Abstract
Antenatal administration of magnesium sulfate is an important part of the neuroprotective strategy for preterm infants. Strong evidence from five randomized controlled trials and five meta-analyses has demonstrated that magnesium sulfate, when administered before preterm delivery, significantly reduces the risk of cerebral palsy at two years. Through secondary analyses of randomized controlled trials and other original clinical studies, this state-of-the-art review highlights the absence of serious adverse effects in both pregnant women and neonates, as well as the impact of maternal body mass index and preeclamptic status on the maternal and neonatal magnesium levels, which could influence the magnitude of the neuroprotective effect. Although antenatal magnesium sulfate is a cost-effective strategy, some practice surveys have demonstrated that the use of magnesium sulfate is not sufficient and that its use is heterogeneous, differing among different maternity wards. Since 2010, an increasing number of obstetrical societies have recommended its use to improve the neurological outcomes of preterm infants, especially the International Federation of Gynecology and Obstetrics and World Health Organization in 2015, and France in 2017. Considering the neuroprotective impact of magnesium sulfate when administered before delivery, postnatal administration should be considered, and its effects should be assessed using randomized controlled trials.
Keywords: magnesium sulfate, preterm birth, neuroprotection, cerebral palsy, neurodevelopment, international recommendations, clinical studies, meta-analysis, preeclampsia, cost-effectiveness
Introduction
Improving the neurological outcomes of children born prematurely remains a crucial issue in perinatal medicine. Antenatal magnesium sulfate (MgSO4) administration is a possible neuroprotective intervention that reduces the incidence of cerebral palsy (CP) at two years of age. Clinical evidence of the neuroprotective impact of MgSO4 is based on five randomized controlled trials (RCTs), and five subsequent meta-analyses of these RCTs, including an individual patient data (IPD) meta-analysis. The purpose of this state-of-the-art review was to: 1) summarize the evidence for the neuroprotective effects of MgSO4 on the immature brain obtained from the RCTs and meta-analyses; 2) resolve some issues through secondary analyses of the RCTs and/or other original clinical studies; 3) analyze the practical use of antenatal MgSO4 in tertiary hospital maternity wards; 4) assess the cost-effectiveness of this intervention; and 5) highlight the increased rates of recommendation of antenatal MgSO4 treatment for fetal neuroprotection since 2010 in many countries, which is key for optimizing individual MgSO4 coverage.
Prevention of CP by Antenatal MgSO4 Administration Before Preterm Delivery
Animal and human observational studies have suggested that MgSO4 is neuroprotective for the immature brain (Marret et al., 1995; Wolf et al., 2012). The mechanisms underlying this neuroprotective effect are not well established; however, studies have indicated several hypotheses. Magnesium can prevent excitotoxicity via N-methyl-D-aspartic acid (NMDA) receptor antagonistic action and a reduction in extracellular glutamate (Nowak et al., 1984; Kang et al., 2011). Further, magnesium can exert anti-inflammatory effects by reducing oxidative stress and pro-inflammatory cytokines (Mazur et al., 2007; Burd et al., 2010; Rayssiguier et al., 2010). In the 2000s, five RCTs (MAGPIE, MAGNET, ACTOMgSO4, PREMAG, and BEAM trials) assessed the impact of antenatal MgSO4 infusion prior to preterm delivery on the incidence of CP at two years (Altman et al., 2002; Mittendorf et al., 2002; Crowther et al., 2003; Marret et al., 2007, 2008; Rouse et al., 2008). These have been described in detail in a previous review (Chollat et al., 2018). Briefly, the rate of motor dysfunction was lower in the MgSO4 group when compared with the control group in the ACTOMgSO4 trial (2.9% versus 5.4% in the placebo group, relative risk [RR]: 0.53, 95% confidence interval [CI]: 0.30–0.92); the rate of combined death or gross motor dysfunction was lower in the MgSO4 group when compared with the control group in the PREMAG trial (25.6% versus 30.8%, odds ratio (OR): 0.62, 95% CI: 0.41–0.93); and the rate of moderate or severe CP was significantly reduced in the MgSO4 group compared with the control group in the BEAM trial (1.9% versus 3.5%, RR 0.55, 95% CI: 0.32–0.95).
