Abstract
Introduction:
Myiasis, tissue infestation by housefly larvae, is commonly found in malignant fungating wounds of cancer patients from climatic condition and lower socio-economic strata.
Aim of study:
It was aimed to study the effectiveness of systemic Ivermectin, Albendazole& Clindamycin (Triple Therapy) in reducing signs & symptoms associated with maggots in malignant head and neck wounds.
Method:
25 adult, advanced head and neck cancer patients presenting with maggots either from wound, oral cavity or nostril, with ECOG score 3 or less were enrolled in this study. Symptoms were assessed using Edmonton Symptom Assessment Scale (ESAS) and wound by Wound Assessment Tool – Hospice, at baseline and then Days 1, 3, 5, and 7. All patients received 3 days course of oral Ivermectin 12 mg per day, Albendazole 400 mg twice per day and Clindamycin 300 mg three times per day for 5 days along with Terpentine oil dressing. All patients received oral Morphine as per their pain score.
Results:
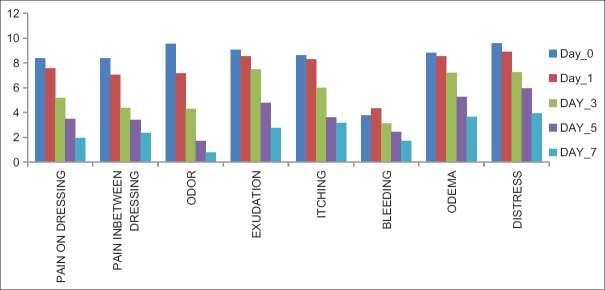
Mean age (yrs) and weight (Kg) were 42.15 ± 8.23 and 52.31 ± 5.18 respectively. 84% patients were male. Mean oral morphine dose was 100.38 mg. There was significant decrease in number of maggots from day 0 (77.28 ± 13.465) to day 1 (20.60 ± 7.263; 73.34% reduction) to day 3 (1.52 ± 2.104; 92.62% reduction). We found statistically significant improvement (P = <0.05) in scores of wound and all other related symptoms on days 1, 3, 5 & 7, except bleeding, edema, nausea, anxiety, appetite loss and feeling of wellbeing, which remained same on Day 1, but improved afterward. Side effects were self-limiting.
Conclusion:
Systemic treatment with Ivermectin, Albendazole and Clindamycin (Triple Therapy) enhances the removal of maggots, early recovery and relief from distress and associated symptoms.
Keywords: Head-and-neck cancer, maggots, malignant wound, myiasis, opioid
INTRODUCTION
Myiasis is a pathological condition caused by the presence of larvae of houseflies in human or animal tissues that evolve to a parasite.[1] It has been reported in malignant fungating wound,[2] gingiva,[3] oral cavity,[1,4] invasive ductal carcinoma of the breast,[5] and cutaneous lesions,[6,7] Tracheostomy,[8] and internal organs such as esophagus and urinary track.[9]
It is frequently found in tropical climate of India.[5] Low socioeconomic status, unhygienic living conditions, immunocompromised state, with fungating lesions, having poor access to health-care facility, and lack of knowledge are predisposing factors. It is commonly seen in patients of head-and-neck cancer in advanced, incurable stage with malignant fungating wound. These patients report to our center, with extensive myiasis of wound, oral cavity, or nasal cavity. It is found to be associated with symptoms such as pain, discharge, odor, swelling, bleeding, and psychosocial issues, which needs assessment, nursing care, and treatment. The presence of maggots in wound often leads to extreme distress to patients and their caregivers.
Treatment of myiasis comprises systemic and local measures. Many agents such as oil of turpentine, mineral oil, ether, chloroform, ethyl chloride, mercuric chloride, creosote, saline, phenol, calomel, olive oil, iodoform applied locally followed by manual removal, or surgical debridement can be used for the removal of maggots.[3] Fewer studies have proved effectiveness of systemic ivermectin[10] and albendazole[6,11] in the treatment of myiasis. Any secondary infection is treated with antibiotics.
This study was aimed to study the effectiveness of systemic ivermectin, albendazole, and clindamycin (triple therapy) in reducing signs and symptoms associated with maggots in the wounds of head-and-neck cancer patients. It was also aimed to assess the associated symptoms of advanced disease in patients. Side effects and other symptoms were treated with appropriate supportive care which was aimed to relieve their suffering.
