Abstract
Background and Objectives:
EUS guided core biopsy was once rarely performed but is now entering mainstream practice. Neuroendocrine tumors often warrant core biopsy as sufficient tissue must be obtained to allow for special staining to ensure a correct diagnosis. Traditionally these lesions were sampled with FNA needles. We performed a retrospective pilot study to evaluate the clinical value and efficacy of the a new EUS core needle biopsy needle as compared to a standard EUS FNA needle in the evaluation of patients with known or suspected neuroendocrine tumors.
Methods:
A retrospective analysis of the first 10 patients (between January 2015 and April 2016) to undergo EUS-FNA with the SharkCore® needle at the University of Utah School of Medicine/Huntsman Cancer Center with neuroendocrine tumors. Each case was retrospectively reviewed by a board certified cytopathologist (BLW) for the following cytologic parameters on the aspirate smears or touch/squash preparations: overall cellularity [1 (low) to 3 (high)], percentage of obtained cells that were lesional/representative (<25%, 26%-50%, and >50%), relative ease of interpretation [1 (difficult) to 3 (easy)]. Pathologic material and reporting records were also reviewed for each case to confirm the number of needle passes to achieve diagnostic adequacy, the presence or absence diagnostic material on H&E slide (from cell block, if prepared), whether a definitive diagnosis was able to be rendered, and the presence or absence of a true core/core fragments (within the cell block, if prepared).
Results:
A total of 20 patients underwent EUS-FNA for suspected neuroendocrine lesions. Ten patients underwent either transgastric or transduodenal EUS-FNA with the 22 gauge SharkCore® needle. The comparison cohort of 10 patients underwent either transgastric or transduodenal EUS-FNA with the standard 22 gauge Echotip® needle. The SharkCore® needle required a fewer mean number of needle passes to obtain diagnostic adequacy than the Echotip® (P=0.0074). For cases with cell blocks, the SharkCore® needle produced diagnostic material in 100% of cases, whereas Echotip® produced diagnostic material in 60% of cases. There was no significant difference between specimen cellularity, percentage of lesional material, or ease of interpretation between the two needle types.
Conclusion:
Our pilot investigation targeting patients with known or suspected pancreatic NETs indicates that the SharkCore® needle shows promise in obtaining suitable tissue for ancillary testing that can allow for more definitive pathologic interpretations on EUS FNA specimens. Fewer passes were needed with the core needle when compared to a standard needle.
Keywords: Fine-needle aspiration needle, Fine-needle biopsy needle, neuroendocrine tumors, SharkCore
INTRODUCTION
Over the past two decades, EUS-FNA has been the most reliable method for sampling a variety of gastrointestinal lesions including solid and cystic pancreatic masses. This technique has been shown to have a high sensitivity (75%–92%) and specificity (82%–100%).[1] A number of different EUS-FNA needles are available, most being designed to obtain cytologic specimens for either aspiration smears or needle rinse specimens. These standard EUS needles work with a high level of clinical efficacy, and meaningful differences between needle types and sizes have been difficult to identify despite extensive efforts to determine which needle type and size is ideal.[2,3,4] Similarly, variations in FNA technique (e.g., use or lack of use of a stylet) have also been shown to have relatively limited impact on final results despite widespread personal preferences among endoscopists.[5,6]
Recently, a new EUS needle (SharkCore®, Beacon Endoscopic, Newton, MA, USA), has been introduced. This device has a novel tip shape designed to improve tissue yield and to potentially obtain a core histologic tissue sample through EUS. The design incorporates two sharp points of different lengths and a multifaceted bevel in an attempt to capture additional (preferably in a core) tissue. Our group recently published a study comparing our experience with the 22-gauge SharkCore® needle to a standard 22-gauge Echotip® needle. Our study showed a trend toward increased production of diagnostic material within prepared cell blocks for the SharkCore® needle compared to the standard needle.[7] In 12 out of 15 pilot cases, EUS-FNA procedures with the SharkCore® needle produced actual tissue cores (or core fragments) compared to no tissue cores with the standard needle.[7]
While the diagnosis of pancreatic adenocarcinoma can be reliably reached based on cytologic preparations (aspirate smears)[8] with good interobserver agreement among cytopathologists,[9] the interpretation of tissue obtained from patients with suspected pancreatic neuroendocrine tumors (NETs) may be more problematic. A recent 15-year retrospective study conducted at our institution on the accuracy of diagnosing pancreatic NETs by EUS-FNA revealed that the method had only 66% sensitivity. By comparison, the detection rate for pancreatic adenocarcinomas was 88% over the same time period.[10] In addition, immunohistochemical staining of representative lesional material is often needed to make a definitive diagnostic interpretation for NETs because they share overlapping cytomorphologic features with acinar cell carcinoma, solid pseudopapillary neoplasm, pancreatoblastoma, and even adenocarcinoma. In reference to this, the Papanicolaou Society of Cytopathology Guidelines advises performing ancillary immunohistochemical stains not only for the cytologic diagnosis of pancreatic NETs but also to aid in awarding these tumors with an accurate grade (by Ki-67 index).[11]
We performed a retrospective pilot study to evaluate the clinical value and efficacy of the SharkCore® needle as compared to a standard EUS-FNA needle in the evaluation of patients with known or suspected NETs.
