sufficient and sustained reductions in weight to reduce an individual’s risk of obesity-related morbidity and mortality [1, 2]. However, a large body of evidence suggests that bariatric surgery can result in sustained weight loss, reduce an individual’s risk of type 2 diabetes mellitus (T2DM) recurrence, and decrease levels of circulating inflammatory markers associated with obesity, the metabolic syndrome, and T2DM [3–5]. Therefore, bariatric surgery is adopted to an increasing extent globally as a treatment for morbid obesity and its secondary complications [6–8].
In recent years, a growing number of studies have begun to realise the potential of microvesicles (MVs) as biomarkers for a number of diseases, including the metabolic syndrome (MetSy) [9], T2DM [10], and atherosclerosis [11]. MVs are a subset of extracellular vesicles (EVs) that can be distinguished by their biogenesis, namely outwards budding of the plasma membrane and subsequent occlusion and shedding of the particle [12]. Like all other extracellular vesicles, MVs are a heterogeneous group of plasma membrane-enclosed particles containing components from their cellular origin, which are shed by most cell types in latent, activated and apoptotic states [13]. A soluble form of CD36 (sCD36) has previously been demonstrated to be present in circulation, and increased levels have been associated with abdominal fat distribution [14], insulin resistance [15], and non-alcoholic fatty liver disease (NAFLD) [16]. Although the exact nature of sCD36 is unknown, studies have previously revealed that, at least in part, sCD36 is associated with circulating MVs [11, 17].
CD36 is a scavenger receptor that has been associated with cellular uptake of lipids in a whole range of cells, and its expression is increased in obesity, MetSy, and diabetes [18–23]. In addition, a large body of evidence suggests that CD36 is involved in pro-inflammatory polarisation of macrophages upon exposure to oxidised LDL cholesterol and therefore contributes to the low-grade inflammatory state observed in diet-induced obesity and MetSy [22, 24]. Thus, measuring CD36 on the surface of MVs might not only yield important information about ectopic fat deposition but also the level of low-grade inflammation.
Therefore, it was hypothesised that significant weight loss and improvement in cardio-metabolic risk factors resulting from bariatric surgery would result in decreased concentrations of circulating MVs, MVs positive for the expression of CD36 on their surface, MVs of monocyte (MMVs) or endothelial (EMV) origin, and MMVs and EMVs positive for CD36. It was further hypothesised that subjects suffering from MetSy at inclusion had increased baseline concentrations of all of the abovementioned MV phenotypes.
Methods
Study design
The study population and design have been described elsewhere [14]. In brief, twenty individuals undergoing bariatric surgery were recruited for the present study. All participants met the Danish requirements for referral to bariatric surgery: ≥ 20 years of age and a body mass index (BMI) ≥ 40 kg m-2 or BMI ≥ 35 with associated co-morbidities. Participants were additionally required to lose approximately 8% of their body weight prior to surgery and inclusion in this study. Bariatric surgery was performed by either one of two surgeons with expertise in Roux-en-Y gastric bypass using a standard laparoscopic Roux-en-Y technique at the Aleris-Hamlet Hospital, Copenhagen, Denmark [14]. All participants were dismissed within 24 h after surgery and no subjects suffered from post-surgical complications.
All twenty subjects were evaluated prior to (baseline) and three months after bariatric surgery. At each visit, following an overnight fast, height and weight were recorded, fat mass and distribution was measured by full body dual-energy x-ray absorptiometry, and venous blood samples were collected for biochemical analyses and flow cytometric measurement of MVs. Haemoglobin, leukocytes, aspartate aminotransferase (AST), alanine aminotransferase (ALT), glycosylated haemoglobin, plasma glucose, C-peptide, serum insulin, total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides were determined with standardised methods in a routine biochemical laboratory. Plasma soluble CD36 (sCD36) was measured with an in-house ELISA as previously described [15]. Serum YKL40 was determined with a commercial ELISA (Quidel, San Diego, CA, USA). High sensitivity C-reactive protein (hsCRP) was measured with a commercial highly sensitive, latex particle-enhanced immunoturbidimetric assay (DAKO, Glostrup, Denmark). Furthermore, insulin sensitivity was determined with the homeostasis model assessment (HOMA-%S, http://www.dtu.ox.ac.uk/homacalculator/index.php), and liver fat percentage (LF%) was predicted using an algorithm based on the presence of MetSy and T2DM as well as fasting insulin and ALT and AST [25].
Flow cytometric analysis of MVs
Blood samples for flow cytometric analysis of MVs were collected into EDTA tubes and the first centrifugation cycle was initiated within two hours after collection. Samples were subjected to centrifugation at 2000 g for 10 min to yield blood plasma. Plasma was stored at − 80 °C until analysis. Flow cytometric analysis of plasma MV content was performed as described previously [26] and in supplementary materials on a BD FACSAria™ III High Speed Cell Sorter (BD Biosciences, San Jose, CA, USA) and data was analysed in FlowJo® version 10.4 (FlowJo LLC, Oregon, USA) as depicted in Fig. 1.
