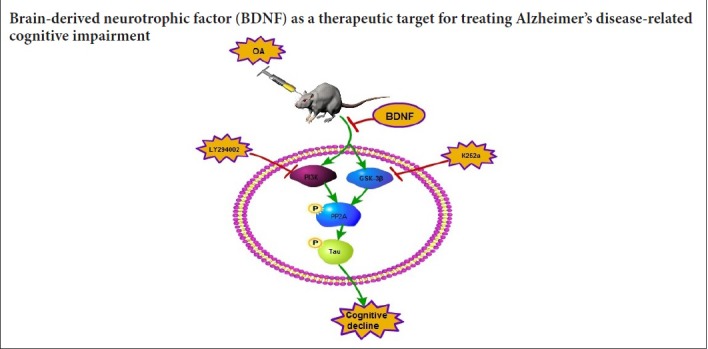
Keywords: nerve regeneration, Alzheimer's disease, exogenous brain-derived neurotrophic factor, Tau protein, okadaic acid, phosphorylation, PP2A-Y307, glycogen synthase kinase-3β, TrkB, cognitive function, brain protection, neural regeneration
Abstract
Decreased expression of brain-derived neurotrophic factor (BDNF) plays an important role in the pathogenesis of Alzheimer's disease, and a typical pathological change in Alzheimer's disease is neurofibrillary tangles caused by hyperphosphorylation of tau. An in vivo model of Alzheimer's disease was developed by injecting okadaic acid (2 μL) and exogenous BDNF (2 μL) into the hippocampi of adult male Wister rats. Spatial learning and memory abilities were assessed using the Morris water maze. The expression levels of protein phosphatase 2A (PP2A), PP2Ac-Yp307, p-tau (Thr231), and p-tau (Ser396/404) were detected by western blot assay. The expression levels of BDNF, TrkB, and synaptophysin mRNA were measured by quantitative real-time polymerase chain reaction. Our results indicated that BDNF expression was suppressed in the hippocampus of OA-treated rats, which resulted in learning and memory deficits. Intra-hippocampal injection of BDNF attenuated this OA-induced cognitive impairment. Finally, our findings indicated an involvement of the PI3K/GSK-3β/AKT pathway in the mechanism of BDNF in regulating cognitive function. These results indicate that BDNF has beneficial effect on Alzheimer's disease, and highlight the potential of BDNF as a drug target for treatment of Alzheimer's disease.
Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disease, accounting for 50–75% of all dementias; about 131 million people worldwide have dementia, and AD is expected to become 3-fold more prevalent by 2050 (Wirz et al., 2014; Alzheimer's Association, 2015; Purnell et al., 2015). Neurofibrillary tangles are typically seen in patients with AD and are strongly associated with dementia severity in these patients (Whittington et al., 2013). The main component of these neurofibrillary tangles is hyperphosphorylated tau protein (Liu et al., 2008; Ye et al., 2017). Tau protein normally exists in the axon and cytoplasm of neurons. The microtubule-associated protein tau stabilizes microtubules, which supports the axonal transport of proteins, vesicles, and organelles (Morris et al., 2011). The biological activity of Tau protein is regulated by phosphorylation and dephosphorylation. Protein phosphatase 2A (PP2A) is the major intracerebral phosphoesterase, which dephosphorylates the tau protein. A previous study showed that the activity and expression of PP2A were reduced by 20% in a mouse model of AD (Zhao et al., 2013). Inhibition of PP2A activity by decreasing PP2A methylation of the catalytic subunit of L309 or increasing Y307 phosphorylation may result in the aggregation and hyperphosphorylation of tau protein (Zhou et al., 2008; Xiong et al., 2013). At present, it is believed that AD and other neurodegenerative diseases are associated with impaired axonal transport, an imbalance of neurotrophic factors (Schindowski et al., 2008; Gan et al., 2015). The over-phosphorylation of Tau protein can lead to abnormal microtubule function, cytoskeleton instability, and axonal transport dysfunction (Lu et al., 2013; Metaxas and Kempf, 2016). Furthermore, it has been reported that abnormal hyperphosphorylation of tau protein is an early manifestation of AD (Caraci et al., 2013; Zhang et al., 2016); thus, inhibition of tau protein phosphorylation is thought to be the key to AD therapy (Iqbal et al., 2014).
Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that can promote the survival and growth of many kinds of neurons, increase the activity of antioxidant enzymes in cells and fight against free radicals, and inhibit the excitatory amino acid cytotoxicity and apoptosis (Huang and Reichardt, 2001; Liu et al.,2016; Sampaio et al., 2017). BDNF also participates in cognition, learning, and memory formation (Yamada and Nabeshima, 2003). The levels of BDNF and its receptor have been found to be markedly decreased in the hippocampus of patients with AD, which may cause the cognitive impairment (Danzer et al., 2004). Conversely, enhanced levels of BDNF may delay age-related cognitive decline, including AD neuropathology, which suggests that BDNF may be a biomarker for the diagnosis and treatment of AD (Beeri and Sonnen, 2016; Buchman et al., 2016). However, the underlying mechanisms by which BDNF affects AD are still not clear.
We used okadaic acid (OA) to establish an animal and cell model of AD, evaluated the beneficial role of exogenous BDNF in OA-induced hyperphosphorylation of tau and the resulting cognitive dysfunction in vivo and in vitro, and also elucidated the possible mechanisms of this effect.
Materials and Methods
Animals
A total of 36 healthy adult male Wister rats aged 7–8 weeks and weighing 200 ± 18 g were provided by the Experimental Animal Center of China Medical University, China (license number: SCXK (Liao) 2003-0001). These rats were housed at 20 ± 2°C with natural ventilation and 12-hour light-dark cycle. Animals had access to standard rat chow and water ad libitum. The present study was approved by the Animal Care and Use Committee of China Medical University (ethical approval number: 1003M) and performed in accordance with the National Institutes of Health Guidelines of the Care and Use of Laboratory Animals.
