Abstract
Background and Objectives:
With improvements in imaging technologies, pancreatic cystic lesions (PCLs) have been increasingly identified in recent years. However, the imaging modalities used to differentiate the categories of pancreatic cysts remain limited, which may cause confusion when planning treatment. Due to progress in endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) technology, auxiliary diagnosis by the detection of cystic fluid has become a recent trend.
Methods:
From March 2015 to April 2016, 120 patients with PCLs were enrolled in this study. According to the results of EUS, cyst fluid carcinoembryonic antigen (CEA) analysis, and cystic fluid cytology, the patients were divided into two groups: a nonmucinous and a mucinous group. Of those, 61 patients who had undergone surgical resection were included in the analysis. The clinical features, biochemical and tumor markers of cyst fluid as well as the cytological test results of the patients were compared with histopathology results.
Results:
A cyst size of 4.0 cm was used as the boundary value; a cyst ≤4.0 cm was defined as a small PCL. 87 (72.5%) lesions were ≤4.0 cm, and 33 (27.5%) lesions were >4.0 cm. Regarding the analysis of CEA and carbohydrate antigens 19-9 (CA19-9), significant differences were found between the nonmucinous and mucinous groups (P < 0.05) according to nonparametric independent samples tests. The EUS, cystic fluid CEA, and cystic fluid cytology results were compared with the tissue pathology findings using McNemar's test (P < 0.05) and showed a sensitivity of 90% and a specificity of 84%.
Conclusion:
A diagnostic combination of EUS, cyst fluid CEA, and cystic fluid cytology could be used to differentiate small pancreatic cystic neoplasms. Cystic fluid cytology analysis is helpful for planning treatment for pancreatic cystic tumors that pose a surgical risk.
Keywords: Carcinoembryonic antigen, cytology, differentiation, endoscopic ultrasonography, mucinous
INTRODUCTION
With improvements in imaging technologies, pancreatic cystic lesions (PCLs) have been increasingly identified in recent years. The prevalence rate of PCLs is 2.5% in the US.[1] PCLs are characterized by a group of common clinical symptoms, for example, abdominal pain or feeling unwell.[2] Due to the risk of the concurrent or later development of malignancy, the diagnosis and discrimination of nonneoplastic from neoplastic cysts or nonmucinous from mucinous cysts are very important. Pancreatic pseudocysts (PPs) constitute a benign disease in which inflammatory fluids are present in approximately 80% of PCLs.[3] The pathological types of pancreatic cystic neoplasms (PCNs) include serous cystic neoplasms (SCNs), mucous cystic neoplasms (MCNs), solid false papilloma neoplasms (SPNs), intraductal papillary mucinous neoplasms (IPMNs), and pancreatic neuroendocrine tumors (PNETs).[4] PPs, SPNs, PNETs, and SCNs are generally regarded as nonmucinous cystic neoplastic lesions and are benign. MCNs and IPMNs are characterized as mucinous cystic neoplastic lesions, and their malignant potential is represented by an adenoma-carcinoma sequence.[5,6,7] However, the development of imaging modalities to accurately characterize particular types of pancreatic cysts remains challenging.[8] Studies have shown that the consistency of preoperative imaging diagnosis and postoperative pathological diagnosis is <50%.[9]
METHODS
Study design
This prospective study was approved by the Chinese PLA General Hospital Ethics Committee (No. S2014-108-01). The clinical records, endoscopic ultrasonography (EUS) images, pathology, and surgical reports included in this study are all true and reliable.