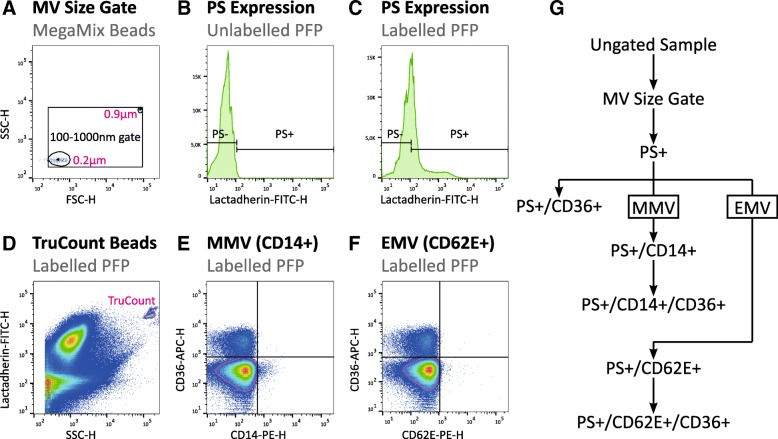
Fig. 1.
Gating strategy for flow cytometric characterisation of MVs. a) A 100-1000 nm MV size gate was established based on 200 nm and 900 nm polystyrene beads (MegaMix) and transferred to all samples. b) Next, a gate was set on FITC-H at the 99th percentile of unlabelled samples. c) MVs were defined as PS+ events based on binding of lactadherin-FITC. e & f) The double negative population was defined based on density (magenta gate), and bi-variate gates were placed at the 99th percentile of the double negative population. Finally, the gates were applied to all PS+ events in the corresponding sample to define MMVs (e), EMVs (f), and the expression of CD36 on these phenotypes (e & f). d) TruCount® beads (magenta gate) were quantified and used to calculate absolute concentrations of MVs. g) Gating hierarchy utilised for defining the MV phenotypes in the current study. PS: Phosphatidylserine; PFP: Platelet-free plasma; MMV: Monocyte microvesicles; EMV: Endothelial microvesicles
Statistical data analysis
All statistical data analyses and plotting was performed in and R 3.2.5 (R Core Team, Vienna, Austria) with xlsx [27], ggplot2 [28], and reshape2 [29] packages installed. The assumption of normality was tested using Shapiro-Wilk’s W-test and confirmed visually for each parameter using QQ-plots and histograms. Paired Student’s t-test or Wilcoxon signed rank test were used to compare pre and post-surgical values for all parameters where appropriate, whereas unpaired Student’s t-test or Mann-Whitney U test were utilized to compare baseline parameters with regards to the presence of MetSy on baseline data. All P-values are two-sided, and statistical significance was defined as p < 0.05.
Results
Anthropometric and biochemical characteristics
This study population has previously been described by Knøsgaard et al. [14] and data are presented in Table 1. In summary, 18 female and 2 male subjects were included into the current study, and subjects were 46.5 (range: 26; 63) years of age at inclusion. Subjects had a median weight of 118 kg (IQR: 108.75; 127) and BMI of 42.1 kg m− 2 (IQR: 40.4; 44.1). At the three-month post-surgical follow-up visit, subjects had incurred significant decreases in body weight (follow-up: 94 kg (IQR: 84.7; 100); n = 20; p < 0.0001) and BMI (follow-up: 33.9 kg m− 2 (IQR: 32.0; 35.0); n = 20; p < 0.0001).
Table 1.