Induction of AD models in vivo and drug intervention
The animal model of AD was prepared by stereotaxic injection technique with OA (Broetto et al., 2016). Rats were anesthetized with intraperitoneal injection of 10% chloral hydrate (300 mg/kg) and mounted on a stereotaxic apparatus (RWD Life Science Co., Ltd., Shenzhen, China). The skulls of the rats were opened and drilled with burr holes on both sides of hippocampal CA3 area according to a rat brain stereotaxic atlas (anteroposterior: −3.8 mm, mediolateral: ±3.8 mm, dorsoventral: 4.0 mm) (Shirazi-Southall et al., 2002).
The 36 rats were randomly and equally divided into six groups (n = 6 per group) as follows: (1) Sham group: Both hippocampi were injected with 2 μL of artificial cerebrospinal fluid; (2) OA group: Both sides of the hippocampus were injected with 2 μL of OA (0.2 μM; Upstate Biotechnology, Inc., Lake Placid, NY, USA) dissolved in dimethyl sulfoxide at 1 μM and diluted to 0.2 μM in artificial cerebrospinal fluid; (3) OA + BDNF group: Both sides of the hippocampus were injected with 2 μL of OA (0.2 μM) and 2 μL of BDNF (50 ng/mL human full-length BDNF protein; Millipore Corp., Billerica, MA, USA) (Yuan et al., 2017); (4) OA + BDNF + K252a group: Both sides of the hippocampus were injected with 2 μL of OA (0.2 μM), 2 μL of BDNF (50 ng/mL), and 2 μL of K252a (0.2 μM, the inhibitor of the BDNF-specific receptor, TrkB; Santa Cruz Biotechnology, Santa Cruz, CA, USA); (5) OA + BDNF + LY294002 group: Both sides of the hippocampus were injected with 2 μL of OA (0.2 μM), 2 μL of BDNF (50 ng/mL), and 2 μL of LY294002 (0.2 μM, the inhibitor of PI3K, Santa Cruz Biotechnology); (6) rats in the normal control group received no treatment. The injection lasted for 5 minutes, and the needle with the syringe was left in place for 2 minutes after the injection to ensure complete infusion of the drug. After surgery, the rats were housed individually and had free access to food and water. Penicillin was applied daily, and the rats were allowed 7 days to recover from surgery. No unintended deaths of animals occurred during the surgery. The general condition of the animals, including body weight, food and water intake, was monitored daily after surgery.
Cell culture, induction of AD model in vitro, and drug intervention
The SH-SY5Y cells (human neuroblastoma cells, derived from human neuroblastoma cell lines SK2N and 2SH) were obtained from Shanghai Institute of Cell Biology (Shanghai, China) and maintained in Dulbecco's modified Eagle's medium (DMEM)-F12 (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal calf serum (Hyclone, Logan, UT, USA), 2 mM L glutamine (Sigma, Santa Clara, CA, USA), and 1% non essential amino acids (100× stock; Euroclone, Italy) in a humidified chamber with 5% CO2 at 37°C. For western blot assay, the cells were cultured in a culture bottle. For immunofluorescence staining, the cells were cultured in six-well plates. The cells in the logarithmic growth phase were treated and grouped as follows: (1) Normal control group without treatment; (2) OA group: treated with OA (40 nM) for 24 hours; (3) OA + BDNF group: treated with OA (40 nM) for 24 hours, followed by adding BDNF (50 ng/mL), and incubated for 15 minutes; (4) OA + BDNF + LY294002 group: treated with OA (40 nM) for 24 hours, followed by adding BDNF (50 ng/mL) and LY294002 (4 μM), and incubated for 15 minutes; (5) OA + BDNF + K252a group: treated with OA (40 nM) for 24 hours, followed by adding BDNF (50 ng/mL) and K252a (4 μM), and incubated for 15 minutes.
Morris water maze test
On day 14 after modeling, spatial learning and memory of the rats was assessed using a Morris water maze test according to a previous study with some modifications (Bromley-Brits et al., 2011). The experimental apparatus (RWD Life Science, Shenzhen, China) was a circular water pool (diameter 150 cm; height 60 cm; containing water at 24 ± 2°C) with four equally spaced quadrants. The pool was placed in a test room containing various prominent visual cues. A translucent 10 cm × 10 cm platform, submerged 1 cm below the water surface, was hidden in the center of quadrant II during the training period and was then removed for the probe task. Memory training was performed 7 days after OA injection. The training was conducted twice every morning and afternoon, for 4 days before the probe task. Each rat was allowed to swim until it found the platform or until 120 seconds had elapsed. The rat was left on the platform for 60 seconds. During the spatial probe task, the platform was removed from the pool and the rats were allowed to swim for 120 seconds. The swim escape latency, average swim speed, time spent in the target quadrant, and number of times the animal crossed the previous location of the platform were recorded by a video tracking system (SMART, Panlab SL, Barcelona, Spain).
Western blot assay
On day 18 after modeling, the hippocampal tissues and the SH-SY5Y cells were used in the western blot assay. At the end of the final behavioral test, the rats were intraperitoneally deeply anesthetized with 10% chloral hydrate 300 mg/kg under non-stress conditions and sacrificed by rapid decapitation. The brains were quickly removed and both sides of hippocampal tissues were carefully dissected on ice. To extract the protein, tissues were homogenized in a precooled RIPA buffer (50 mM Tris-HCl, 50 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 10 mM ethylene glycol tetraacetate, 2 mM sodium pyrophosphate, 4 mM paranitrophenylphosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 2 μg/mL pepstatin, pH 7.5). The homogenates were incubated on ice for 30 minutes and centrifuged at 12,000 × g for 15 minutes at 4°C.