METHODOLOGY
This single-center, prospective observational study was conducted on 25 adult, advanced head-and-neck cancer patients who attended outpatients' clinic of the Department of Palliative Medicine at a tertiary cancer center in India.
Patients presented with a chief complaint of maggots coming out either from wound, oral cavity, or nostril. These patients were admitted to hospice for wound care and symptom control.
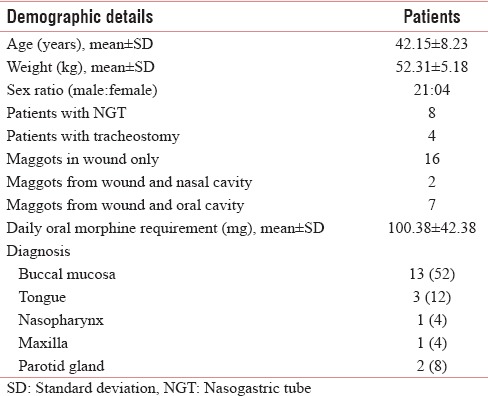
In hospice, patients stayed with a caregiver and were attended by the palliative care team that consists of physician, nurses, nursing students, and counselor. Patients were evaluated for stage of the disease, treatment received, and their performance status. The site of maggots was noted as few patients had more than one site for maggot infestation. Many patients had tracheostomy or/and nasogastric tube (NGT) in place. Patients unable to swallow liquids were inserted with NGT before starting the care. Patients with Eastern Cooperative Oncology Group (ECOG) score 3 or less and able to take at least liquids with or without NGT were included in the study.
BSc nursing students, who were trained in wound assessment and management, were allotted one patient at a time. The students helped patients and caregivers to complete the wound assessment tool (WAT) describing wound-related symptoms. A simple tool, WAT-hospice, is designed by our team, keeping socioeconomic, cultural, and educational background of our patients in mind. The Edmonton Symptom Assessment System (ESAS) tool was used to assess symptom profile in these patients. ESAS consists of symptoms common in cancer patients: pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being and shortness of breath, and “other problems.” WAT and ESAS scored from 0 to 10 with “0” meaning no symptom at all, while “10” suggesting worst possible symptom ever experienced. These tools were translated in local language, i.e., Gujarati for better patient understanding. These assessment tools were completed by caregiver and patient themselves, with the help of assigned nursing student, everyday morning, before wound dressing. Patients were assessed at baseline and followed up every alternate days, i.e., days 1, 3, 5, and 7 of the treatment. The same nursing student assisted the same patient for assessment throughout the study period of 7 days. They were unaware of treatment regimen for myiasis.
All patients received standard drug regimen for treating myiasis, which is as follows:
Tablet ivermectin 12 mg per day for 3 days
Tablet albendazole 400 mg twice per day for 3 days
Tablet clindamycin 300 mg three times per day for 5 days.
Wound was irrigated with turpentine oil twice a day and maggots were manually removed with the help of tweezers, without rupture of larvae as it may cause foreign body reaction.[4] Pallets soaked in diluted turpentine oil were inserted in nostrils for few minutes in patients who had maggots coming out from nostrils. Even dead larvae were removed because it can cause local inflammation[5] and foreign body granuloma formation.[12] Wounds were kept clean, dry, and covered till all maggots were removed. Metronidazole ointment dressing was started only after the removal of all maggots.
Most of the patients were on tablet morphine IR or CR for pain management. Morphine dose was revised and all were put on tablet morphine 10 mg IR as per visual analog scale score. Other symptoms were controlled with supportive medications. Oral care, general hygiene, nutrition, and other issues were also addressed. Patients were encouraged to do gargle with metronidazole solution and betadine mouthwash, alternatively, every 4 h.
Wound assessment, total number of maggots removed over previous 24 h, and ESAS were noted on days 0, 1, 3, 5, and 7. ECOG scoring was done on arrival and on day 7. Other treatment-related side effects such as diarrhea, headache, nausea, and vomiting were noted and treated.
Results of days 1, 3, 5, and 7 were compared with the baseline records of day 0, i.e., the day of arrival. The results were subjected to statistical analysis using Student's t-test. Statistical analysis was done using IBM Software SPSS version 22.0, (Chicago, USA).