Characterization of patients by a set of conditions
From March 2015 to April 2016, 120 patients with PCNs were enrolled in the study. Before enrollment, the patients were diagnosed with PCNs by abdominal ultrasound, computed tomography, or magnetic resonance cholangiopancreatography. After the establishment of a multidisciplinary collaboration with the Department of Surgery and a review of previous literature, patients with a cyst size of 2.0–7.0 cm were enrolled in the study. The first step was performed by two experienced endoscopists (EnQiang LingHu and Ningli Chai) who independently perform more than 300 endoscopic resections for gastroesophageal neoplasia annually. We also invited doctors who specialize in interventional ultrasonography to assist in jointly determining the definitive diagnosis of PCNs. The EUS imaging characteristics of MCNs, SCNs, SPNs, and PNETs were initially used. GF-UE260-AL5/GF-UM200 endoscopy (Olympus Co., Tokyo, Japan) and EU-ME2 endoscopic ultrasonography (Olympus Co.) were used to determine the feasibility of performing EUS-guided fine-needle aspiration (EUS-FNA) with a 19G/22G needle (Cook Co., Boston, USA). Patients underwent needle puncture to acquire fluid samples. The collected cystic fluid was used for biochemical tests, molecular diagnosis, and cytological examinations. All patients with the following contraindications were excluded: (i) PPs, (ii) severe acute pancreatitis, (iii) malignant tumors, (iv) severe cardiopulmonary circulatory system disease, (v) blood coagulation dysfunction, or (vi) mental illness. Each patient provided informed consent. All patients who did not undergo surgery were followed up every 3 months, and EUS findings were reviewed if the cyst became too large to conduct EUS-FNA. Patients who exhibited neoplasia growth underwent surgery.
Definitions
The pathological results were determined based on the WHO classification of pancreatic tumors. Dysplasia grading was performed by pathological diagnosis of PCNs. Benign PCNs were classified as low grade or moderate grade, and malignant PCNs were classified as invasive carcinoma, carcinoma in situ, or high-grade dysplasia. According to the Sendai 2012 International Consensus Guidelines for Cyst Management, when a cyst is >3 cm, the following signs could represent a high risk of IPMN or MCN: enhanced cystic walls, main pancreatic duct size of 5–9 mm, internal nipple sample structure, and main pancreatic duct expansion.[10,11,12] Therefore, we collected patient information, including clinical symptoms, weight loss, abdominal pain, obstructive jaundice, history of pancreatitis, EUS findings, the size and number of lesions, lesions within the thickened/enhanced walls, separations, and mural lesions.[3,13,14]
Diagnostic criteria
Endoscopic ultrasonography criteria
The main EUS finding for SCNs was multiple, small, anechoic cystic areas with a “honeycomb” appearance, sometimes with central fibrosis or calcification.[15,16] MCNs were indicated by macrocystic lesions with few separations, focal peripheral calcification in some cases, and no ductal dilation; atypical mural lesions were observed in some cases.[7] IPMN microbubbles were indicated by hyperechoic mural lesions, raised ductal walls, and small clusters of grape-like dilations of IPMN-branch ducts.[9]
Carcinoembryonic antigen criteria
Brugge et al.[12,17] analyzed receiver operator curves of tumor markers in 341 cases and demonstrated that the accuracy of carcinoembryonic antigen (CEA) was 79% using a cutoff of 192 ng/mL. Talar-Wojnarowska et al.[4] included 52 patients with PCLs and found that CEA was elevated to 238 ± 12.5 ng/mL in patients with malignant cysts. Alkaade et al.[8] also used a cutoff of 192 ng/mL for selected patients with malignant cysts. Given the above findings and considering our small number of fluid samples, we selected 192 ng/mL as the cutoff to exclude a type I statistical error.
CA19-9 and CA72-4 criteria
Cancer antigen 19-9 (CA19-9) and cancer antigen 72-4 (CA72-4) have been widely used for the diagnosis of different types of cancers, including hepatic, colorectal, and pancreatic cancers.[18] Serum levels of CA 19-9 (normal range, 0–37 U/mL) and CA72-4 (normal range, 0–12 U/mL) as well as pancreatic juice cytology obtained endoscopically during endoscopic retrograde cholangiopancreatography have also been analyzed.[19] However, the use of serum CA19-9 in discriminating between benign and malignant pancreatic disease remains controversial. In our study, we attempted to use cyst fluid to differentiate between benign and malignant pancreatic disease.