Five meta-analyses of these RCTs have demonstrated a neuroprotective effect of antenatal MgSO4 infusion on CP at two years of age (Conde-Agudelo and Romero, 2009; Costantine et al., 2009; Doyle et al., 2009; Zeng et al., 2016; Crowther et al., 2017). In the IPD meta-analysis, Crowther et al. (2017) demonstrated that MgSO4 treatment significantly reduced CP in survivors (RR 0.68, 95% CI: 0.54–0.87, n = 4601 neonates, number of RCTs = 5, number of pregnant women who needed the treatment to avoid one CP = 46), regardless of the reason of preterm birth, gestational age at time of MgSO4 treatment, or cumulative dose.
Despite this strong evidence, some questions have remained unaddressed by RCTs or meta-analyses. Some relevant clinical studies are seeking the resolution of these issues, as described in the following sections.
Fetal Neuroprotection by MgSO4: Persisting Questions and Concerns Minor maternal side effects
In a recent systematic review, maternal life-threatening side effects, such as death, cardiac or respiratory arrest, and intensive care admission were not associated with MgSO4 treatment (Bain et al., 2013b). These data are consistent with the results of RCTs and meta-analyses. However, women receiving MgSO4 had an increased risk of developing minor side effects. Specifically, they had double the risk of hypotension, tachycardia, respiratory depression, discomfort at the injection site, drowsiness, headache, dizziness, mouth dryness or thirst, and blurred vision; five times the risk of nausea and/or vomiting, flushing and warmth, and sweating; and 15 times the risk of itching, tingling, and muscle weakness. These adverse effects are transient and disappear with treatment cessation. A decrease in the dose administered could limit the occurrence of these side effects. One trial has shown that a lower dose regimen (2 g/3 hours) significantly reduced treatment cessation due to side effects when compared with a higher dose regimen (5 g/4 hours) (Malapaka and Ballal, 2011). In addition, extending the duration of the loading dose from 20 to 60 minutes reduces flushing and warmth, but has no impact on other side effects (Bain et al., 2014).
Low maternal body mass index (BMI) may also influence the occurrence of side effects. For example, more maternal adverse effects were observed in underweight women than in normal or overweight women (Vilchez et al., 2018).
Neonatal side effects
Results of the meta-analyses conducted on the RCTs did not show any adverse outcomes for neonates, including respiratory distress syndrome, need for mechanical ventilation, or necrotizing enterocolitis (Conde-Agudelo and Romero, 2009; Zeng et al., 2016). However, one trial showed a higher incidence of spontaneous intestinal perforation after antenatal MgSO4 administration among extremely low birthweight infants (Rattray et al., 2014). In this monocentric retrospective study, spontaneous intestinal perforation and death among extremely low birthweight (ELBW) infants were evaluated before, during, and after the initiation of a neuroprotection protocol involving antenatal magnesium. One hundred and fifty-five ELBW infants were included: 81 before, 23 during, and 51 after establishing the MgSO4 protocol. Overall, 78.3% of ELBW infants were exposed to MgSO4 during the study, compared to 50.6% and 60.8% exposed before and after the protocol, respectively. The incidence of spontaneous intestinal perforation in the MgSO4 protocol was 30.4% versus 12.9% in the group not receiving MgSO4 (P = 0.03). The experimental design of this study makes these results difficult to interpret. First, the protocol used by Rattray et al. included a loading dose of 6 g and maintenance dose of 2 g per hour, whereas current protocols include a loading dose of 4 g and maintenance dose of 1 g per hour. Second, all groups were exposed to MgSO4. Third, in the placebo group, the incidence of spontaneous intestinal perforation was higher (12.9%) than usual (< 1%) (Suply et al., 2015). Finally, the sample size was small. In addition, this adverse effect was not reported in other RCTs or meta-analyses. Therefore, this effect may be influenced by local or study design factors and may not be representative of current MgSO4 treatment practices.
Antenatal MgSO4 administration did not influence neonatal vital parameters, such as heart and respiratory rates, temperature, oxygen saturation, and glycemia (Nunes et al., 2017). The assessment of hemodynamic parameters by echocardiography one day after antenatal MgSO4 infusion showed lower systemic vascular resistance and higher myocardial function in preterm infants born before 29 weeks of gestation (WG) (James et al., 2015).
Two meta-analyses have shown that antenatal MgSO4 exposure does not improve 5-minute Apgar scores that are < 7 (Doyle et al., 2009; Zeng et al., 2016). A secondary analysis of the BEAM cohort did not show any difference in rates of intubation, chest compressions, hypotension, or mechanical ventilation between the MgSO4 and placebo groups (Drassinower et al., 2015). These findings support the safety of antenatal MgSO4 exposure on short-term neonatal outcomes.