RESULTS
All patients belonged to middle age ranged from 29 to 60 years. Mean age (in years) and weight (in kg) were 42.15 ± 8.23 and 52.31 ± 5.18, respectively. There were 4 female (16%) and 21 male (84%) patients. Eight had NGT and four patients had tracheostomy in situ. Two patients had maggots in wound and nasal cavity and seven patients of buccal mucosa had maggots from wound as well as from oral cavity. All patients received oral morphine (10 mg), and dose ranged from 60 to 180 mg/day with mean ± standard deviation (SD) dose (in mg) of 100.38 ± 42.38. Table 1 shows baseline characteristics of study participants.
Table 1.
Baseline characteristics of study participants
There was a significant improvement in pain on dressing, pain in between dressing, odor, exudation, and itching, from day 1 onward. Bleeding and edema around the wound increased or remained same on day 1. The mean number of maggots was (mean ± SD) 77.28 ± 13.465 on day 0, which reduced to 20.60 ± 7.263 (73.34%) on day 1, and 1.52 ± 2.104 (92.62%) on day 3. None of the patients had maggots from day 5 onward [Table 2 and Figure 1].
Table 2.
Wound assessment score-hospice
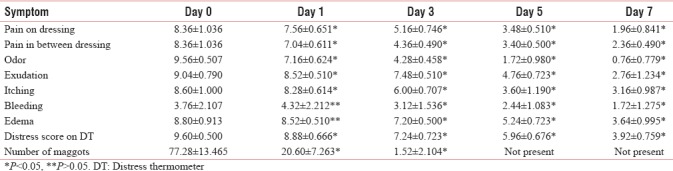
Figure 1.
Comparison of symptom profile of wound assessment tool-hospice
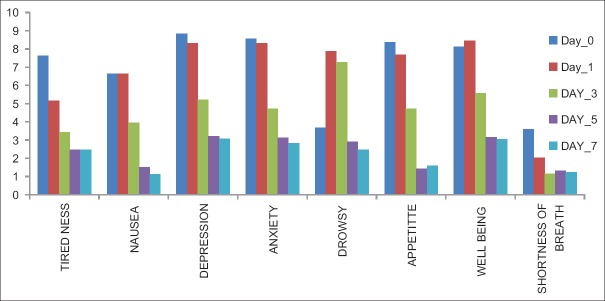
Common symptoms such as overall pain, tiredness, depression, anxiety, and shortness of breath improved significantly (P < 0.05) on days 1, 3, 5, and 7. Nausea, anxiety, appetite, and feeling of well-being remained same on day 1 (P > 0.05), which improved from day 3 onward [Table 3 and Figure 2].
Table 3.
Eastern Cooperative Oncology Group score
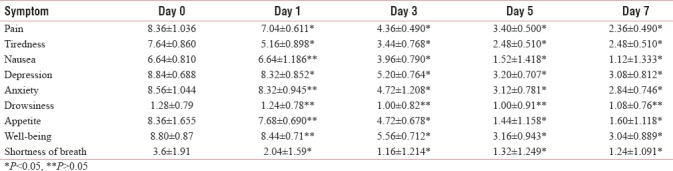
Figure 2.
Comparison of symptom profile of Edmonton Symptom Assessment System
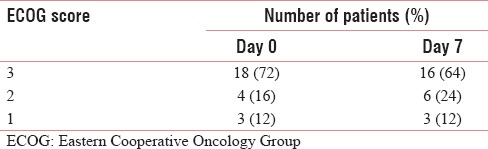
Adverse effects were seen in study participants in the form of diarrhea in 7 patients, headache in 10 patients, and giddiness in 5 patients [Table 4]. ECOG score improved in 2 patients from 3 to 2 on day 7 of the admission [Table 5].
Table 4.
Adverse effects
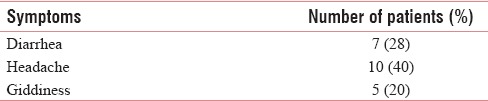
Table 5.