Characteristics of the study population
| Baseline | 3 monthFollow-up | Δ | P-value | |
|---|---|---|---|---|
| (n = 20) | (n = 20) | |||
| Age [Years] | 46.5 ± 11.2 | |||
| Sex [M/F] | 2/18 | |||
| Metabolic Syndrome [n (%)] | 13 (65%) | 2 (10%) | ||
| NASH [n (%)] | 13 (65%) | 11 (55%) | ||
| Haemoglobin [mmol l−1] | 8.5 ± 0.5 | 8.4 ± 0.6 | −0.1 ± 0.4 | 0.1405 |
| Weight [kg] | 118 (108.75; 127) | 94 (84.7; 100) | −23.33 ± 4.21 | 0.0001 |
| BMI [kg m− 2] | 42.1 (40.4; 44.1) | 33.9 (32.0; 35.0) | −8.4 ± 1.4 | < 0.0001 |
| Total Fat Mass [kg] | 54.8 ± 11.0 | 40.2 (32.9; 42.3) | −15.6 ± 35. 6 | < 0.0001 |
| Body Fat Percentage [%] | 45.3 ± 4.9 | 39.4 ± 6.5 | −5.6 ± 2.4 | < 0.0001 |
| Fat Mass/Fat Free Mass [AU] | 0.84 ± 0.16 | 0.67 ± 0.17 | −0.16 ± 0.06 | < 0.0001 |
| Android Fat [kg] | 48.2 ± 8.2 | 31.9 ± 9.6 | −16.8 ± 4.7 | < 0.0001 |
| Android Fat Percentage [%] | 46.8 ± 4.0 | 39.5 ± 6.0 | −7.3 ± 3.8 | < 0.0001 |
| Truncal Fat [kg] | 26.6 ± 40.3 | 17.9 ± 49.2 | −8.4 ± 2.9 | < 0.0001 |
| Truncal Fat Percentage [%] | 44.5 ± 3.7 | 37.5 ± 6.4 | −6.6 ± 3.9 | < 0.0001 |
| Total Cholesterol [mmol l−1] | 4.92 ± 1.00 | 4.49 ± 0.88 | −0.44 ± 0.82 | 0.0275 |
| HDL Cholesterol [mmol l−1] | 1.14 ± 0.27 | 1.12 ± 0.28 | −0.02 ± 0.15 | 0.659 |
| LDL Cholesterol [mmol l−1] | 2.99 ± 0.87 | 2.82 ± 0.69 | −0.18 ± 0.73 | 0.2992 |
| Oxidized LDL Cholesterol | 4.53 ± 1.13 | 4.34 ± 0.93 | −0.19 ± 0.84 | 0.3203 |
| Triglycerides [mmol l−1] | 1.72 ± 0.62 | 1.15 (1.05; 1.45) | −0.46 ± 0.52 | 0.001 |
| Triglyceride/HDL [AU] | 1.38 (1.09; 1.89) | 1.03 (0.86; 1.41) | −0.41 ± 0.54 | 0.0023 |
| Glycated Haemoglobin [%] | 5.9 ± 0.43 | 5.6 (5.5; 5.8) | −0.14 ± 0.40 | 0.0792 |
| Fasting Glucose [mmol l−1] | 5.45 (5.18; 6.03) | 5.1 (4.78; 5.33) | −0.45 (− 0.8; − 0.15) | 0.0157 |
| Fasting C-Peptide [pmol l− 1] | 1061 (921; 1422) | 745 (566; 935) | − 360 (− 542; −36.3) | 0.0094 |
| Fasting Insulin [pmol l− 1] | 131.7 (97.5; 201.5) | 63.7 (53.7; 77.8) | −69.7 (− 105.7; −40.8) | 0.0014 |
| HOMAIR | 2.55 (2.05; 3.2) | 1.6 (1.3; 2.05) | −0.85 (− 1.43; − 0.075) | 0.0149 |
| HOMASE | 42.7 ± 16.4 | 60.4 ± 20.7 | 17.7 ± 26.3 | 0.0072 |
| YKL40 [ng ml−1] | 57.5 (47; 68.3) | 58 (45; 72.8) | 1.1 ± 14.8 | 1 |
| Liver Fat Percentage [%] | 6.7 (4.54; 9.99) | 2.82 (2.23; 3.79) | −2.78 (−6.23; −1.84) | 0.0002 |
| ALT [U I−1] | 30.9 ± 13.6 | 29.4 ± 15.8 | −1.45 ± 20.4 | 0.7537 |
| AST [U I−1] | 28 (25; 32.3) | 26 (22; 37) | 0.65 ± 12.1 | 0.9826 |
| AST/ALT [AU] | 0.97 (0.88; 1.36) | 0.97 (0.92; 1.24) | 0.06 (−0.11; 0.30) | 0.33 |
| Leukocytes [mia l−1] | 8.37 ± 2.24 | 6.65 (5.73; 8.08) | −1.34 ± 1.15 | 0.0003 |
| High-sensitivity CRP [mg l−1] | 4.67 (2.78; 9.99) | 2.16 (1.28; 5.16) | −3.91 ± 6.15 | 0.0073 |
| Soluble CD36 [AU] | 0.48 ± 0.20 | 0.37 (0.24; 0.40) | −0.13 (−0.19; − 0.06) | 0.0008 |
Data are depicted as mean ± SD or median (Q25%; Q75%)
p-values < 0.05 in bold text
Effect of bariatric surgery on microvesicle phenotypes
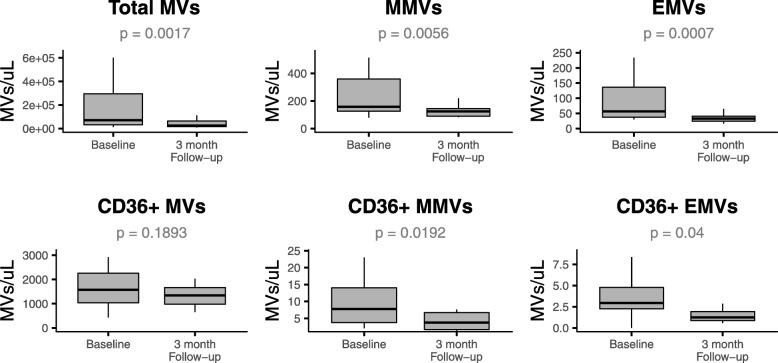
In the present study, plasma MV content and phenotypic origin of EVs were determined by flow cytometry. At the three-month post-surgical follow-up visit, significant decreases were observed in all of the investigated MV phenotypes with the exception of CD36+ MVs, which remained unaltered (Fig. 2 and Additional file 1: Table S2).