METHODS
A retrospective analysis of the first ten patients (between January 2015 and April 2016) to undergo EUS-FNA with the SharkCore® needle at the University of Utah School of Medicine/Huntsman Cancer Center with NCTs appearing either in the diagnostic line or within the diagnostic comment section was performed. For comparison, a parallel group of the ten most recent preceding patients receiving the same diagnostic impression who underwent EUS-FNA by the same endoscopists using a standard needle (EchoTip®, Wilson-Cook, Winston-Salem, NC, USA) were included in the study. The latter specimens were collected between January 2010 and December 2014. This study was approved by the Institutional Review Board at the University of Utah/ARUP Laboratories.
EUS-FNA with both needle types was performed in the standard manner using linear endoscopes and procedures were performed by two endosonographers, with one (DGA) with over 15 years of experience, acting as the operator in 18 out of the 20 cases. The technique for both needles was to insert the needle and take several slow passes whereas the stylet was slowly withdrawn (referred to as the “slow pull” technique). Rapid on-site evaluation (ROSE) by a cytopathologist was performed on all of the twenty included cases.
Slides were prepared for on-site evaluation using the rapid Diff-Quik® stain, whereas alcohol-fixed slides were submitted for Papanicolaou staining. For the standard needle specimens, FNA direct smears were created at the time of the examination and excess needle rinse, if present, was submitted into a saline tube for cell block preparation. For the SharkCore® specimens, either touch or squash preparation slides were created, and the core (or core fragments) was submitted in formalin fixation for cell block preparation. The techniques for on-site slide preparations were either to move the core/core fragments around the surface of the slide with a needle cap or gently press the sample between two slides and then put the core/core fragments into formalin (touch PREP method). The squash preparation entails compressing the core/core fragments between two slides and using shear force (in the direction of the long axis of the slides) to help disperse cells for on-site cytologic evaluation. This method typically disrupted the core/core fragments to the extent that no remaining tissue fragments were available to be placed into fixative. In some cases, one or more passes from the SharkCore® needle were placed directly into formalin and cell blocks were prepared. This technique was employed only after adequate lesional tissue was obtained on a prior touch preparation or squash prepared slide during on-site cytologic evaluation.
Each case was retrospectively reviewed by a board certified cytopathologist (BLW) for the following cytologic parameters on the aspirate smears or touch/squash preparations: overall cellularity (1 [low] to 3 [high]), percentage of obtained cells that were lesional/representative (<25%, 26%–50%, and >50%), and relative ease of interpretation (1 [difficult] to 3 [easy]). Pathologic material and reporting records were also reviewed for each case to confirm the number of needle passes to achieve diagnostic adequacy, the presence or absence of diagnostic material on hematoxylin and eosin slide (from cell block, if prepared), whether a definitive diagnosis was able to be rendered, and the presence or absence of a true core/core fragments (within the cell block, if prepared). The cytologic parameters of cellularity, production of diagnostic material in cell blocks, and production of core/core fragments were evaluated using Fisher's exact test. The number of passes was evaluated using the paired t-test.