The incidence of side effects due to hypermagnesemia is anecdotal and often secondary to administration errors (Rigo et al., 2017). Some case reports have reported an association between major side effects, including hypotension, QT interval prolongation, intraventricular conduction delay, respiratory distress, apnea, lethargy hypotonia, neuromuscular blockade, and coma, and severe hypermagnesemia (18–22 mM) (Huey et al., 1995; Ali et al., 2003; Hyun et al., 2011). In these case reports, neonates were not exposed to MgSO4 before delivery, and the etiology of hypermagnesemia was unknown or caused by a malfunction of an automated parenteral nutrition-mixing device. Therefore, an appropriate administration protocol for MgSO4 is essential to limit MgSO4 administration errors.
Dosage, duration of infusion, and serum levels of magnesium: what is the impact on the neuroprotective effect of MgSO4?
Serum magnesium levels decrease in women during pregnancy from 0.75–0.95 mM, which is the range found in healthy adults (Costello et al., 2016), to 0.59–0.95 mM during gestation and 0.54 to 0.90 mM (mean 0.74 mM, 95% CI: 0.43–1.04) at delivery (Rigo et al., 2017). In a prospective pharmacokinetic cohort of 111 pregnant women, the steady state level of magnesium before birth ranged from 2.0 in non-preeclamptic women to 3.5 mM in preeclamptic women after MgSO4 administration. The placental transfer of MgSO4 was excellent and resulted in a ratio of the mean magnesium levels of the neonate to the mother at delivery of 0.94 ± 0.15 mM (Brookfield et al., 2016). A meta-analysis has found that neonatal magnesium levels at birth are estimated to be 0.76 mM (95% CI: 0.52–0.99) or 1.29 mM (95% CI: 0.50–2.08) with or without maternal magnesium supplementation, respectively (Rigo et al., 2017).
Several factors affect maternal and neonatal MgSO4 levels. These include:
-A decrease in MgSO4 clearance in preeclamptic women (3.98 L/h versus 5.88 L/h for non-preeclamptic women), which directly affects magnesium levels (3 mM in preeclamptic women versus 2.1 mM in non-preeclamptic women).
-The linear relationship between maternal weight and the time required to reach a steady state. BMI has a significant effect on magnesium levels, and obese women (in particular, those with BMI > 30 kg/m2) could be subject to subtherapeutic magnesium levels (Tudela et al., 2013).
-A significant correlation between neonatal magnesium levels in the first 24 hours of life and the total dose of MgSO4 received by the mother (Borja-Del-Rosario et al., 2014; García Alonso et al., 2018).
Considering these findings, questions arise regarding the impact of the mother's weight (especially if the mother is obese) on the neurological outcome of the infant. A secondary analysis of the BEAM trial showed that MgSO4 significantly reduced CP, but only in non-obese women (Vilchez et al., 2018). This finding suggests that a dosage adjustment based on maternal weight is required. More studies are required to explore this association.
Similarly, a retrospective study on 304 mother-baby dyads found a correlation between neurological outcomes and serum magnesium levels. Neonates with low (< 1.0 mM) or high (> 1.9 mM) serum magnesium levels had a higher OR for grade 3 or 4 intraventricular hemorrhage than neonates with magnesium levels between 1 and 1.9 mM (Narasimhulu et al., 2017). In this study, the neonatal magnesium levels were dependent on the maternal magnesium dose, maternal serum concentration, and duration of therapy. In a retrospective study with 88 infants exposed to MgSO4, elevated magnesium levels (> 1.5 mM) were associated with lower locomotor scores in the first year of life (Morag et al., 2017). Conversely, another retrospective cohort that included 75 preterm infants exposed to MgSO4 revealed that higher levels of MgSO4 (> 1 mM) were associated with a significantly decreased risk of abnormal motor examination findings between 20 and 36 months of age (Doll et al., 2014).
The relationship between neonatal magnesium levels and neurological outcomes is unclear. There may be a range of serum magnesium levels that elicit the neuroprotective effects of MgSO4 therapy. The recommended dose of MgSO4 used for fetal neuroprotection was obtained based on the dose used for tocolysis or prevention of preeclampsia, as a preliminary consensus based on previous clinical trials is lacking. Studies assessing the optimal target level of magnesium are necessary to optimize the neuroprotective effects of MgSO4 and limit any possible side effects.