Fig. 2.
Baseline (n = 20) and three-month follow-up (n = 20) concentrations of MV phenotypes in 20 individuals. Significant decreases from baseline to follow-up were observed for PS+ MVs, MMVs, EMVs, and CD36+ MMVs and CD36+ EMVs, however no differences were observed in CD36+ MV concentration. Outliers are not depicted due to their extreme nature
Specifically, concentrations of phosphatidylserine positive (PS+) MVs decreased by a median of 66.55% from baseline to follow-up (p = 0.0017). This was equally accompanied by altered concentrations of MMV sub-phenotypes, where MMVs decreased by 36.11% (p = 0.0056) and CD36+ MMVs by 60.04% (p = 0.0192). Similar results were observed for EMV sub-phenotypes, where EMV concentrations decreased by 40.10% (p = 0.0007) and CD36+ EMVs by 54.93% (p = 0.04).
Microvesicles and metabolic syndrome
At baseline, thirteen subjects were defined as having MetSy [30], while only two subjects were defined as having MetSy at the three-month post-surgical follow-up visit. In order to investigate the impact of MetSy on MV phenotypes, subjects were initially stratified into metabolically healthy (MH; n = 7) and metabolically unhealthy (MuH; n = 13) groups based on the presence of MetSy prior to surgery (characteristics presented in Additional file 1: Table S3), and baseline concentrations of MV phenotypes compared between the two groups. However, no significant differences could be observed in baseline concentrations of any of the investigated MV phenotypes between MH and MuH groups (Additional file 1: Table S3).
Discussion
Previously, in the cohort of this study, where severely obese subjects underwent bariatric surgery, we investigated how weight loss secondary to bariatric surgery affects levels of circulating sCD36 [14]. In the present study on the other hand, we investigated how different MV phenotypes specifically focussing on MMVs, EMVs and their respective phenotypes positive for the expression of CD36 are affected by weight loss following bariatric surgery. In this study,, the results were two-fold. First, concentrations of total MVs, MMVs, EMVs, CD36+ MMVs and CD36+ EMVs decreased significantly following bariatric surgery. Second, and somewhat conversely, there were no differences in baseline concentrations of any of the investigated MV phenotypes between MH and MuH subjects.
In the present study, the concentration of PS+ MVs was found to decrease significantly following bariatric surgery. In contrast to the present study, Witczak et al. described that the total number of extracellular vesicles were unaltered following bariatric surgery [31]. However, this discrepancy could be due to different pre-analytical methodology and different methods for characterising EVs, which might result in a different subset of EVs being analysed. It has previously been established that platelet MVs are the most abundant phenotype of MVs present in plasma [32], and that platelet MVs are up-regulated in obese subjects [33, 34]. Levels of platelet MVs seem to correlate with body composition [33], plasma lipids [35], and hyperglycaemia [36], all attributing to oxidative stress [37]. These parameters also seem to affect several other MV phenotypes including leukocyte MVs [38, 39], EMVs [33], and MMVs [35]. Thus, it can be inferred that the improvements observed in body composition, plasma lipid profile, and insulin sensitivity result in decreased activation of platelets, leukocytes, vascular endothelial cells, and monocytes/macrophages, which in turn could explain the significant reduction in PS+ MVs.
An interesting observation in the present study was that CD36+ MVs did not differ significantly from baseline to the three-month follow-up. This result is in contrast to a study conducted by Campello et al., in which CD36+ MVs decreased significantly from baseline to three and twelve-months follow-up visits [39]. Interestingly, although CD36+ MVs were unaltered, a significant decrease was seen in sCD36 in the present cohort. Levels of sCD36 have previously been suggested to reflect those of tissue expression [15] and further associated with unhealthy fat distribution [14], insulin resistance [15], and hepatic fat accumulation [16], all complications of obesity. It can thus be argued that sCD36 might be a more sensitive marker for cardio-metabolic complications than MV-associated CD36.