RESULTS
A total of twenty patients underwent EUS-FNA for suspected neuroendocrine lesions. Ten patients underwent either transgastric or transduodenal EUS-FNA with the 22-gauge SharkCore® needle between January 2015 and February 2016. Seven patients underwent EUS-FNA evaluation for solid pancreatic masses, one for a solid duodenal wall mass, and two patients underwent EUS-FNA evaluation for peripancreatic lymphadenopathy. The comparison cohort of ten patients underwent either transgastric or transduodenal EUS-FNA with the standard 22-gauge Echotip® needle. Nine patients in this group underwent EUS-FNA evaluation for solid pancreatic masses and one had the procedure for peripancreatic lymphadenopathy. All cases (except for one deemed less than optimal with the standard needle) were deemed satisfactory for interpretation during ROSE. There were no adverse events seen in any patient.
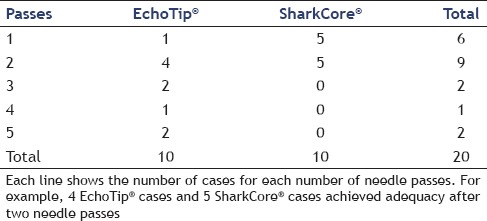
The SharkCore® needle required a fewer mean number of needle passes to obtain diagnostic adequacy than the Echotip® (P = 0.0074). The SharkCore® needle required a mean of 1.5 passes to reach adequacy whereas the Echotip® required a mean of 2.9 passes [Table 1].
Table 1.
Comparison of needle passes for SharkCore® needle and EchoTip® needle
All twenty cases had cell blocks prepared. With regard to cell blocks, the SharkCore® needle showed a trend toward increased material within the cell blocks (P = 0.0867). For cases with blocks, the SharkCore® needle produced diagnostic material in 100% of cases, whereas Echotip® produced diagnostic material in 60% of cases. Eight of the ten cell blocks made using the SharkCore® contained either cores or core fragments within the block, whereas the remaining two had scant diagnostic material comprised mostly of single lesional cells. All six of the successful cell blocks using the Echotip® needle had cohesive fragments of diagnostic tissue [Table 2]. All cases with diagnostic tissue in the cell block had immunohistochemical stains performed to support the cytodiagnosis including all ten cases using the SharkCore® needle. Figure 1 shows photomicrographs of cell block material from a representative case using the each needle type. Figures 2 and 3 show cytologic preps with the standard needle and SharkCore®, respectively.
Table 2.
Production of diagnostic material in cell blocks
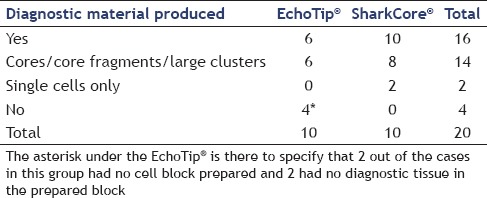
Figure 1.
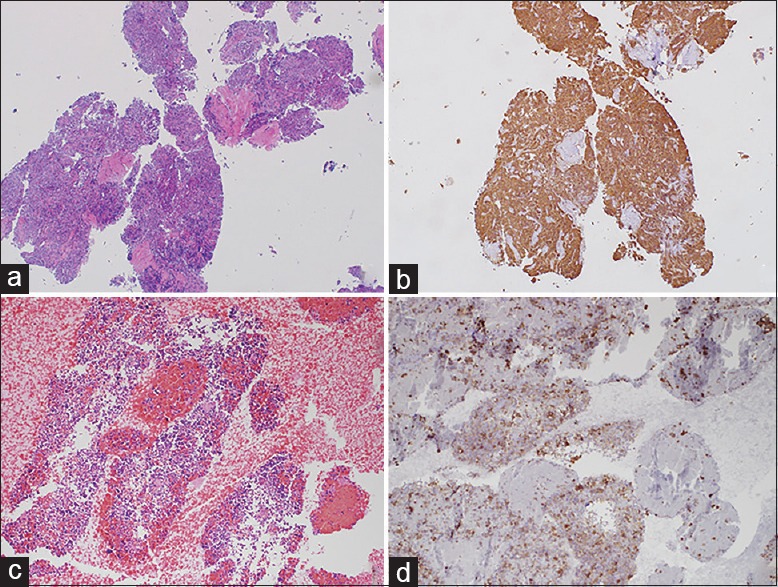
(a) Lesional core material from a case using the SharkCore® needle (H and E, ×10). (b) Synaptophysin stain on the same tissue block. (c) A large cluster of lesional tissue from a case using the standard needle (H and E, ×10). (d) Synaptophysin stain on the same tissue block
Figure 2.