The cells for western blot assay were harvested by scraping, washed in PBS, resuspended, then homogenized and sonicated in RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 1 mM sodium orthovanadate, 10 mg/mL aprotinin, and 100 mg/mL phenylmethyl sulphonyl fluoride. Lysates were then centrifuged at 12,000 × g for 10 minutes at 4°C, and supernatants were collected for analysis.
The protein contents in the supernatants were determined by Bradford reagent assay (Ku et al., 2013). Protein from the hippocampal and the treated cells was mixed with loading buffer, boiled for 5 minutes, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to an Immobilon-P polyvinylidene difluoride (Millipore Corp.) membrane. Membranes were blocked with 5% dry milk in PBS. Proteins were probed with specific antibodies Tau (Ser199/202) (1:500; rabbit, Millipore Corp.), pTau (Ser199/202) (1:500; rabbit, Millipore Corp.), phospho-glycogen synthase kinase (pGSK)-3β (1:1000; rabbit, Cell Signaling Technology, Danvers, MA, USA), phospho-AKT (pAKT) (1:1000; rabbit, Santa Cruz Biotechnology), PP2A (1:1000; goat, Santa Cruz Biotechnology), PP2Ac-Yp307 (1:500; goat, Santa Cruz Biotechnology), Tau-5 (1:1000; mouse, Santa Cruz Biotechnology), p-tau (Thr231) (1:1000; mouse, Santa Cruz Biotechnology, p-tau (Ser396/404) (1:1000; goat, Santa Cruz Biotechnology, and β-actin (1:5000; mouse, Sigma, Santa Clara, CA, USA), and incubated overnight at 4°C. The next day, the membranes were washed four times with 0.1% Tween-20 TBS (pH 7.6) and incubated with horseradish peroxidase-conjugated anti-rabbit, anti-goat, or anti-mouse secondary antibodies (1:2000; Beyotime, Haimen, China) at room temperature for 2 hours. An enhanced chemiluminescence kit (Millipore Corp.) was used to detect immunoreactive protein bands. Protein levels were normalized to β-actin. Relative optical density of protein bands was measured following subtraction of the film background using Scion Image software (Scion Corporation, Frederick, MD, USA).
Quantitative real-time polymerase chain reaction
The fresh hippocampal tissues were put in commercial RNA extraction reagent (Roche Applied Science, Indianapolis, IN, USA) for quantitative polymerase chain reaction (qPCR) analysis. First-strand cDNA was synthesized with the use of 1 μg of total RNA (Transcriptor First Strand cDNA Synthesis Kit, Roche Applied Science). The qPCR was performed with Applied Biosystems Step One (Applied Biosystems, Foster City, CA, USA) using SYBR Green Master solution (Roche Applied Science) to measure the fluorescence intensity of amplified products. Reactions were as follows: 55°C for 2 minutes, 95°C for 10 minutes, and then 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute. Data were analyzed using the 2−ΔΔCt method, as previously described (Avnet et al., 2017), with β-actin as a housekeeping gene. Fold change of all groups was normalized to those of the control group. The sequences of primers are shown in Table 1. All the sequence specificities of the primers used in the current study have been verified by Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/).
Table 1.
Oligonucleotide primer sets for real-time polymerase chain reaction

Immunofluorescence staining
SH-SY5Y cells were cultured in the six-well plates. During the logarithmic phase, the cells were received different treatments. After being fixed with 4% paraformaldehyde for 1 hour, cells were perforated with 0.5% Triton for 15 minutes, blocked with TBS (pH 7.5) at room temperature for 30 minutes, and then incubated with either anti-PP2Ac-Yp307 (1:100; goat, Santacruz Biotechnology, Santa Cruz, CA, USA) or anti-pTau (Ser199/202) (1:100; rabbit, Chemicon, Bilka, MA, USA) at 37°C for 2 hours. After washing twice with PBS for 10 minutes, the Cy3-labeled goat anti-rabbit IgG (1:200; Beyotime, Haimen, China) or FITC-labeled rabbit anti-goat IgG (1:200; Heowns, Tianjin, China) was added and incubated at 37°C for 30 minutes. Finally, after four washes with TBS for 5 minutes, the cells were sealed with quenching agent and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (Ver. 14.0; SPSS Inc., Chicago, IL, USA). Experiments was repeated three times. Data are shown as the mean ± SD. One-way analysis of variance followed by Bonferroni confidence interval adjustment tests was conducted to evaluate dynamic changes in all experimental data. P < 0.05 was considered statistically significant.
Results
Hippocampal injection of BDNF attenuates OA-induced cognitive decline
The Morris water maze test was performed to assess the protective effect of OA on spatial learning ability of OA-treated rats. Results of navigation paths indicated that spatial learning capacity of OA-treated rats was remarkably compared with the sham and normal control groups. The escape latencies were longer in the OA-treated rats than those in sham group (P < 0.001). In probe trials, OA-treated rats showed a fewer number of platform crosses than the sham group (P < 0.001), which indicates that OA administration impaired memory and spatial learning ability of the rats. However, administration of BDNF remarkably improved spatial learning ability in rats. BDNF-treated groups showed significantly shorter escape latencies and more platform crosses compared with the OA group (P < 0.001). Nevertheless, when K252a was added, the effect of BDNF was blocked; the escape latencies were longer and the number of platform crossings was lower than those of the OA + BDNF group (P < 0.001 and P < 0.01, respectively). When LY294002, the specific inhibitor of PI3K was added, there was no notable difference in escape latencies or the number of platform crossings compared with the OA + BDNF group (P > 0.05; Figure 1).
Figure 1.