Cytology criteria
The official cytology report was used as the data source to detect mural exfoliated cells. The possibility of samples being classified as nondiagnostic (acellular and nonmucinous) was 30%, while the other cases were classified as diagnostic (mucinous or nonmucinous epithelium, extracellular mucin, or inflammatory cells).[20] Cysts were categorized as mucinous if mucinous epithelium or extracellular mucin without gastrointestinal contaminant cells was documented, and they were categorized as nonmucinous if diagnostic cytology documented only inflammatory cells or nonmucinous epithelium.[21]
Statistical analysis
The SPSS Version 22.0 (IBM, NY, USA) was used for the statistical analysis. Because the continuous variables did not exhibit a normal distribution, a nonparametric rank sum test was used. The Chi-square test was used for single-factor qualitative data. A single-factor logistic regression was used for the analysis of risk factors. McNemar's test was used to compare the diagnostic test results with tissue pathology findings after surgery. A value of P < 0.05 represented a statistically significant difference.
RESULTS
Patient characteristics
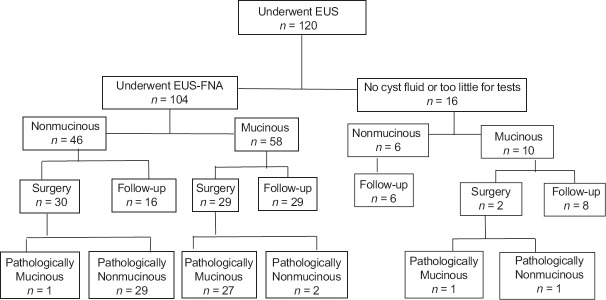
Of the 120 included patients, 49 were men and 71 were women, with an age range of 18–87 years. The average age was 50.93 ± 17.66 years. A cyst size of 4.0 cm was used as the boundary value, and cysts ≤4.0 cm were defined as small PCLs. In 87 cases (72.5%), cysts were ≤4.0 cm, and in 33 cases (27.5%), cysts were >4.0 cm. Using a combination of EUS findings, cystic fluid CEA analysis, and cystic fluid cytology, 46 cases were diagnosed as PNETs, SPN, or SCN in the nonmucinous group. Of these, 36 cases included histopathology results. A total of 58 cases were diagnosed as MCN or IPMN; all 58 cases included histopathology results. A total of 16 cases had no cyst fluid or an insufficient amount to be distinguished by EUS, including 10 mucinous cases and 2 cases with histopathology results [Figure 1].
Figure 1.
Diagram of the clinical course of all patients with pancreatic cystic neoplasms
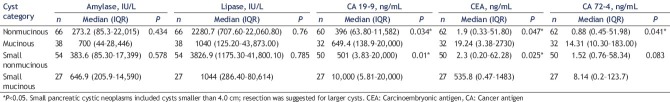
The contents of cyst fluid amylase, lipase, CEA, CA19-9, and CA72-4 in 104 cases were analyzed using nonparametric independent samples tests. In the nonmucinous group, the values were as follows: CEA: 85.3–22,015 ng/mL; CA19-9: 63.7–11, 582 ng/mL; CA72-4: 0.45–5796 ng/mL; amylase: 1.2–275, 020 IU/L; and lipase: 707.6–22,060.8 IU/L. In the mucinous group, the values were as follows: CEA: 3.38-2730 ng/mL; CA19-9: 138.9–20,000 ng/mL; CA72-4: 10.3–183 ng/mL; amylase: 44–28,446 IU/L; and lipase: 125.2–43,873 IU/L. The CA19-9 content was significantly different between the two groups (P < 0.05) [Table 1].
Table 1.