Bariatric surgery led to reductions of MMVs and CD36+ MMVs in the present study. In addition to demonstrating that bariatric surgery reduces MMVs, Cheng et al. also demonstrated that MMVs were associated with BMI and HbA1c [40]. CD36+ MMVs were further demonstrated to correlate with BMI, waist circumference, total fat mass, triglycerides, and fasting levels of C-peptide and insulin in another study [35]. A growing body of evidence is starting to recognise that macrophages play a central role in the development of insulin resistance and T2DM (reviewed in [41]). In obese individuals, steatotic cells secrete chemokines that promote migration of monocytes into tissues and their subsequent polarisation into a more pro-inflammatory phenotype [42, 43]. Apart from this, a growing body of evidence has implicated CD36 on the surface of macrophages and its ability to interact with oxidised LDL cholesterol in the pro-inflammatory polarisation of macrophages [22]. Moreover, the expression of CD36 on the surface of macrophages is increased in obese individuals [44]. Thus, it is possible that the significant weight loss observed following bariatric surgery and the concurrent improvements in cardio-metabolic health leads to decreased recruitment of monocytes to steatotic tissues, increased proportion of anti-inflammatory macrophages, and overall decreased activity in macrophages. This could in turn lead to a reduction in the release of MMVs and CD36+ MMVs.
EMVs and CD36+ EMVs were found to be significantly reduced following bariatric surgery in the current study. Studies that have previously examined the impact of bariatric surgery on EMV concentrations have yielded conflicting results. In line with the present study, both Campello et al. [39] and Cheng et al. [40] reported that levels of various phenotypes of MVs including platelet MVs, EMVs and MMVs decreased significantly in obese subjects after bariatric surgery. Conversely, Stepanian et al. [34] reported no differences in platelet MVs and EMVs one year after subjects had undergone bariatric surgery, which was later supported by a study conducted by Witczak et al. [31]. However, a growing body of evidence is in support of there being a relationship between EMVs and obesity, MetSy, and T2DM [33, 45–48]. Ectopic fat deposition and hyperglycaemia have long been known to contribute to endothelial dysfunction and subsequent atherosclerosis by means of dysregulation of circulating lipids [49]. A common feature of endothelial dysfunction is the production of ROS resulting from altered intracellular metabolism [50], which in turn leads to mitochondrial fragmentation, increased endothelial permeability, and up-regulation of adhesion molecules and pro-inflammatory cytokines [51]. In turn, this promote the attachment, migration and polarisation of monocytes into underlying tissues and a subsequent inflammatory response. Some doubt has to be cast on the concentrations of EMV sub-phenotypes due to similar binding patterns of the EMV antibody and its isotype control utilised in the current assay (Additional file 2: Figure S1). It can therefore not be ruled out that the current results could have arisen from non-specific binding of the antibody and subsequent inclusion of false positive events. However, the stark differences between baseline and the three-month follow-up visit are striking nonetheless.
Somewhat controversially, baseline concentrations of MV phenotypes did not differ between subjects with and without MetSy. This result is in support of observations by Stepanian et al. [34], who also observed no differences in levels of platelet MVs and EMVs between metabolically healthy subjects and subjects with MetSy. On the other hand, Chironi et al. [38] reported that leukocyte MVs were higher in individuals with MetSy in a non-obese cohort, however EMVs did not differ between groups in their study. Conversely, Amabile et al. [47] and Arteaga et al. [52] demonstrated that several phenotypes of EMVs were associated with the MetSy and correlated with number of components of MetSy. Although it was hypothesised that MuH subjects had increased levels of the investigated MV phenotypes, the authors recognise that several confounding factors might have influenced the results of the present study including BMI and body composition. To a certain extent explain, this might also differences observed between studies, as mean BMI differs greatly between studies.
A significant strength of the current study is that it employs flow cytometry to quantify and characterise MVs from subjects undergoing bariatric surgery. Although conventional flow cytometry lacks the sensitivity to measure the smallest of extracellular vesicles [53], it remains a preferred method of characterising MVs and holds great advantages over a multitude of other methods for characterising and quantifying MVs including nano-particle tacking analysis, Western blot, and ELISA. This is due to its ability to simultaneously quantify MVs and characterise their expression of multiple surface markers in a high-throughput manner, thus allowing for discrimination between large numbers of MVs with different phenotypic origins [26, 54–56]. Several limitations should, however, also be addressed in the present study. First, the authors recognise that the sample size in the present study is a limitation, which could give the study insignificant power to discover significant differences in baseline concentrations of MVs between MH and MuH subjects. In addition, this could explain the lack of correlations between changes of MV phenotypes on the one hand and body composition, plasma lipids, insulin sensitivity, and inflammatory markers on the other (data not shown). Second, the follow-up in this study is relatively short, and it is therefore impossible to make inferences about the long-term effects of bariatric surgery on levels of circulating MVs. Third, due to constraints, isotype controls were prepared for baseline samples only, thus limiting the interpretability of flow cytometric characterisation of MVs in samples from follow-up visits, as the extent of non-specific binding of antibodies to MVs cannot be examined. Nonetheless, the magnitude of decrease in MV concentrations from baseline to follow-up would arguably imply that the importance of this is minimal if not insignificant.