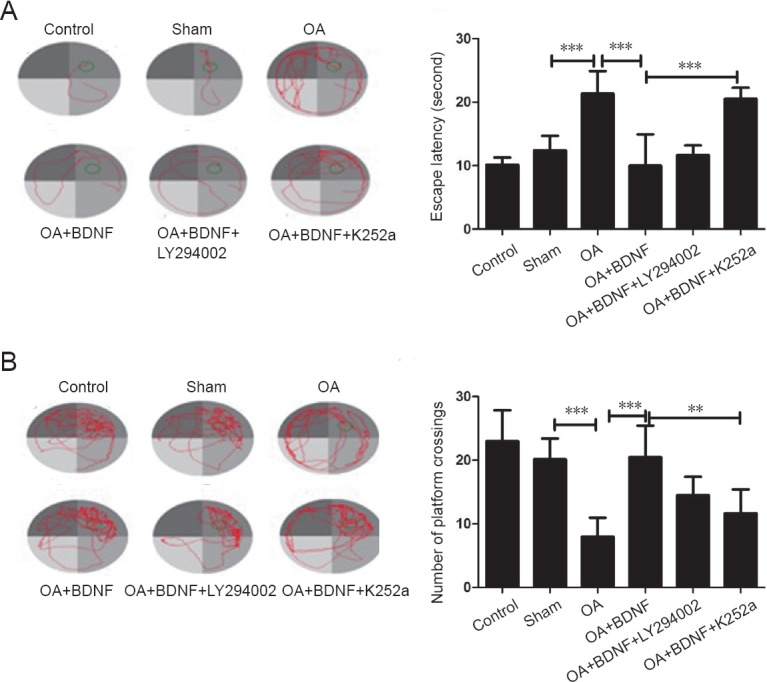
Exogenous BDNF improves cognitive function in a rat model of Alzheimer's disease induced by OA.
(A) The swim traces and escape latencies in the navigation experiment. (B) The swim traces and number of platform crossings in the space exploration experiment. Data are expressed as the mean ± SD (one-way analysis of variance followed by the Bonferroni confidence interval adjustment test). **P < 0.01, ***P < 0.001. OA: Okadaic acid; BDNF: brain-derived neurotrophic factor; LY2940002: inhibitor of PI3K; K252a: inhibitor of the BDNF-specific receptor.
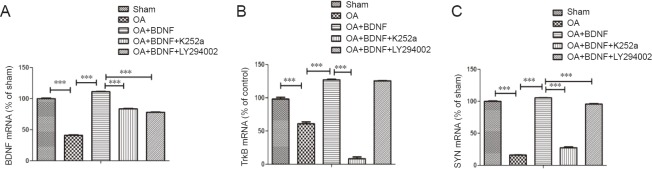
Exogenous BDNF reverses the cognitive dysfunction of AD rats by increasing the BDNF/TrkB/SYN mRNA levels
Normal BDNF levels and synaptic function are important for learning (Caroni et al., 2014; Petzold et al., 2015). Our qPCR results showed that compared with the sham group, the mRNA levels of BDNF, TrkB, and SYN, were significantly decreased in the OA group (all P < 0.001) and were up-regulated by administration of BDNF (all P < 0.001). However, both LY294002 and K252a inhibited the effect of BDNF on BDNF, TrkB, and SYN mRNA expressions (all P < 0.001; Figure 2). These results revealed that the exogenous BDNF may ameliorate cognitive function in AD rats by increasing BDNF levels and promoting synaptic function.
Figure 2.
Exogenous BDNF increases BDNF/TrkB/SYN mRNA levels of Alzheimer's disease rats.
BDNF (A), TrkB (B), and SYN (C) mRNA expression in the rat hippocampus of each group was examined using quantitative polymerase chain reaction. Data are expressed as the mean ± SD (one-way analysis of variance followed by the Bonferroni confidence interval adjustment test). ***P < 0.001. BDNF: Brain-derived neurotrophic factor; SYN: synaptophysin; OA: okadaic acid; LY294002: inhibitor of PI3K; K252a: inhibitor of the BDNF-specific receptor.
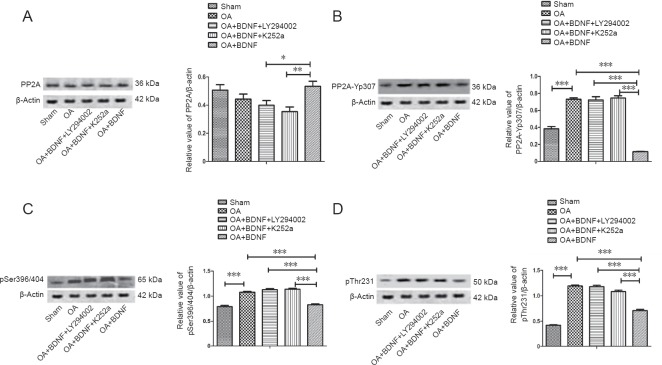
Exogenous BDNF rescues the phosphorylation of PP2A and tau protein by OA in vivo
To test the protective effect of BDNF on the over-phosphorylation of PP2A and tau protein induced by OA, we examined the levels of PP2A, PP2Ac-Yp307, p-tau (Ser396/404), and p-tau (Thr231) using a western blot assay after the stereotaxic injection. BDNF treatment inhibited the OA-induced decrease in PP2A expression and the increase in PP2Ac-Yp307, p-tau (Ser396/404), and p-tau (Thr231) levels compared with the sham group. LY294002 and K252a treatment obviously attenuated the beneficial effect of BDNF, as compared with the OA + BDNF group (P < 0.001; Figure 3).
Figure 3.
Exogenous BDNF rescues the OA-induced phosphorylation of PP2A and tau protein in vivo.