Eastern Cooperative Oncology Group score
DISCUSSION
Myiasis is a term derived from the Greek word “myia,” meaning invasion of vital tissue of humans or other mammals by fly larvae.[13] Hope[14] described the first incidence of this parasitosis in 1840. Cases of myiasis have been reported from tropical countries such as India,[5] Nepal,[15] and Sri Lanka.[12,16] Hot and humid climate provides favorable environment for growth of house fly, which lays eggs in the dead and decaying tissues of malignant fungating wounds.
As per the National Cancer Registry, Population Based Cancer Registry (PBCR) 2012-2014, incidence of mouth cancer is highest in Ahmedabad.[17] We have highest number of head-and-neck cancer patients, having malignant fungating wounds, and few of them are infested with maggots. Hence, this study was taken up for the treatment of myiasis in advanced head-and-neck cancer patients.
Many WATs have been described and evaluated for nurses' needs.[18] The study of Greatrex-White and Moxey[18] has shown that of 14 selected WATs, no tool was identified which fulfilled all the criteria. Only the Applied Wound Management and National Wound Assessment Form met most of nurses' needs in carrying out wound assessment. Our team at hospice has designed a simple, WAT-hospice, which was used for wound assessment. Most of our patients and caregivers are illiterate. Hence, BSc nursing students posted for hospice training were assigned a patient to help the patient and caregiver in wound assessment and for filling up the form everyday morning before the change of dressing. They also assessed the common symptoms, using ESAS scale.[19]
The standard treatment for myiasis is the manual removal, associated or not with topical and systemic asphyxiating drugs that force the larvae to come out.[10] Various local agents recommended are turpentine, mineral oil, ether, chloroform, ethyl chloride, mercuric chloride, creosote, saline, phenol, calomel, olive oil, and iodoform, but they have controversial results. In this study, wound was flushed with turpentine oil or pallets soaked in turpentine oil as it irritates the maggots causing larval asphyxia and forcing them out of their hiding place.[3]
All patients received common regimen of ivermectin, albendazole, and clindamycin, orally. In 1993, ivermectin has been reported to be safe for human use[20] and usually well tolerated in dose of 15–200 mg/kg.[21] Ivermectin, a macrolide that is activated by gamma-aminobutyric acid liberation, leads to parasitic death and their spontaneous elimination by washing out larvae and has been found out to be effective in humans.[22,23]
Ivermectin is effective in the treatment of several myiasis and it is a good alternative when surgical removal is unfeasible.[24] It is recommended to give repeatedly till the site is cleared off all maggots.[6] Hence, we gave ivermectin for 3 consecutive days. Even topical treatment with ivermectin has been used for myiasis.[25]
All patients received tablet albendazole 400 mg, given twice a day for 3 days. Albendazole is a broad-spectrum antihelminthic-antiparasitic agent and has been used for treatment of intestinal myiasis[11] and cutaneous myiasis.[6] All patients in this study were from rural area or slum area where soil contamination of wound, clothes, and other parts of body is very likely. Few of them had larvae in nasal and oral cavity. There is a possibility that they might have engulfed parasites leading to intestinal infestation. Hence, it was justified to give antihelminthic agent with other treatment. It has also been reported that deworming treatment might mobilize parasites toward the body surface, allowing them to be surgically removed.[6]
Malignant fungating wound is likely to have anaerobic environment and ideal for growth of house fly larvae. This secondary infection requires treatment with antibiotics. Clindamycin is used primarily to treat anaerobic infections caused by susceptible anaerobic bacteria and may be used to treat infections caused by susceptible aerobic bacteria as well.[26] Other studies have reported using augmentin and metronidazole,[2] polymyxin B,[5] and cefazolin and metronidazole.[27] The common side effect of clindamycin, like any other antibiotics, is diarrhea. Seven patients in the study had diarrhea, which was treated with probiotics.
Patients were submitted to local treatment and systemic regimen immediately after admission to hospice. Number of maggots removed decreased by 73.34% on day 1 and 92.62% on day 3. All patients' wound, nasal cavity, and oral cavity were clear of maggots from day 4 onward. There was significant improvement in wound-related symptoms on day 1 onward. Studies have reported that symptomatic improvement occurs immediately with the removal of the larvae.[28] Ivermectin given orally in just one dose of 150–200 mcg/kg body weight and repeated after 24 h has been reported to be effective in severe cases.[29] Shinohara et al.[10] reported a moderate success when treating oral myiasis with ivermectin 6 mg given orally and repeated after 24 h. Some authors have suggested that the treatment should start with doses of up to 300 mcg/kg, that is, for patients from 40 to 60 kg – 2 tablets (12 mg) are indicated and 3 tablets (18 mg) should be used for patients weighing from 60 to 90 kg.[27]
There was no improvement in bleeding from the wound and edema around wound on day 1. This may be because of manual removal of maggots with tweezers. Bleeding and edema improved on days 3, 5, and 7 when the wound was clear of maggots. There was a significant improvement in odor and exudation as clindamycin helped in reducing the anaerobic infection in the wound.