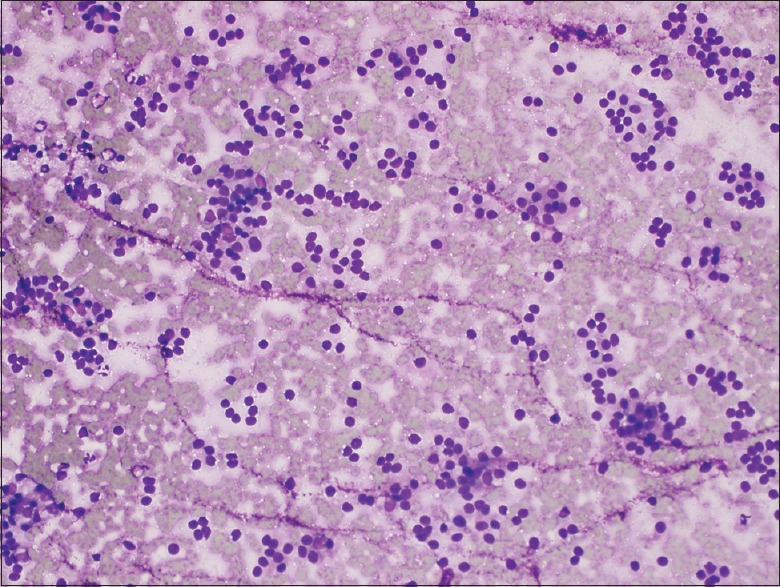
Diff-Quik® stained aspirate smear from a case using the standard needle showing characteristic cytologic features of neuroendocrine tumor. Specifically, a somewhat monotonous proliferation of loosely clustered cells with plasmacytoid features (×20)
Figure 3.
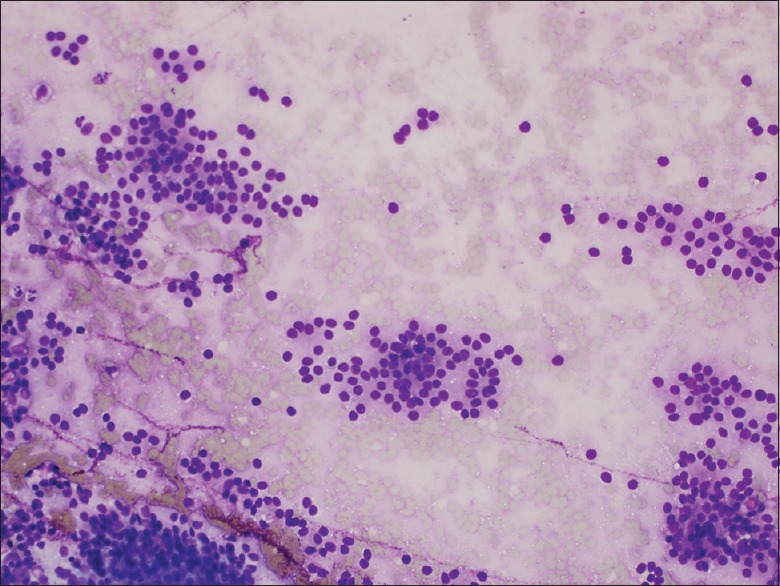
Diff-Quik® stained touch preparation from a case using the SharkCore® needle showing characteristic cytologic features of neuroendocrine tumor. Specifically, a somewhat monotonous proliferation of loosely clustered cells with plasmacytoid features (×20)
In terms of diagnostic reporting between the two groups, 9/10 cases using the SharkCore® needle were reported as NET or consistent with NET, whereas the remaining case was called “neoplastic cells present, favor NET.” In cases performed using the standard needle, 5/10 cases were reported as NET or consistent with NET, 4/10 were designated as favor NET, and one was reported as tumor cells present (with NET listed in the differential diagnosis in the comment section).