The expression of PP2A (A), PP2Ac-Yp307 (B), p-tau (Ser396/404) (pSer396/404) (C), and p-tau (Thr231) (D) protein of each group was analyzed using western blot assay. Data are expressed as the mean ± SD (one-way analysis of variance followed by the Bonferroni confidence interval adjustment test). *P < 0.05, **P < 0.01, ***P < 0.001. BDNF: Brain-derived neurotrophic factor; PP2A: protein phosphatase 2A; OA: okadaic acid; LY294002: inhibitor of PI3K; K252a: inhibitor of the BDNF-specific receptor.
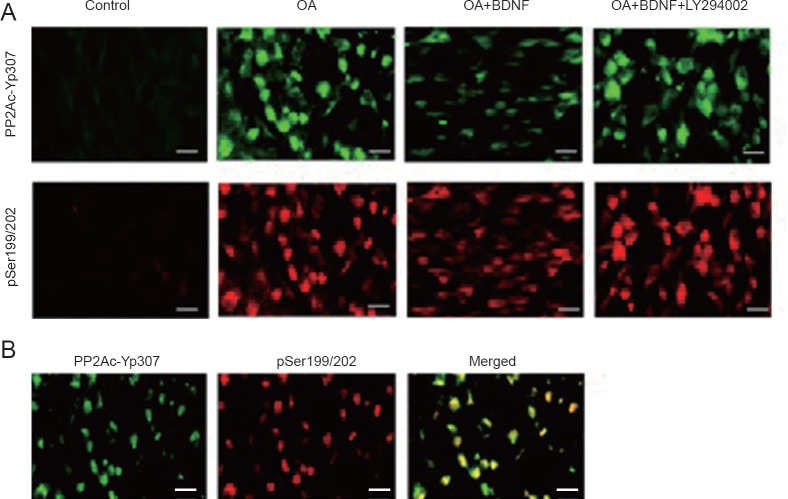
Exogenous BDNF reduces phosphorylation of tau by enhancing the activity of PP2A in vitro
Immunofluorescence staining results showed that In the normal control group, there were hardly any PP2Ac-Yp307- positive nd pTau (Ser199/202)-positive cells; after OA treatment, the number of both PP2Ac-Yp307-positive and pTau (Ser199/202)-positive cells was obviously increased, and the staining of pTau (Ser199/202) and PP2Ac-Yp307 proteins was highly co-localized. The addition of exogenous BDNF markedly reduced positive staining of both PP2Ac-Yp307 and pTau (Ser199/202); however, when LY294002 was present, the effect of BDNF was weakened, as evidenced by increased positive staining of PP2Ac-Yp307 and pTau (Ser199/202) (Figure 4).
Figure 4.
Correlation between phosphorylated PP2Ac (PP2Ac-Yp307) and phosphorylated tau, and the effect of exogenous BDNF in vitro.
(A) PP2Ac-Yp307-positive cells (green, FITC) and pTau (Ser199/202) (pSer199/202)-positive cells (red, Cy3) were revealed through the immunofluorescence technique. (B) The pSer199/202 and PP2Ac-Yp307 were highly co-locative in the OA group. Scale bars: 50 μm. BDNF: Brain-derived neurotrophic factor; OA: okadaic acid; FITC: fluorescein isothiocyanate; Cy3: Cyanine3; LY294002: inhibitor of PI3K; K252a: inhibitor of the BDNF-specific receptor.
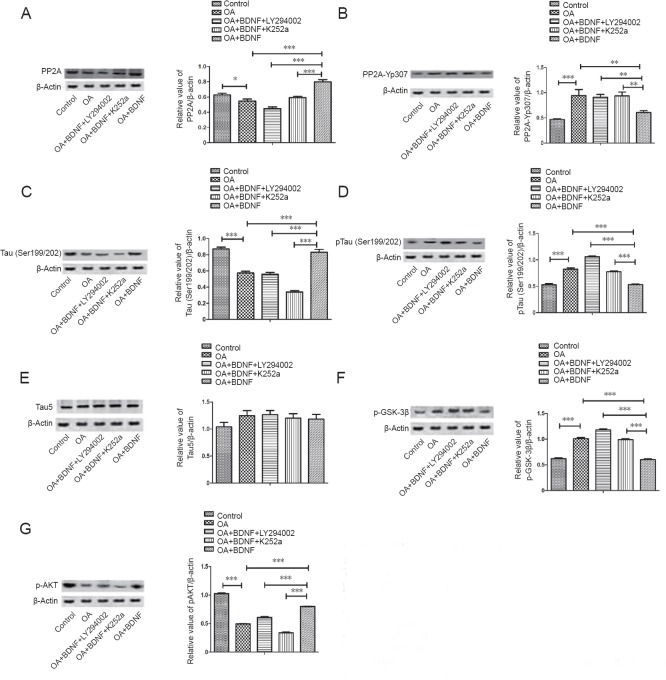
BDNF activates PP2A and inhibits tau phosphorylation through the PI3K/GSK-3β pathway in vitro
To further study how BDNF regulated PP2A and tau phosphorylation, western blot assay was performed to examine the associated proteins. In the OA group, PP2A expression was significantly lower (P < 0.05 and P < 0.001; Figure 5A) and the level of PP2Ac-Yp307 was markedly higher (P < 0.01 and P < 0.001; Figure 5B) than the normal control and BDNF groups, but the level of tau (Ser199/202) (Figure 5C) was lower in the OA group, and the level of ptau (Ser199/202) (Figure 5D) was higher in the OA-treated group (P < 0.05 and P < 0.001) compared with the normal control and BDNF groups. No between-group differences were found in total tau protein expression (Figure 5E). In the LY294002 group, PP2A and Tau (Ser199/202) levels were significantly reduced (all P < 0.001l), and the levels of PP2Ac-Yp307 and pTau (Ser199/202) were obviously enhanced compared with the BDNF group (Figure 5A–D) (for PP2Ac-Yp307, P < 0.01; for pTau (Ser199/202), P < 0.001). Compared with the normal control and BDNF groups, the OA group showed a higher level of pGSK-3β all P < 0.001; Figure 5F), and a lower level of pAKT (all P < 0.001; Figure 5G).