Cyst fluid amylase, lipase, carcinoembryonic antigen, cancer antigen 19-9, and cancer antigen 72-4 contents in the mucinous and nonmucinous groups
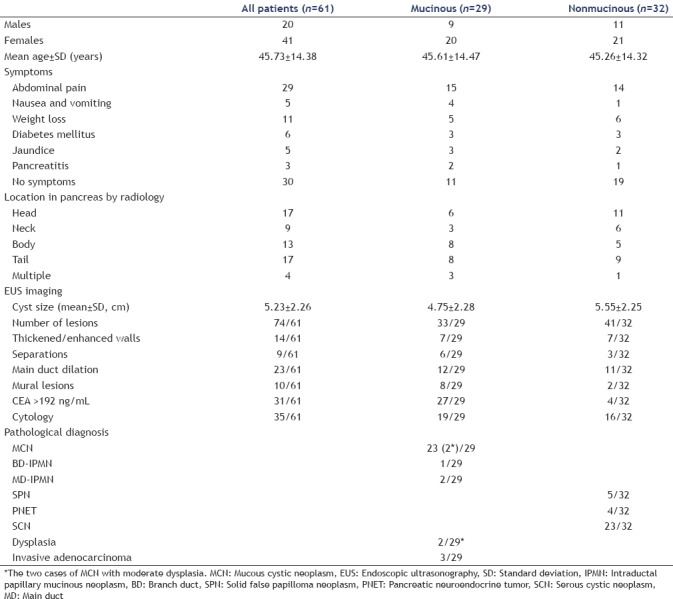
Sixty-one patients were pathologically diagnosed through surgical resection. Twenty-three of patients (37.7%) were diagnosed with MCNs, and two of them had moderate dysplasia. The patients included three cases (5%) of mucinous cystadenoma (MCA), three cases (5%) of IPMN, five cases (8%) of SPN, four cases (6.5%) of PNET, and 23 cases (37.7%) of SCN. The clinical features of these cases are presented below. Thirty-one patients (50.8%) were identified with tumor detection factors according to physical examination, and 29 patients (47.5%) had epigastric abdominal pain. Eleven patients (18%) had lost weight during the last year, three patients (5%) had a history of pancreatitis, five patients (8.3%) had jaundice, and five patients (8.3%) had nausea and vomiting during the last year. Regarding the location of cysts, 17 cases (27.8%) occurred in the head of the pancreas, nine cases (14.7%) occurred in the neck of the pancreas, 13 cases (21.3%) occurred in the body of the pancreas, and 17 cases (27.8%) occurred in the tail of the pancreas. The mean cyst sizes were 4.75 ± 2.28 cm in the mucinous group and 5.55 ± 2.25 cm in the nonmucinous group. Twelve patients (41.3%) in the mucinous group had main duct dilation, and 11 patients (34.7%) in the nonmucinous group had main duct dilation. The above characteristics were analyzed using a single-factor Chi-square analysis (P > 0.05). Twenty-seven patients (93%) in the mucinous group had a CEA concentration >192 ng/mL. Thirty-five patients (57.3%) were identified by fluid cytology [Table 2].
Table 2.
Clinical features and risk status of pancreatic cystic neoplasms
In our study, we used three strategies to analyze the differences between the mucinous group and nonmucinous group. In the first strategy, EUS imaging findings and histopathology results after surgical resection were compared in 61 cases in the mucinous and nonmucinous groups using McNemar's test (P < 0.05), resulting in a sensitivity of 76% and specificity of 72%. In the second strategy, EUS imaging + cyst fluid CEA analysis was compared with histopathology results after surgical resection in 61 cases in the mucinous and nonmucinous groups using McNemar's test (P < 0.05), resulting in a sensitivity of 83% and specificity of 78%. In the third strategy, EUS imaging + cyst fluid CEA + cyst fluid cytology findings were compared with histopathology results after surgical resection in 61 cases in the mucinous and nonmucinous group using McNemar's test (P = 0.001 [< 0.05]), resulting in a sensitivity of 90% and specificity of 84% [Table 3].