Clinical and pathologic follow-up for the SharkCore® group showed that 2/10 patients had subsequent resections confirming NETs, 4/10 had clinical progression of disease including metastases, and 4/10 have clinically stable disease on imaging and are being managed by surveillance. Follow-up for the Echotip® group showed that 2/10 patients had subsequent resections confirming NET, 2/10 had clinical progression of disease including radiologic metastases, 2/10 had clinically stable disease on surveillance, one died of unrelated causes, and 3 were lost to follow up.
In terms of the retrospectively assessed cytologic parameters on the aspirate smears and touch/squash preparations, there was no significant difference between specimen cellularity, percentage of lesional material, or ease of interpretation between the two needle types (based on a blinded review by a practicing, board certified cytopathologist).
DISCUSSION
This pilot study evaluated the clinical utility of incorporating the novel SharkCore® needle into the evaluation of patients with known or suspected NETs. Our preparations with the SharkCore® needle allowed for simultaneous cytology material (touch or squash preps) in all cases, diagnostic core biopsy/core fragment material in a significant proportion of cases (8/10), and stainable lesional cell block material in all cases (allowing for delineation of immunohistochemical markers to prove neuroendocrine differentiation). The quantity of diagnostic material in cell blocks provided by the SharkCore® needle, to go along with the standard cytomorphologic features compatible with NETs, enabled the pathologist to render a definitive report of NET in 9/10 cases.
A comparison group of the same number of patients with known or suspected NETs who underwent EUS-FNA with a standard 22-gauge needle had the same retrospective evaluation performed. While the cytologic preparations were equivalent in this group when compared to the SharkCore® group, four of the ten cell blocks lacked any lesional material for further immunohistochemical evaluation (markers to prove neuroendocrine differentiation). This resulted in a trend toward less definitive interpretations in this group as only 5/10 (50%) of these patients received a definitive diagnosis of NET in the pathology report. Along the same lines, a recent study by Dwyer et al. conducted on 56 intra-abdominal masses comparing standard EUS needles to a biopsy type needle also showed more material for ancillary studies for the biopsy needle type.[12]
Another notable finding is our result that the cytologic material garnered on site by the SharkCore® needle allowed for significantly fewer passes to reach specimen adequacy (P = 0.0074). The reduction in the number of needle passes to reach adequacy was nearly 2-fold less with the SharkCore® (average 1.5 passes/case) versus the standard needle (2.9 passes/case). This parallels our findings from our recent pilot study comparing the SharkCore® to the conventional needle in a cohort of thirty patients.[7]
While the standard cytologic morphologic analysis functions well in the majority of EUS FNAs performed on solid pancreatic lesions, a subset of these lesions present diagnostic dilemmas when tissue obtained is analyzed using cytologic preparations (such as aspirate smears) alone. Pancreatic NETs are one such entity where having a robust cell block for ancillary studies is critical to navigate the primary differential diagnosis between NETs, acinar cell carcinomas, solid pseudopapillary neoplasms, and even metastatic melanoma or plasma cell dyscrasias.[11]
In a recent retrospective study of patients with histologically confirmed pancreatic NETs who had prior EUS-FNAs performed, Chen et al. found that a definitive diagnosis of NET was only able to be rendered on 13/21 (61.9%) of EUS-FNA specimens. Each of the 13 cases that had a definitive diagnosis had adequate cell block material obtained for ancillary testing, underpinning the need for robust cell block material to render a conclusive determination of a pancreatic NET.[13] In addition, Deshpande and Lauwers in their evaluation of pancreatic NETs with a cystic component highlighted the potential cytomorphologic overlap of cystic NETs with adenocarcinoma. In their review of histologically confirmed cases of cystic NETs, three of the five were misdiagnosed on EUS-FNA as either adenocarcinoma or atypical glandular cells (a term that can be taken by a clinician as suspicious for adenocarcinoma). The authors suggested that appropriate immunostaining of cell block material would help avoid this potential pitfall as none of the miscategorized cases had cell block material available at the time of diagnosis.[14] Conversely, in a recent 15-year retrospective study conducted at our own institution, 8 of the 26 (30%) false-positive EUS-FNA diagnoses of adenocarcinoma proved to be NETs on resection. In fact, NETs accounted for the most common cause of false-positive adenocarcinomas in this study.[10] It is worth noting that this study was performed before the introduction of the SharkCore® needle at our institution.
