INTRODUCTION
“Good morning Doctor. I had an abdominal ultrasound for dyspepsia and they found I have a pancreatic cyst. I’m scared! Could it be or become a cancer?”
Pancreatologists are currently called to face this clinical scenario more and more frequently. The number of incidentally discovered pancreatic cyst is growing mostly due to the increased use of cross-sectional imaging but also for a lengthening of life and aging of the population and maybe for a real increased incidence. However, several questions remain unsolved. What is the real risk of malignant transformation? When to employ invasive investigations? When to resect?
THE FALSE WORRY GENERATED BY SURGICAL SERIES
In the past decades, high-grade dysplasia or malignancy was reported in 40.4% of resected intraductal papillary mucinous neoplasia (IPMN), higher in main-duct IPMN (MD-IPMN) (62.2%) than in branch-duct IPMN (BD-IPMN) (24.4%).[1] In 2012, Fritz et al. reported the prevalence of malignancy in about 25% of Sendai-negative BD-IPMN[2] and an involvement of the main pancreatic duct in 29% in suspected BD-IPMN.[3] These studies generated alarm among physicians because this information was translated to the general population. However, these results come from retrospective surgical series and are burdened by an important selection bias. Indeed, patients included in these studies received surgery for suspicion of cancer based on symptoms (e.g., jaundice or weight loss), elevated CA 19.9 or worrisome features. Moreover, retrospective studies could not differentiate malignancy arising from IPMN from distinct pancreatic ductal carcinoma developed besides IPMN. Indeed, considering the high prevalence of pancreatic cyst, it is probable that the rising of a pancreatic cancer is associated with preexisting pancreatic cyst(s).
LESSONS FROM EPIDEMIOLOGICAL STUDIES
A recent study from the Surveillance, Epidemiology, and End Results (SEER) population-based data reported an incidence of pancreatic cancer of 10–20/100,000 person/years in the USA.[4] Interestingly, this study evaluated pancreatic ductal adenocarcinoma incidence according to histology. The incidence of nonsecretory endocrine cancers, ductal adenocarcinomas, and adenocarcinoma not otherwise specified increased over the time, whereas the incidence of mucinous adenocarcinomas decreased and was estimated around 1/100,000 person/years. Moreover, the number of cases was too few for temporal trend analysis for IPMN as well as for the other rare adenocarcinomas.
Looking at cross-sectional imaging studies on general population, the prevalence of pancreatic cyst widely ranges between 2.4% and 49% with a mean value estimated in about 12%[5,6,7,8,9,10,11,12,13,14,15] [Table 1]. This impressive number becomes even more clinically significant, considering that only about 1% of patients have a cyst >2 cm. Moreover, the prevalence of cyst directly correlates with age.[8] It is estimated that 80-year-old patients have a pancreatic cyst in >35% of cases. In the study of Chang et al., analyzing >20,000 healthy people, the pretest probability of a cyst to be an IPMN was higher than 80% whereas it was lower than 5% for serous lesions.[16] We can speculate that BD-IPMN represents >80% of cases of incidentally discovered pancreatic cyst(s).
Table 1.
Prevalence of pancreatic cyst in large series of cross-sectional studies on healthy patients
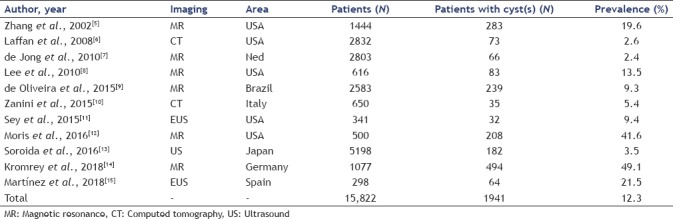
The incidence of pancreatic cyst has been reported in 12.9% in a population-based study over a period of 5-year follow-up (2.6% per year).[14] In other words, about 2600/100,000 persons per year will develop a pancreatic cyst. The incidence of pancreatic cancer is about 10–20/100,000, but for adenocarcinoma, mucinous type is only 1/100,000. Therefore, malignant transformation of pancreatic cyst seems to be a very rare event [Figure 1]. This speculation is supported by another recent study from the SEER that estimated a prevalence rate of mucin-producing adenocarcinoma arising in patients with pancreatic cysts of 33.2/100,000 persons.[17]
Figure 1.