Conclusion
In conclusion, this study reports that concentrations of MVs and specifically those of monocyte and endothelial origin decrease significantly in the follow-up period following bariatric surgery. Moreover, the concentration of the bulk of CD36+ MVs remained unaltered, while significant decreases are seen in sCD36, CD36+ MMVs, and CD36+ EMVs. These changes likely reflect the significant weight loss incurred by the subjects following bariatric surgery, which in turn leads to reduction in ectopic deposition of fat, improved plasma lipid profile, decreased low-grade inflammation, and oxidative stress. At the cellular level, decreased amount of lipids in the extracellular space reduce CD36-specific uptake of lipids by endothelial cells and macrophages, which in turn reduces cell stress and CD36 expression on the cell membrane, thereby resulting in decreased shedding of MVs. However, baseline levels of MV phenotypes did not differ between subjects with MetSy and those without MetSy.
Finally, in order to further investigate the potential of MMVs, EMVs and their respective phenotypes positive for the expression of CD36, larger studies have to be conducted in which associations between their concentration and components of the metabolic syndrome and type 2 diabetes are thoroughly investigated.
Additional files
Supplementary methodological information on MV characterisation by flow cytometry and antibody panels used in this study. Table S1. Antibodies and concentrations used for flow cytometric characterisation of MVs. Table S2. Baseline and three-month follow-up concentrations of MV phenotypes in study participants. Table S3. Characteristics of the metabolically healthy and metabolically unhealthy participants. (DOCX 25 kb)
Figure S1. Typical scatter plots for the two different antibody panels used to characterise MV phenotypes. (EPS 1189 kb)
Acknowledgements
The authors would like to thank Dr. Charlotte Christie Petersen and the staff at the FACS Core Facility at the Institute for Biomedicine, Aarhus University for their expert assistance with setting up and performing flow cytometric analysis of all samples.
Funding
The study was supported by a grant from The Novo Nordisk Foundation (NNF13OC0007713). None of the funding bodies had any role in the design or conduct of this study nor in the preparation of this manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study as well as analysis scripts are available from the corresponding author on reasonable request.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
Body mass index
- CD
Cluster of differentiation
- EMV
Endothelial-derived microvesicle
- EV
Extracellular vesicle
- HDL
High density lipoprotein
- HOMA-%S
homeostasis model assessment of sensitivity
- hsCRP
Highly sensitive C-reactive protein
- IQR
Inter-quartile range
- LDL
Low density lipoprotein
- LF%
liver fat percentage
- MetSy
Metabolic syndrome
- MH
Metabolically healthy
- MMV
Monocyte-derived microvesicle
- MuH
Metabolically unhealthy
- MV
Microvesicle
- NAFLD
Non-alcoholic fatty liver disease
- PS
phosphatidylserine
- sCD36
Soluble CD36
- T2DM
Type 2 diabetes mellitus
Authors’ contributions
HV supervised the collection of patient samples and anthropometric/body composition data at baseline and follow-up. MHC, MHN and AH were all major contributors in designing the present study. MHC and MHN designed the antibody panels and conducted flow cytometry experiments. JB analysed all data and was the major contributor in writing the manuscript. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
All procedures performed in the present study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. The Danish Data Protection Agency (id. 00908 HEK.750.86–4) and The Ethics Committee in the Capital Region of Denmark, Committee E (id. H-3-2009-100) approved the present study, and all participants gave informed written consent prior to inclusion.
Consent for publication
Not applicable.
Competing interests
Dr. Handberg reports grants from The Novo Nordisk Insulin Foundation during the conduct of the study. In addition, Dr. Handberg has a patent WO2005/116644 issued. All other authors declare that they have no competing interest pertaining to the current study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaco Botha, Phone: (+45) 6165 7004, Email: j.botha@rn.dk.
Morten Hjuler Nielsen, Email: mohn@rn.dk.
Maja Høegh Christensen, Email: majahoegh@gmail.com.
Henrik Vestergaard, Email: henrik.vestergaard@sund.ku.dk.
Aase Handberg, Email: aaha@rn.dk.