Limitations of this study include its single center and retrospective nature. To date, there is very limited published literature on the SharkCore® needle, and this study represents the first on the SharkCore® needle as it functions in the role of diagnosing NETs. Larger studies, multicenter studies, and studies comparing the device to other EUS-FNA needles as well as needles designed to obtain a core sample are warranted.
EUS needles continue to be an area of development. The desire to possess a needle that is easy for the endoscopist to maneuver, inexpensive, and has the ability to obtain histologic core tissue in selected cases where ancillary studies are needed by the pathologist has been longstanding. Our pilot investigation targeting patients with known or suspected pancreatic NETs indicates that the SharkCore® needle shows promise in obtaining suitable tissue for ancillary testing that can allow for more definitive pathologic interpretations on EUS-FNA specimens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–52. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Witt BL, Adler DG, Hilden K, et al. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–74. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 3.Songür N, Songür Y, Bırcan S, et al. Comparison of 19- and 22-gauge needles in EUS-guided fine needle aspiration in patients with mediastinal masses and lymph nodes. Turk J Gastroenterol. 2011;22:472–8. doi: 10.4318/tjg.2011.0322. [DOI] [PubMed] [Google Scholar]
- 4.Imazu H, Uchiyama Y, Kakutani H, et al. Aprospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract 2009. 2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimeno-García AZ, Paquin SC, Gariépy G, et al. Comparison of endoscopic ultrasonography-guided fine-needle aspiration cytology results with and without the stylet in 3364 cases. Dig Endosc. 2013;25:303–7. doi: 10.1111/j.1443-1661.2012.01374.x. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Gupta N, Gaddam S, et al. Acomparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sc i. 2011;56:2409–14. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 7.Adler DG, Witt B, Chadwick B, et al. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc Ultrasound. 2016;5:178–83. doi: 10.4103/2303-9027.183976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: The M. D. Anderson cancer center experience. Cancer. 2002;96:174–80. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 9.Kurtycz D, Tabatabai L, Khalbuss W, et al. Pancreatic fine-needle aspiration cytopathology: An analysis of the CAP NGC program for pancreatic FNA 2003-2011. J Am Soc Cytopathol. 2015;4:327–34. doi: 10.1016/j.jasc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Beal HL, Shea JE, Witt BL, et al. Accuracy of diagnosing PDA, neuroendocrine, and IPMN by EUS-FNA at a single institution. J Gastroenterol Hepatol Res. 2015;4:1844–9. [Google Scholar]
- 11.Layfield LJ, Ehya H, Filie AC, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11:4. doi: 10.4103/1742-6413.133352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer J, Pantanowitz L, Ohori NP, et al. Endoscopic ultrasound-guided FNA and proCore biopsy in sampling pancreatic and intra-abdominal masses. Cancer Cytopathol. 2016;124:110–21. doi: 10.1002/cncy.21623. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Nassar A, Kommineni VT, et al. Endoscopic ultrasonography-guided fine-needle aspiration cytology of surgically confirmed cystic pancreatic neuroendocrine tumors: A Mayo Clinic experience. J Am Soc Cytopathol. 2015;4:335–43. doi: 10.1016/j.jasc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande V, Lauwers GY. Cystic pancreatic endocrine tumor: A variant commonly confused with cystic adenocarcinoma. Cancer. 2007;111:47–53. doi: 10.1002/cncr.22422. [DOI] [PubMed] [Google Scholar]