Table 3.
Three strategies used to analyze differences between the mucinous and nonmucinous groups

Complications
Of the 120 cases, none of the patients had acute or late sepsis that was attributable to the EUS-FNA procedure, including FNA-introduced intraabdominal abscess, endoscope trauma, or systemic adverse events. Only two patients (1.7%) had transiently high amylase; after 3 days of medical treatment, the levels returned to normal.
DISCUSSION
The annual death toll due to pancreatic cancer is reported to be 200,000 people worldwide. Pancreatic cancer ranks fifth in the number of cancer deaths worldwide, and the 5-year survival rate is <5%.[22] SCNs, MCNs, SPNs, nerve internal secretory tumors, and IPMNs can all occur before the development of pancreatic cancer.[23] According to the Sendai 2012 International Consensus Guidelines for Management, if a cyst is >3 cm, the following signs could represent a high risk of IPMN or MCN: enhanced cyst walls, main pancreatic duct size of 5–9 mm, internal nipple sample structure, and main pancreatic duct expansion.[24,25] Therefore, in the detection of pancreatic cystic tumors, it is necessary to differentiate between benign and malignant tumors to provide optimal treatment. Studies have reported that fluid cytology analysis is of great importance for the diagnosis of pancreatic lymphangioma. Cytological analysis can be combined with clinical, imaging, and fluid characteristics for lesions such as pancreatic lymphangioma and other common pancreatic cysts to avoid unnecessary surgery. In addition, studies have shown that fluid cytology analysis helps determine the degree of malignancy of PCLs, especially IPMN in the small branches of the pancreatic duct.[26,27]
The levels of blood and urine amylase are often used to determine whether the pancreas catheter is unobstructed, and the measurement of cystic fluid amylase can be used to determine PCLs properties. Cystic fluid amylase levels are often high in most PPs and in patients with IPMN and MCN.[28] Fluid amylase levels <250 U/L are found in serous gland diseases, mucus gland diseases, or cancer and have a specificity of 98% and a sensitivity of 44%.[8,10] Some studies have reported that SPNs are low-grade malignant neoplasms composed of monomorphic epithelial cells that form solid and pseudopapillary structures, and malignancy is rare.[3] Our study found that seven cases of SPNs were benign.
MCN epithelia produce mucin more often in females than males and typically occur during middle age (average age 48–55 years). The body and tail of the pancreas are the most common locations, and lesions in these areas have no communication with the duct. The ovarian stroma is pathognomonic, and the malignancy rate is 10%–17%.[14] Our study found that two (8.7%) of the 23 MCN cases were premalignant.
Early studies analyzed 450 patients with PCLs. A fluid CEA level <5 ng/mL was used to classify SCA or pancreatic pseudocyst (PPC), resulting in a sensitivity of 50% and a specificity of 95%. A cystic fluid CEA level >800 ng/mL was used to distinguish MCA and mucinous cystadenocarcinoma, resulting in a sensitivity of 48% and specificity of 98%. A CA19-9 level of <37 U/mL was used to distinguish PPC and SCA, resulting in a sensitivity of 19% and specificity of 98%.[12] Our study found that the CEA and CA19-9 levels were different between the mucinous and nonmucinous groups (P < 0.05). The CA19-9 cutoff value was 364.55 ng/mL, and malignant pancreatic cystic tumors were diagnosed with a sensitivity of 72.2% and a specificity of 75%. Data based on surgical histopathological analyses have shown that a cystic fluid CEA level of 192 ng/mL can be used to diagnose MCA with a sensitivity of 75% and a specificity of 83.6%. The association between the cystic fluid CEA value and the degree of malignancy and disease progression requires further study.[13] Our study found that when a cutoff value of CEA of 133 ng/mL was used, pancreatic cystic tumors could be diagnosed with a sensitivity of 72.2% and specificity of 82.1%. These estimates will become more accurate as the data continue to accumulate.