References
- 1.Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369:145–54 Available from: http://www.nejm.org/doi/10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed]
- 2.Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, et al. Meta-analysis: pharmacologic treatment of obesity. Ann. Intern. Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Journal of Internal Medicine. 2013;273(3):219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 4.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen Stine Brinkløv, Rathcke Camilla Noelle, Jørgensen Nils Bruun, Madsbad Sten, Vestergaard Henrik. Effects of Roux-en-Y Gastric Bypass on Fasting and Postprandial Levels of the Inflammatory Markers YKL-40 and MCP-1 in Patients with Type 2 Diabetes and Glucose Tolerant Subjects. Journal of Obesity. 2013;2013:1–10. doi: 10.1155/2013/361781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 8.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and Endoluminal procedures: IFSO worldwide survey 2014. Obes. Surg. 2017;27:2279–2289. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agouni A, Ducluzeau P-H, Benameur T, Faure S, Sladkova M, Duluc L, et al. Microparticles from patients with metabolic syndrome induce vascular hypo-reactivity via Fas/Fas-ligand pathway in mice. Reitsma PH, editor. PLoS One. 2011;6:e27809. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3217000&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 10.Kurtzman N, Zhang L, French B, Jonas R, Bantly A, Rogers WT, et al. Personalized cytomic assessment of vascular health: evaluation of the vascular health profile in diabetes mellitus. Cytometry B. Clin. Cytom. 2013;84:255–266. doi: 10.1002/cyto.b.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hjuler Nielsen M, Irvine H, Vedel S, Raungaard B, Beck-Nielsen H, Handberg A. Elevated atherosclerosis-related gene expression, monocyte activation and microparticle-release are related to increased lipoprotein-associated oxidative stress in familial hypercholesterolemia. Boissonnas A, editor. PLoS One . 2015;10:e0121516. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4395270&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 12.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 14.Knøsgaard L, Thomsen SB, Støckel M, Vestergaard H, Handberg A. Circulating sCD36 is associated with unhealthy fat distribution and elevated circulating triglycerides in morbidly obese individuals. Nutr. Diabetes. 2014;4:e114. doi: 10.1038/nutd.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handberg A, Levin K, Højlund K, Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006;114:1169–1176. doi: 10.1161/CIRCULATIONAHA.106.626135. [DOI] [PubMed] [Google Scholar]
- 16.Heebøll S, Poulsen MK, Ornstrup MJ, Kjær TN, Pedersen SB, Nielsen S, et al. Circulating sCD36 levels in patients with non-alcoholic fatty liver disease and controls. Int J Obes (Lond) [Internet] Nature Publishing Group. 2017;41:262–267. doi: 10.1038/ijo.2016.223. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen M. H., Irvine H., Vedel S., Raungaard B., Beck-Nielsen H., Handberg A. The Impact of Lipoprotein-Associated Oxidative Stress on Cell-Specific Microvesicle Release in Patients with Familial Hypercholesterolemia. Oxidative Medicine and Cellular Longevity. 2016;2016:1–8. doi: 10.1155/2016/2492858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A, Murugesan G, Chen K, Zhang L, Wang Q, Febbraio M, et al. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011;117:6355–6366. doi: 10.1182/blood-2011-02-338582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IOCM V, van Klinken JB, van Diepen JA, van den Berg SAA, Febbraio M, LKM S, et al. CD36 is important for adipocyte recruitment and affects lipolysis. Obesity (Silver Spring). 2013;21:2037–2045. doi: 10.1002/oby.20354. [DOI] [PubMed] [Google Scholar]
- 20.Adachi H, Tsujimoto M. Endothelial scavenger receptors. Prog. Lipid Res. [Internet]. England. 2006;45:379–404. doi: 10.1016/j.plipres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Corpeleijn E, Pelsers MMAL, Soenen S, Mensink M, Bouwman FG, Kooi ME, et al. Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. J. Physiol. Pharmacol. [internet]. 2008;59:77–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22584574. [PubMed]
- 22.Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metab. Syndr. Relat. Disord. 2011;9:239–245. doi: 10.1089/met.2011.0003. [DOI] [PubMed] [Google Scholar]
- 23.Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Söderström I, Edlund H. Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes Hepatosteatosis and hepatic insulin resistance. J Biol Chem. 2015;290:19034–19043. doi: 10.1074/jbc.M115.640292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. Nature Publishing Group. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 25.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. Elsevier Inc. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J. Extracell. vesicles [Internet]. 2014;3:1–12. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3916676&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 27.Dragulescu AA. xlsx: Read, write, format Excel 2007 and Excel 97/2000/XP/2003 files [Internet]. 2014. Available from: https://cran.r-project.org/package=xlsx
- 28.Wickham H. ggplot2: Elegant Graphics for Data Analysis [Internet]. Springer-Verlag New York; 2009. Available from: http://ggplot2.org
- 29.Wickham H. Reshaping Data with the reshape Package. J. Stat. Softw. [Internet]. 2007;21. Available from: http://www.jstatsoft.org/v21/i12