Figure 5.
BDNF activates PP2A and inhibits tau phosphorylation via the PI3K/GSK-3β pathway in vitro.
The protein levels of PP2A (A), PP2Ac-Yp307 (B), Tau (Ser199/202) (C), pTau (pSer199/202) (D), Tau5 (E), p-GSK-3β (F), and p-AKT (G) in cells with different treatments were analyzed using western blot assay. Data are expressed as the mean ± SD (one-way analysis of variance followed by the Bonferroni confidence interval adjustment test). *P < 0.05, **P < 0.01, ***P < 0.001. BDNF: Brain-derived neurotrophic factor; PP2A: protein phosphatase 2A; PI3K: phosphoinositide 3-kinase; GSK: glycogen synthase kinase; OA: okadaic acid; LY294002: inhibitor of PI3K; K252a: inhibitor of the BDNF-specific receptor.
Discussion
In this study, OA was used to induce the phosphorylation of PP2A and tau protein in rats and human SH-SY5Y cells. OA caused cognitive dysfunction in rats, which we suggest was the result of significantly decreased PP2A activity, an increase in tau phosphorylation, and subsequent synapse dysfunction. Exogenous BDNF reversed the cognitive dysfunction in AD rats by increasing PP2A activity and then decreasing tau phosphorylation, and also by reversing synapse dysfunction. The protective effect of BDNF on the AD brain was achieved through the PI3K/GSK-3β/AKT pathway. Our results indicate that BDNF may be a potential therapeutic target for AD.
In this study, we found that OA treatment decreased PP2A activity and dramatically increased tau protein phosphorylation both in rats and SH-SY5Y cell models of AD. Expression of the inactive PP2A was highly correlated with phosphorylated tau in SH-SY5Y cell models of AD. Tau protein is known to be involved in dementia-associated disorders, including AD, progressive supranuclear palsy, chronic traumatic encephalopathy, Pick's disease, and corticobasal degeneration (Arendt et al., 2016). Tau protein is also known to play crucial roles in axonal transport and microtubule stabilization (Kneynsberg et al., 2017). Hyperphosphorylation of tau protein causes disruption of axonal transport, which results in an imbalance of neurotrophic factors and neuronal death (Baird and Bennett, 2013; Le et al., 2016). PP2A contributes to the regulation of tau phosphorylation in the brain, and determines an estimated 70% of tau phosphatase activity (Liu et al., 2005; Martin et al., 2013a; Martin et al., 2013b; Sontag and Sontag, 2014). PP2A mRNA and protein expression in the AD brain is confirmed to be below normal levels (Vogelsberg-Ragaglia et al., 2001; Sontag et al., 2004), while the expression of its inhibitor 2 (I2PP2A) is increased (Tanimukai et al., 2005). Our results were consistent with these reports, so in vivo and in vitro AD models were successfully established.
Moreover, the present study found that OA injection into the CA3 area of hippocampus obviously accelerated tau protein phosphorylation, and markedly suppressed the expression of SYN. Emerging data have suggested that tau plays a critical role in synaptic plasticity via binding related partners (Ittner et al., 2010; Morris et al., 2011; Nisbet et al., 2015; Regan et al., 2016). Synapses form the structural basis for memory formation. Indeed, synaptic loss and dysfunction are important pathological features in the early AD brain (Pozueta et al., 2013). In one study, tau was revealed to be involved in early synaptic deficits before tau tangles formed and obvious neurodegenerative disorder occurred (Hoover et al., 2010). The multiple cellular events caused by tau hyperphosphorylation can eventually result in synaptic dysfunction and neurodegeneration (Morris et al., 2011), while inhibiting tau phosphorylation can block the mislocalization of endogenous tau (Miller et al., 2014). SYN is a specific marker for synaptic structure, constitutes synaptic vesicle-specific membrane channels, and is involved in vesicle transport and emission; SYN density has been found to be negatively correlated with the severity of dementia of AD (Wang et al., 2016). When the over-phosphorylated tau was dephosphorylated, SYN expression also increased, and the cognitive dysfunction was reversed. Thus, synaptic function may be affected by the phosphorylation of tau in the AD brain. Consistent with these reports, our results demonstrated that tau and SYN were involved in OA-induced AD.
BDNF is a key regulator of AD-associated cognitive disorders; it affects the survival of neurons, synaptic plasticity, and the memory function (Lu et al., 2014). BDNF participates in neuronal connectivity and neuroplasticity, which contributes to synaptic efficacy (Duman et al., 2000; Cotman and Berchtold, 2002). BDNF is also a promising drug target of AD because it protects adult neurons from injury in the hippocampus, cerebral cortex, and basal forebrain (Connor and Dragunow, 1998; Murer et al., 2001). Atasoy et al. (2017) reported that tau hyperphosphorylation reduced BDNF secretion and decreased BDNF levels in cortical neurons. Furthermore, the loss of BDNF has been found to promote neurite atrophy in patients with AD, and increasing BDNF levels may delay the course of AD (Nagahara et al., 2009; Nagahara et al., 2013). However, the underlying mechanism by which BDNF works on tau is unclear. We hypothesized that BDNF protects against cognitive decline via promoting neuronal survival and facilitating synaptic plasticity. The present results provide direct support for this; hippocampal OA injection obviously impaired spatial learning and memory abilities, which were strongly correlated with decreased BDNF levels. These results suggest that the inhibition of BDNF expression by over-phosphorylated tau impairs mnemonic processes in animals. Furthermore, the infusion of exogenous BDNF into the CA3 area of the hippocampus reversed the cognitive impairment induced by OA in rats via activation of PP2A and consequent dephosphorylation of tau protein, and a subsequent increase in the expression of SYN; all these effects were dependent on activation of the PI3K/AKT/GSK-3β pathway. Furthermore, in this study, when the PI3K inhibitor LY294002 was introduced, the dephosphorylation effect of BDNF on tau protein dramatically decreased, while the effect of BDNF on SYN expression and the improvement in learning and memory of AD rats were not affected. This suggests that other pathways are also involved in the effect of BDNF in the AD brain.