Research has shown that PCN progression to pancreatic cancer is related to gene mutations and missing chromosome hybrids. DNA analyses of pancreatic cystic fluid, including K-ras, GNAS gene mutation analysis, tumor suppressor genes, and loss of heterozygosity, can be used for the identification of benign and malignant lesions.[29] Therefore, in the long-term follow-up of pancreatic cysts, focal monitoring of fluid, including changes in certain molecules (BRAF, CDKN2A, CTNNB1 GNAS, KRAS, NRAS, PIK3CA, RNF43, SMAD4, TP53, and VHL) is very important.[30] Pancreatic mucins are highly glycosylated high-molecular-weight glycoproteins and can be used in the determination of the malignant potential of precancerous or malignant lesions.[31] Therefore, follow-up studies should use pancreatic cystic fluid DNA analysis and the pancreatic protein glycosylation phenotype to identify new tumor markers for the accurate classification of pancreatic cystic adenoma.
The analysis of PCL fluid can indicate the nature of the disease. EUS-FNA of amylase, lipase, CEA, CA19-9, and CA72-4 as well as genetic and cytological analyses can improve the accuracy of the diagnosis of pancreatic cysts. The CEA level in the cystic fluid is the most accurate marker for the diagnosis of pancreatic mucous cysts. The reasonable application of cystic fluid analysis can significantly improve the clinical understanding of pancreatic cysts and guide subsequent therapy. Positive cytology tests have important clinical significance for the diagnosis of malignant tumors. Cytology can determine whether a cyst has secretory glands or epithelial cells, which indicate mucinous cystic tumors. In patients at risk for pancreatic cystic tumors or in cases of an unclear diagnosis, fluid analysis is helpful for selecting and developing the best treatment plan.
CONCLUSION
A diagnostic combination of EUS, cyst fluid CEA, and cystic fluid cytology could be used to differentiate small pancreatic cystic neoplasms. Cystic fluid cytology analysis is helpful for planning treatment for pancreatic cystic tumors that pose a surgical risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–84. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 2.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Cauley CE, Pitman MB, Zhou J, et al. Circulating epithelial cells in patients with pancreatic lesions: Clinical and pathologic findings. J Am Coll Surg. 2015;221:699–707. doi: 10.1016/j.jamcollsurg.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talar-Wojnarowska R, Pazurek M, Durko L, et al. Pancreatic cyst fluid analysis for differential diagnosis between benign and malignant lesions. Oncol Lett. 2013;5:613–6. doi: 10.3892/ol.2012.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gbormittah FO, Haab BB, Partyka K, et al. Characterization of glycoproteins in pancreatic cyst fluid using a high-performance multiple lectin affinity chromatography platform. J Proteome Res. 2014;13:289–99. doi: 10.1021/pr400813u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuyama M, Ueda T. Endoscopic ultrasound-guided transjejunal puncture of the main pancreatic duct as an alternative treatment for strictured pancreatojejunal anastomosis. Pancreatology. 2014;14:107–8. doi: 10.1016/j.pan.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Jang JY, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131–6. doi: 10.1016/j.pan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Alkaade S, Chahla E, Levy M. Role of endoscopic ultrasound-guided fine-needle aspiration cytology, viscosity, and carcinoembryonic antigen in pancreatic cyst fluid. Endosc Ultrasound. 2015;4:299–303. doi: 10.4103/2303-9027.170417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harima H, Kaino S, Shinoda S, et al. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2015;21:6252–60. doi: 10.3748/wjg.v21.i20.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan XB, Wang B, Liu F, et al. Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography-guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms. J Dig Dis. 2013;14:191–5. doi: 10.1111/1751-2980.12027. [DOI] [PubMed] [Google Scholar]