ESAS is designed to assist in the assessment of nine symptoms common in cancer patients: pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath (there is also a line labeled “other problem”). There was a significant improvement in score of all the symptoms on days 1, 3, 5, and 7, except nausea, anxiety, appetite, and feeling of well-being, which remained same on day 1, but improved afterward.
There was improvement in pain, depression, and tiredness from day 1 onward in all the patients. Seven patients had diarrhea, which responded to treatment of probiotics. Ten patients had headache, which was treated with nonsteroidal anti-inflammatory drugs. Five patients complained of giddiness, which was self-limiting. This suggests that the local and systemic treatment given for maggots was effective and well tolerated by patients. There was no need to increase the dose of oral morphine during study period.
Systemic treatment with ivermectin, albendazole, and clindamycin helped to flush out the maggots within 3 days. It helped to relieve the associated symptoms and improved the overall well-being by improvement in depression, distress, and tiredness in all the patients. These drugs were very well tolerated by cancer patients and side effects were self-limiting. This regimen is recommended for treatment of myiasis in cancer patients.
CONCLUSION
Our study suggests role of regular assessment of wound and its related symptoms in head-and-neck cancer patients. This study also showed that systemic treatment with ivermectin, albendazole, and clindamycin (triple therapy) along with turpentine oil dressing enhances the removal of maggots, early recovery and relief from distress, and other associated symptoms. However, more robust studies are required to establish effectiveness of triple therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rey L. Parasitologia. 2nd ed. Rio de Janeiro: Editora Guanabara Koogan; 1991. [Google Scholar]
- 2.Biradar S, Wankhede P, Munde A, Shaikh S. Extensive myiasis infestation associated with oral squamous cell carcinoma: Report of two cases. Dent Res J (Isfahan) 2015;12:100–5. doi: 10.4103/1735-3327.150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SL, Manuel S, John TV, Sivan MP. Extensive gingival myiasis – Diagnosis, treatment, and prevention. J Oral Maxillofac Pathol. 2011;15:340–3. doi: 10.4103/0973-029X.86715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhagawati BT, Gupta M, Singh S. Oral myiasis: A rare entity. Eur J Gen Dent. 2013;2:312–4. [Google Scholar]
- 5.Kumar N, Parameshwaran Nair R, Sinha A, Kumar A. Myiasis in a case of invasive ductal carcinoma breast – A rare Presentation. Med Res Chron. 2014;1:208–212. [Google Scholar]
- 6.Puente S, Otranto D, Panadero R, Herrero MD, Rivas P, Ramírez-Olivencia G, et al. First diagnosis of an imported human myiasis caused by Hypoderma sinense (Diptera: Oestridae), detected in a European traveler returning from India. J Travel Med. 2010;17:419–23. doi: 10.1111/j.1708-8305.2010.00464.x. [DOI] [PubMed] [Google Scholar]
- 7.Caissie R, Beaulieu F, Giroux M, Berthod F, Landry PE. Cutaneous myiasis: Diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560–8. doi: 10.1016/j.joms.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Blejter J. Tracheostomy Wound Myiasis in a Child: Case Report and Review of the Literature. 2012. [Last accessed on 2018 Jun 01]. p. 2. Article ID 317862. Available from: https://www.hindawi.com/journals/cripe/2012/317862/ [DOI] [PMC free article] [PubMed]
- 9.Demir AD, Iraz M, İpek DN. Urogenital myiasis caused by Psychoda albipennis in a child. Turk Pediatri Ars. 2015;50:65–8. doi: 10.5152./tpa.2015.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinohara EH, Martini MZ, de Oliveira Neto HG, Takahashi A. Oral myiasis treated with ivermectin: Case report. Braz Dent J. 2004;15:79–81. doi: 10.1590/s0103-64402004000100015. [DOI] [PubMed] [Google Scholar]