This study has some limitations that should be noted. First, we were not able to fully elucidate the detailed mechanisms of dephosphorylation of tau protein by BDNF treatment. We will investigate this in future experiments. Second, the in vitro experiments were performed on only one cell line, so the results should be verified using more cell lines in future work.
Taken together, this study found that BDNF expression was suppressed in the hippocampus of OA-treated rats, which resulted in obvious cognitive deficits. Intra-hippocampal injection of BDNF attenuated OA-induced cognitive impairment. Finally, the PI3K/GSK-3β/AKT pathway was involved in the mechanism of BDNF in regulating cognitive function. Our results offer a theoretical basis for BDNF as a therapeutic target for treating AD-related cognitive impairment.
Additional file: Open peer review report 1 (5.4KB, pdf) .
Footnotes
Conflicts of interest: There are no conflictes of interest associated with the paper.
Financial support: None.
Institutional review board statement: The study was approved by the Animal Care and Use Committee of China Medical University (ethical approval number: 1003M).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Gaoshang Chai, Jiangnan University, China.
P-Reviewer: Chai G; C-Editor: Zhao M; S-Editor: Yu J, Li CH; L-Editor: Qiu Y; T-Editor: Liu XL
References
- 1.Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016;126:238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Atasoy IL, Dursun E, Gezen-Ak D, Metin-Armagan D, Ozturk M, Yilmazer S. Both secreted and the cellular levels of BDNF attenuated due to tau hyperphosphorylation in primary cultures of cortical neurons. J Chem Neuroanat. 2017;80:19–26. doi: 10.1016/j.jchemneu.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Avnet S, Di Pompo G, Chano T, Errani C, Ibrahim-Hashim A, Gillies RJ, Donati DM, Baldini N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int J Cancer. 2017;140:1331–1345. doi: 10.1002/ijc.30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird FJ, Bennett CL. Microtubule defects & neurodegeneration. J Genet Syndr Gene Ther. 2013;4:203. doi: 10.4172/2157-7412.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeri MS, Sonnen J. Brain BDNF expression as a biomarker for cognitive reserve against Alzheimer disease progression. Neurology. 2016;86:702–703. doi: 10.1212/WNL.0000000000002389. [DOI] [PubMed] [Google Scholar]
- 7.Broetto N, Hansen F, Brolese G, Batassini C, Lirio F, Galland F, Dos Santos JP, Dutra MF, Goncalves CA. Intracerebroventricular administration of okadaic acid induces hippocampal glucose uptake dysfunction and tau phosphorylation. Brain Res Bull. 2016;124:136–143. doi: 10.1016/j.brainresbull.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. 2011:2920. doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–741. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caraci F, Bosco P, Leggio GM, Malaguarnera M, Drago F, Bucolo C, Salomone S. Clinical pharmacology of novel anti-Alzheimer disease modifying medications. Curr Top Med Chem. 2013;13:1853–1863. doi: 10.2174/15680266113139990141. [DOI] [PubMed] [Google Scholar]
- 11.Caroni P, Chowdhury A, Lahr M. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 2014;37:604–614. doi: 10.1016/j.tins.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 13.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 14.Danzer SC, Pan E, Nef S, Parada LF, McNamara JO. Altered regulation of brain-derived neurotrophic factor protein in hippocampus following slice preparation. Neuroscience. 2004;126:859–869. doi: 10.1016/j.neuroscience.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 16.Gan KJ, Morihara T, Silverman MA. Atlas stumbled: kinesin light chain-1 variant E triggers a vicious cycle of axonal transport disruption and amyloid-beta generation in Alzheimer's disease. Bioessays. 2015;37:131–141. doi: 10.1002/bies.201400131. [DOI] [PubMed] [Google Scholar]
- 17.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal K, Gong CX, Liu F. Microtubule-associated protein tau as a therapeutic target in Alzheimer's disease. Expert Opin Ther Targets. 2014;18:307–318. doi: 10.1517/14728222.2014.870156. [DOI] [PubMed] [Google Scholar]
- 20.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Kneynsberg A, Combs B, Christensen K, Morfini G, Kanaan NM. Axonal degeneration in tauopathies: disease relevance and underlying mechanisms. Front Neurosci. 2017;11:572. doi: 10.3389/fnins.2017.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku HK, Lim HM, Oh KH, Yang HJ, Jeong JS, Kim SK. Interpretation of protein quantitation using the Bradford assay: comparison with two calculation models. Anal Biochem. 2013;434:178–180. doi: 10.1016/j.ab.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Le MH, Weissmiller AM, Monte L, Lin PH, Hexom TC, Natera O, Wu C, Rissman RA. Functional impact of corticotropin-releasing factor exposure on tau phosphorylation and axon transport. PLoS One. 2016;11:e0147250. doi: 10.1371/journal.pone.0147250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu HY, Wang PQ, Bian Y, Wang JC, Wei YH. Effects of eye acupuncture therapy on neurological function and brain-derived neurotrophic factor expression in a rat model of cerebral ischemia/reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:2634–2641. [Google Scholar]