- 11.Jabbar KS, Verbeke C, Hyltander AG, et al. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Natl Cancer Inst. 2014;106:djt439. doi: 10.1093/jnci/djt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375–88. doi: 10.3978/j.issn.2078-6891.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das A, Brugge W, Mishra G, et al. Managing incidental pancreatic cystic neoplasms with integrated molecular pathology is a cost-effective strategy. Endosc Int Open. 2015;3:E479–86. doi: 10.1055/s-0034-1392016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell JJ. Prevalence, diagnosis and management of pancreatic cystic neoplasms: Current status and future directions. Gut Liver. 2015;9:571–89. doi: 10.5009/gnl15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandula M, Moole H, Cashman M, et al. Success of endoscopic ultrasound-guided ethanol ablation of pancreatic cysts: A meta-analysis and systematic review. Indian J Gastroenterol. 2015;34:193–9. doi: 10.1007/s12664-015-0575-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim J. Endoscopic ultrasound-guided treatment of pancreatic cystic and solid masses. Clin Endosc. 2015;48:308–11. doi: 10.5946/ce.2015.48.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Fritz S, Hackert T, Hinz U, et al. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg. 2011;98:104–10. doi: 10.1002/bjs.7280. [DOI] [PubMed] [Google Scholar]
- 19.Cao S, Hu Y, Gao X, et al. Serum carbohydrate antigen 19-9 in differential diagnosis of benign and malignant pancreatic cystic neoplasms: A meta-analysis. PLoS One. 2016;11:e0166406. doi: 10.1371/journal.pone.0166406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saul A, Ramirez Luna MA, Chan C, et al. EUS-guided drainage of pancreatic pseudocysts offers similar success and complications compared to surgical treatment but with a lower cost. Surg Endosc. 2016;30:1459–65. doi: 10.1007/s00464-015-4351-2. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-D’Jesús A, Fernández-Esparrach G, Marra-Lopez C, et al. Adverse events of EUS-guided FNA of pancreatic cystic and solid lesions by using the lexicon proposed in an ASGE workshop: A prospective and comparative study. Gastrointest Endosc. 2016;83:780–4. doi: 10.1016/j.gie.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Pan S, Brentnall TA, Chen R. Proteomics analysis of bodily fluids in pancreatic cancer. Proteomics. 2015;15:2705–15. doi: 10.1002/pmic.201400476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan M, Karanovic D, Chalhoub W, et al. Gastric duplication cyst with elevated amylase: An unusual presentation mimicking pancreatic cystic neoplasm. ACG Case Rep J. 2015;2:86–8. doi: 10.14309/crj.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultana A, Jackson R, Tim G, et al. what is the best way to identify malignant transformation within pancreatic IPMN: A systematic review and meta-analyses. Clin Transl Gastroenterol. 2015;6:e130. doi: 10.1038/ctg.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140–8. doi: 10.1016/j.gie.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Zhan X, Li Z. Molecular analyses of aspirated cystic fluid for the differential diagnosis of cystic lesions of the pancreas: A systematic review and meta-analysis. Gastroenterol Res Pract. 2016;2016:3546085. doi: 10.1155/2016/3546085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishna SG, Lee JH. Appraisal of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic cysts. World J Gastroenterol. 2016;22:1701–10. doi: 10.3748/wjg.v22.i4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai Y, Iwashita T, Shinoura S, et al. Role of serial EUS-guided FNA on pancreatic cystic neoplasms: A retrospective analysis of repeat carcinoembryonic antigen measurements. Gastrointest Endosc. 2016;84:780–4. doi: 10.1016/j.gie.2016.03.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frampton AE, Stebbing J, Gall TM, et al. Activating mutations of GNAS and KRAS in cystic fluid can help detect intraductal papillary mucinous neoplasms of the pancreas. Expert Rev Mol Diagn. 2015;15:325–8. doi: 10.1586/14737159.2015.1002771. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ling-Hu E, Guo Y. The diagnosis of pancreatic cystic neoplasm. Chin J Gastrointest Endosc. 2015;2:3–5. [Google Scholar]
- 31.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]