Risk of malignant transformation of pancreatic cyst based on published studies
INTRADUCTAL PAPILLARY MUCINOUS NEOPLASIA NATURAL HISTORY
Different observational studies demonstrated the not aggressive natural history of IPMN. In the study of Kromrey et al., no patient died for pancreatic diseases and no pancreatic cancer was observed during a follow-up of 5 years with only a minimal progress in about 50% of patients.[14] The study of Kayal et al. demonstrated no progression to malignancy of low-risk BD-IPMN during a follow-up longer than 50 months.[18] Moreover, in a cohort of patients with worrisome features or high-risk stigmata at the time of diagnosis who underwent nonoperative management, only 12% developed an invasive cancer during 50-month follow-up.[19] In the latter study, independent predictors of poor disease-specific survival were age >70 years, atypical/malignant cyst fluid cytology, jaundice, and MD >15 mm. Patients with worrisome features had better 5-year disease-specific survival compared with those with high-risk stigmata, and in elderly patients with IPMNs with worrisome features, the 5-year disease-specific survival was 96%. The authors concluded that conservative management is appropriate in elderly patients. On the other hand, the presence of high-risk stigmata was associated with a 40% risk of IPMN-related death, strengthening that surgical resection should be offered to fit patients.
Despite low progression, pancreatic cystic lesions generate patients’ anxiety because of risk of pancreatic cancer, perceived as lethal condition. A recent study investigating the patients’ mood after surgery of pancreatic cyst concluded that patients are highly satisfied with their decision to have surgery, regardless of the final diagnosis, because the fear of cancer and anxiety of the cyst greatly affected their quality of life.[20] However, there is a significant gap among patients about cyst knowledge, and greater emphasis on patient education could improve patients’ knowledge by reducing anxiety and fear.
WHAT CLINICIAN NEEDS
We must face and manage a huge number of patients, mainly elderly with comorbidities, often unfit for surgery or with high surgical risk, and with a disease characterized by indolent natural history and rare malignant transformation.
However, BD-IPMNs may develop malignancy, though rarely, and special care must be taken in younger patients. The lack of test that accurately defines the cystic type and predicts its biologic behavior implies many years of follow-up with expensive imaging (e.g., magnetic resonance imaging) and invasive procedures (e.g., EUS). Indeed, current international guidelines for the management of IPMN are mainly based on cyst morphology at imaging and to promptly identify the onset of worrisome features or high-risk stigmata that are associated with malignancy.[1]
Furthermore, pancreatic cysts are not always IPMNs. In the absence of communication with the ductal system, a different diagnosis between serous cystadenoma (SCA) and mucinous cystic neoplasm (MCN) is the main goal because of implication on the management. MCN is the only cyst type most of the times recommended for resection for its malignant potential, whereas SCA is always benign and surgery is reserved to symptomatic large neoplasm. Moreover, a delayed follow-up schedule is reasonable for SCA. Unfortunately, among uniloculated/oligocystic lesions, there is a large radiological and cystic fluid analysis overlap that makes the differential diagnosis often difficult. No diagnostic test or tool can, nowadays, unequivocally define the cyst type that often remains undetermined. A new micro-forceps suitable to be passed through a 19G needle under EUS guidance that can provide a fragment of the cyst wall improving the accuracy in differential diagnosis between mucinous and nonmucinous cyst has recently become available.[21] Moreover, progression in the field of molecular biology could define a panel of mutations identifiable on cystic fluid able to define the diagnosis and to predict the prognosis of the lesion.[22] However, micro-forceps biopsy is skill demanding, and DNA molecular analysis remains expensive and not widely available. Therefore, these tools are not currently applicable in clinical practice. Meanwhile, large prospective studies are warranted to validate their clinical impact. Today, based on our knowledge, a follow-up strategy is mandatory in a patient considered to be fit for pancreatic surgery. Since timing and modality of the follow-up strictly depend on presumptive diagnosis, classification of the cyst at the time of diagnosis is crucial, and a multidisciplinary evaluation is, therefore, recommended in difficult cases. Moreover, it is extremely important to evaluate the person “around the cyst.” Indeed, the diagnostic/therapeutic workup is strictly related to the patients’ age and comorbidities. Last but not least, physicians must keep in mind that a new onset of pancreatic cyst may represent an epiphenomenon of a missed pancreatic cancer at imaging. Therefore, a first short-time (3 months) follow-up to promptly identify any underlying solid tumor, even in the absence of worrisome features at first observation, should be reasonable.