- 11.Shivekar S, Senthil K, Srinivasan R, Sureshbabu L, Chand P, Shanmugam J, et al. Intestinal myiasis caused by Muscina stabulans. Indian J Med Microbiol. 2008;26:83–5. doi: 10.4103/0255-0857.38869. [DOI] [PubMed] [Google Scholar]
- 12.Kumarasinghe SP, Karunaweera ND, Ihalamulla RL. A study of cutaneous myiasis in Sri Lanka. Int J Dermatol. 2000;39:689–94. doi: 10.1046/j.1365-4362.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- 13.Pereira T, Tamgadge AP, Chande MS, Bhalerao S, Tamgadge S. Oral myiasis. Contemp Clin Dent. 2010;1:275–6. doi: 10.4103/0976-237X.76401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hope FW. On insects and their larvae occasionally found in human body. Trans R Soc Entomol. 1840;2:256–71. [Google Scholar]
- 15.Yadav SK, Shrestha S, Sah AK. Extensive myiasis infestation over a malignant lesion in maxillofacial region: Report of cases. Int J Pharm Biol Arch. 2012;3:530–33. [Google Scholar]
- 16.Bayer HG. Myiasis maligna of nose and ears in Ceylon; recommendation of a new treatment. AMA Arch Otolaryngol. 1954;59:104–7. doi: 10.1001/archotol.1954.00710050116014. [DOI] [PubMed] [Google Scholar]
- 17. [Last accessed on 2018 Jun 01]. Available from: http://www.ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/ALL_CONTENT/PDF_Printed_Version/Chapter7_Printed.pdf .
- 18.Greatrex-White S, Moxey H. Wound assessment tools and nurses' needs: An evaluation study. Int Wound J. 2015;12:293–301. doi: 10.1111/iwj.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton symptom assessment system (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 20.Martin-Prevel Y, Cosnefroy JY, Tshipamba P, Ngari P, Chodakewitz JA, Pinder M, et al. Tolerance and efficacy of single high-dose ivermectin for the treatment of loiasis. Am J Trop Med Hyg. 1993;48:186–92. doi: 10.4269/ajtmh.1993.48.186. [DOI] [PubMed] [Google Scholar]
- 21.Rao GS, Chatra L, Prashanth SK. Oral myiasis: A rare entity. J Maxillofac Oral Surg. 2009;8:398–400. doi: 10.1007/s12663-009-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatt AP, Jayakrishnan A. Oral myiasis: A case report. Int J Paediatr Dent. 2000;10:67–70. doi: 10.1046/j.1365-263x.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu DB, Bhaskar H. Musca: The housefly. In: Sandhu DB, Bhaskar H, editors. Textbook of Intervertebrate Zoology. New Delhi: Campus; 2004. pp. 704–9. [Google Scholar]
- 24.Bolognia JL, Jorizzo JL, Rapini R. Dermatology. 2nd ed. Vol. 1. St Louis, MO: Mosby Elsevier; 2008. Cutaneous myiasis; pp. 1300–1. [Google Scholar]
- 25.Victoria J, Trujillo R, Barreto M. Myiasis: A successful treatment with topical ivermectin. Int J Dermatol. 1999;38:142–4. doi: 10.1046/j.1365-4362.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 26.Brook I, Lewis MA, Sándor GK, Jeffcoat M, Samaranayake LP, Vera Rojas J, et al. Clindamycin in dentistry: More than just effective prophylaxis for endocarditis? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:550–8. doi: 10.1016/j.tripleo.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 27.Gealh WC, Ferreira GM, Farah GJ, Teodoro U, Camarini ET. Treatment of oral myiasis caused by Cochliomyia hominivorax: Two cases treated with ivermectin. Br J Oral Maxillofac Surg. 2009;47:23–6. doi: 10.1016/j.bjoms.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Biswas S, McNerney P. Myiasis on a giant squamous cell carcinoma of the scalp: A Case report and review of relevant literature. World J Oncol. 2016;7:34–9. doi: 10.14740/wjon966w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar JS. Oral myiasis: A case report. Pac J Med Sci. 2012;10:47–50. [Google Scholar]