- 30.KGMM A, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 31.Witczak JK, Min T, Prior SL, Stephens JW, James PE, Rees A. Bariatric surgery is accompanied by changes in extracellular vesicle-associated and plasma fatty acid binding protein 4. Obes Surg. 2017:1–8. Available from: https://link.springer.com/article/10.1007/s11695-017-2879-z. [DOI] [PubMed]
- 32.Berckmans RJ, Nieuwland R, Böing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. doi: 10.1055/s-0037-1615646. [DOI] [PubMed] [Google Scholar]
- 33.Helal O, Defoort C, Robert S, Marin C, Lesavre N, Lopez-Miranda J, et al. Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2011;21:665–671. doi: 10.1016/j.numecd.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L, et al. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring) 2013;21:2236–2243. doi: 10.1002/oby.20365. [DOI] [PubMed] [Google Scholar]
- 35.Botha J, Velling Magnussen L, Nielsen MH, Nielsen TB, Højlund K, Andersen MS, et al. Microvesicles correlated with components of metabolic syndrome in men with type 2 diabetes mellitus and lowered testosterone levels but were unaltered by testosterone therapy. J Diabetes Res. 2017;2017:1–9. doi: 10.1155/2017/4257875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamant M. Elevated Numbers of Tissue-Factor Exposing Microparticles Correlate With Components of the Metabolic Syndrome in Uncomplicated Type 2 Diabetes Mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.CIR.0000036596.59665.C6. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa Shigetada, Fujita Takuya, Shimabukuro Michio, Iwaki Masanori, Yamada Yukio, Nakajima Yoshimitsu, Nakayama Osamu, Makishima Makoto, Matsuda Morihiro, Shimomura Iichiro. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chironi Gilles, Simon Alain, Hugel Bénédicte, Del Pino Muriel, Gariepy Jérôme, Freyssinet Jean-Marie, Tedgui Alain. Circulating Leukocyte-Derived Microparticles Predict Subclinical Atherosclerosis Burden in Asymptomatic Subjects. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(12):2775–2780. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- 39.Campello E, Zabeo E, Radu CM, Spiezia L, Foletto M, Prevedello L, et al. Dynamics of circulating microparticles in obesity after weight loss. Intern Emerg Med. 2016;11:695–702. doi: 10.1007/s11739-016-1397-7. [DOI] [PubMed] [Google Scholar]
- 40.Cheng V, Kashyap SR, Schauer PR, Kirwan JP, McCrae KR. Restoration of glycemic control in patients with type 2 diabetes mellitus after bariatric surgery is associated with reduction in microparticles. Surg. Obes. Relat. Dis. Elsevier Inc. 2013;9:207–212. doi: 10.1016/j.soard.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castoldi A, Naffah de Souza C, Câmara NOS, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol [Internet] 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 43.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity Elsevier Inc. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Kashyap SR, Ioachimescu AG, Gornik HL, Gopan T, Davidson MB, Makdissi A, et al. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity (Silver Spring). Nature Publishing Group. 2009;17:2142–2148. doi: 10.1038/oby.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab. 2006;91:3676–3679. doi: 10.1210/jc.2006-0851. [DOI] [PubMed] [Google Scholar]
- 46.Tramontano a F, Lyubarova R, Tsiakos J, Palaia T, Deleon JR, Ragolia L. Circulating endothelial microparticles in diabetes mellitus. Mediators Inflamm. 2010;2010:250476. doi: 10.1155/2010/250476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amabile N, Cheng S, Renard JM, Larson MG, Ghorbani A, McCabe E, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014;35:2972–2979. doi: 10.1093/eurheartj/ehu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J Pathol. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tchernof A, Despres J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 50.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y, Jiang L-LI, Maimaitirexiati X-MZY, Zhang Y, Wu L. Irbesartan attenuates TNF-α-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-κB pathway in HUVECs. Eur. Rev. med. Pharmacol. Sci. 2015;19:3295–3302. [PubMed] [Google Scholar]
- 52.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am. J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 53.Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 54.Arraud N, Gounou C, Linares R, Brisson AR. A simple flow cytometry method improves the detection of phosphatidylserine-exposing extracellular vesicles. J Thromb Haemost. 2015;13:237–247. doi: 10.1111/jth.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arraud N, Gounou C, Turpin D, Brisson AR. Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytometry. 2016;89:184–195. doi: 10.1002/cyto.a.22669. [DOI] [PubMed] [Google Scholar]
- 56.Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J. Extracell. vesicles 2015;4:25530. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4382613&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methodological information on MV characterisation by flow cytometry and antibody panels used in this study. Table S1. Antibodies and concentrations used for flow cytometric characterisation of MVs. Table S2. Baseline and three-month follow-up concentrations of MV phenotypes in study participants. Table S3. Characteristics of the metabolically healthy and metabolically unhealthy participants. (DOCX 25 kb)
Figure S1. Typical scatter plots for the two different antibody panels used to characterise MV phenotypes. (EPS 1189 kb)
Data Availability Statement
The datasets used and/or analysed during the current study as well as analysis scripts are available from the corresponding author on reasonable request.