FINAL CLINICAL CONSIDERATIONS
In conclusion, it is likely that the majority of patients with asymptomatic cyst will never undergo surgical resection and will never develop malignancy. However, progression to pancreatic mucinous adenocarcinoma is possible. Until a safe, feasible, and reproducible test able to accurately predict cyst type and behavior will become available, considering the need of resources optimization, we must try:
To differentiate serous with mucinous lesions
To diagnose malignancy or alterations highly suggestive for malignancy
To use the cheaper and less invasive imaging modality
-
To limit invasive tests (biopsies):
- To a number of very selected patients
- Only if they change the clinical decision (i.e., surgery).
Conflict of Interest
There are no conflicts of interest.
REFERENCES
- 1.Tanaka M, Fernández-del Castillo C, et al. International consensus guidelines, 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: Not harmless. Ann Surg. 2012;256:313–20. doi: 10.1097/SLA.0b013e31825d355f. [DOI] [PubMed] [Google Scholar]
- 3.Fritz S, Klauss M, Bergmann F, et al. Pancreatic main-duct involvement in branch-duct IPMNs: An underestimated risk. Ann Surg. 2014;260:848–55. doi: 10.1097/SLA.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 4.Gordon-Dseagu VL, Devesa SS, Goggins M, et al. Pancreatic cancer incidence trends: Evidence from the surveillance, epidemiology and end results population-based data. Int J Epidemiol. 2018;47:427–39. doi: 10.1093/ije/dyx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–53. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 6.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–84. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10:e0121317. doi: 10.1371/journal.pone.0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanini N, Giordano M, Smerieri E, et al. Estimation of the prevalence of asymptomatic pancreatic cysts in the population of san marino. Pancreatology. 2015;15:417–22. doi: 10.1016/j.pan.2015.05.461. [DOI] [PubMed] [Google Scholar]
- 11.Sey MS, Teagarden S, Settles D, et al. Prospective cross-sectional study of the prevalence of incidental pancreatic cysts during routine outpatient endoscopic ultrasound. Pancreas. 2015;44:1130–3. doi: 10.1097/MPA.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 12.Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol. 2016;14:585–93. doi: 10.1016/j.cgh.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Soroida Y, Sato M, Hikita H, et al. Pancreatic cysts in general population on ultrasonography: Prevalence and development of risk score. J Gastroenterol. 2016;51:1133–40. doi: 10.1007/s00535-016-1196-y. [DOI] [PubMed] [Google Scholar]
- 14.Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–45. doi: 10.1136/gutjnl-2016-313127. [DOI] [PubMed] [Google Scholar]
- 15.Martínez B, Martínez JF, Aparicio JR. Prevalence of incidental pancreatic cyst on upper endoscopic ultrasound. Ann Gastroenterol. 2018;31:90–5. doi: 10.20524/aog.2017.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YR, Park JK, Jang JY, et al. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore) 2016;95:e5535. doi: 10.1097/MD.0000000000005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol. 2013;108:1546–50. doi: 10.1038/ajg.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayal M, Luk L, Hecht EM, et al. Long-term surveillance and timeline of progression of presumed low-risk intraductal papillary mucinous neoplasms. AJR Am J Roentgenol. 2017;209:320–6. doi: 10.2214/AJR.16.17249. [DOI] [PubMed] [Google Scholar]
- 19.Crippa S, Pezzilli R, Bissolati M, et al. Active surveillance beyond 5 years is required for presumed branch-duct intraductal papillary mucinous neoplasms undergoing non-operative management. Am J Gastroenterol. 2017;112:1153–61. doi: 10.1038/ajg.2017.43. [DOI] [PubMed] [Google Scholar]
- 20.Puri PM, Watkins AA, Kent TS, et al. Decision-making for the management of cystic lesions of the pancreas: How satisfied are patients with surgery? J Gastrointest Surg. 2018;22:88–97. doi: 10.1007/s11605-017-3564-1. [DOI] [PubMed] [Google Scholar]
- 21.Barresi L, Crinò SF, Fabbri C, et al. Endoscopic ultrasound-throughthe-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig Endosc. 2018 doi: 10.1111/den.13197. doi: 10.1111/den.13197. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2017 doi: 10.1136/gutjnl-2016-313586. pii: gutjnl-2016-313586. [DOI] [PMC free article] [PubMed] [Google Scholar]