- 25.Liu Q, Lee HG, Honda K, Siedlak SL, Harris PL, Cash AD, Zhu X, Avila J, Nunomura A, Takeda A, Smith MA, Perry G. Tau modifiers as therapeutic targets for Alzheimer's disease. Biochim Biophys Acta. 2005;1739:211–215. doi: 10.1016/j.bbadis.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Zhou XW, Tanila H, Bjorkdahl C, Wang JZ, Guan ZZ, Cao Y, Gustafsson JA, Winblad B, Pei JJ. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med. 2008;12:241–257. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, He HJ, Zhou J, Miao JY, Lu J, He YG, Pan R, Wei Y, Liu Y, He RQ. Hyperphosphorylation results in tau dysfunction in DNA folding and protection. J Alzheimers Dis. 2013;37:551–563. doi: 10.3233/JAD-130602. [DOI] [PubMed] [Google Scholar]
- 29.Martin L, Page G, Terro F. Tau phosphorylation and neuronal apoptosis induced by the blockade of PP2A preferentially involve GSK3β. Neurochem Int. 2011;59:235–250. doi: 10.1016/j.neuint.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Terro F. Tau protein phosphatases in Alzheimer's disease: the leading role of PP2A. Ageing Res Rev. 2013a;12:39–49. doi: 10.1016/j.arr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F. Tau protein kinases: involvement in Alzheimer's disease. Ageing Res Rev. 2013b;12:289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Metaxas A, Kempf SJ. Neurofibrillary tangles in Alzheimer's disease: elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen Res. 2016;11:1579–1581. doi: 10.4103/1673-5374.193234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller EC, Teravskis PJ, Dummer BW, Zhao X, Huganir RL, Liao D. Tau phosphorylation and tau mislocalization mediate soluble Abeta oligomer-induced AMPA glutamate receptor signaling deficits. Eur J Neurosci. 2014;39:1214–1224. doi: 10.1111/ejn.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 36.Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, Koo EH, Masliah E, Tuszynski MH. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci. 2013;33:15596–15602. doi: 10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nisbet RM, Polanco JC, Ittner LM, Gotz J. Tau aggregation and its interplay with amyloid-β. Acta Neuropathol. 2015;129:207–220. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petzold A, Psotta L, Brigadski T, Endres T, Lessmann V. Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiol Learn Mem. 2015;120:52–60. doi: 10.1016/j.nlm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 41.Purnell JQ, Herrick C, Moreland-Russell S, Eyler AA. Outside the exam room: policies for connecting clinic to community in diabetes prevention and treatment. Prev Chronic Dis. 2015;12:E63. doi: 10.5888/pcd12.140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regan P, Whitcomb DJ, Cho K. Physiological and pathophysiological implications of synaptic Tau. Neuroscientist. 2016 doi: 10.1177/1073858416633439. doi: 10.1177/1073858416633439. [DOI] [PubMed] [Google Scholar]
- 43.Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer's and Parkinson's diseases: implications for pathogenesis and therapy. Neural Regen Res. 2017;12:549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindowski K, Belarbi K, Buée L. Neurotrophic factors in Alzheimer's disease: role of axonal transport. Genes Brain Behav. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 46.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 47.Sontag JM, Sontag E. Protein phosphatase 2A dysfunction in Alzheimer's disease. Front Mol Neurosci. 2014;7:16. doi: 10.3389/fnmol.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer's disease. Am J Pathol. 2005;166:1761–1771. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Yu L, Yang H, Li C, Hui Z, Xu Y, Zhu X. Oridonin attenuates synaptic loss and cognitive deficits in an Abeta1-42-induced mouse model of Alzheimer's disease. PLoS One. 2016;11:e0151397. doi: 10.1371/journal.pone.0151397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittington RA, Bretteville A, Dickler MF, Planel E. Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:147–155. doi: 10.1016/j.pnpbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wirz KT, Keitel S, Swaab DF, Verhaagen J, Bossers K. Early molecular changes in Alzheimer disease: can we catch the disease in its presymptomatic phase? J Alzheimers Dis. 2014;38:719–740. doi: 10.3233/JAD-130920. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Jing XP, Zhou XW, Wang XL, Yang Y, Sun XY, Qiu M, Cao FY, Lu YM, Liu R, Wang JZ. Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol Aging. 2013;34:745–756. doi: 10.1016/j.neurobiolaging.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 56.Ye S, Wang TT, Cai B, Wang Y, Li J, Zhan JX, Shen GM. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer's disease model rats. Neural Regen Res. 2017;12:1479–1484. doi: 10.4103/1673-5374.215260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan J, Zhang Y, Wang X, Ma H. Exogenous brain-derived neurotrophic factor at a 50 ng/mL concentration has a significant protective effect on bilirubin-induced cerebral cortex neuronal injury. Clin Lab. 2017;63:1421–1429. doi: 10.7754/Clin.Lab.2017.170303. [DOI] [PubMed] [Google Scholar]
- 58.Zhang SG, Wang XS, Zhang YD, Di Q, Shi JP, Qian M, Xu LG, Lin XJ, Lu J. Indirubin-3′-monoxime suppresses amyloid-beta-induced apoptosis by inhibiting tau hyperphosphorylation. Neural Regen Res. 2016;11:988–993. doi: 10.4103/1673-5374.184500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao HH, Di J, Liu WS, Liu HL, Lai H, Lu YL. Involvement of GSK3 and PP2A in ginsenoside Rb1's attenuation of aluminum-induced tau hyperphosphorylation. Behav Brain Res. 2013;241:228–234. doi: 10.1016/j.bbr.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 60.Zhou XW, Gustafsson JA, Tanila H, Bjorkdahl C, Liu R, Winblad B, Pei JJ. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis. 2008;31:386–394. doi: 10.1016/j.nbd.2008.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.