The duration of MgSO4 infusion does not affect its neuroprotective effects. A secondary analysis of the BEAM trial found no change in neurological outcomes when comparing the administration durations of < 12 hours, between 12 and 18 hours, or > 18 hours (McPherson et al., 2014). These findings are consistent with the IPD AMICABLE meta-analysis that showed a neuroprotective effect against CP regardless of the total dose received (Crowther et al., 2017). However, the proximity of magnesium exposure to delivery could be crucial. A final MgSO4 exposure < 12 hours before delivery was associated with significantly reduced odds of CP compared with exposure > 12 hours before delivery (Turitz et al., 2016).
Use of MgSO4: Terms of Use, Feasibility, and Safety of Therapeutic Protocols
No studies have compared the neurological effects of different MgSO4 regimens. All currently published RCTs report a loading dose range of 4–6 g over 15–30 minutes (Bain et al., 2012). Maintenance doses of 1–2 g per hour until birth or for up to 12–24 hours have been used in all trials, except the PREMAG trial. Re-treatment was considered only in the BEAM trial. Furthermore, the IPD meta-analysis found no significant difference in the incidence of CP among different MgSO4 regimens (Crowther et al., 2017).
Two national surveys have shown heterogeneous clinical practices regarding the maintenance dose (1 or 2 g/h), duration of treatment (until delivery, or for a maximum of 12 or 24 hours), consideration of re-treatment, and minimal interval between the two treatments (De Silva et al., 2015; Chollat et al., 2017). This heterogeneity is likely to be a result of differences in the regimens that are reported in the literature.
Consequently, the use of MgSO4 for fetal neuroprotection must be optimized. In Europe, only 7.6% of all maternity units reported the use of MgSO4 in 2012, resulting in only 14.3% of eligible women receiving MgSO4 for fetal neuroprotection (Wolf et al., 2017). Individual MgSO4 coverage improves following the implementation of a standard protocol. In Australia and New Zealand, the proportion of eligible women who did not receive antenatal MgSO4 decreased from 69.7% in 2011 to 22.5% in 2013 after the development of a standard protocol (Siwicki et al., 2015). A French study showed that 68% of eligible women received MgSO4 before delivery during the first year of implementing a protocol in a tertiary obstetric unit (Bouet et al., 2015). A similar study in an Australian maternity unit showed that 74% of preterm infants born at < 32 WG were antenatally exposed to MgSO4 in the first 12 months following the implementation of a national guideline (Ow et al., 2012). A qualitative study highlighted that information dissemination was a key enabler for increasing the knowledge of and skills for the use of MgSO4 in order to optimize MgSO4 administration. Forgetting to prescribe MgSO4, difficulty in predicting preterm birth, and complex administration processes were the most significant barriers perceived by health professionals precluding the use of MgSO4 (Bain et al., 2015). In Canada, a multifaceted knowledge translation strategy was implemented to improve MgSO4 administration for eligible women. This program included national clinical guidelines and online e-learning modules, educational rounds, focus group discussions, and surveys focused on barriers and facilitators. This intervention was associated with an improvement of 84% in the odds of optimal use in 10 years (Teela et al., 2015; De Silva et al., 2018). Many audits have shown that the implementation of an administration protocol for MgSO4 for fetal neuroprotection is safe and feasible (Ow et al., 2012; Bain et al., 2013a; Bouet et al., 2015; Tan and Groom, 2015).
Economic Analysis: Is the MgSO4 Strategy Cost-Effective?
CP is a life-long condition that deeply affects infants and their families, and generates costs for both the healthcare system and society. A recent systematic review provided information on the economic aspects of CP, including interventions for its prevention. The authors concluded that the “administration of magnesium sulfate for imminent preterm birth is a dominant strategy resulting in less cost and more benefit compared with no treatment” (Shih et al., 2018). In addition, two studies have analyzed the cost-effectiveness of MgSO4 treatment (Cahill et al., 2011; Bickford et al., 2013). Cahill et al. demonstrated that for every 10,000 women at risk for preterm birth treated with MgSO4, $1.8 million was saved and 52 quality-adjusted life years (QALY) were gained. Bickford et al. (2013), moreover, showed that MgSO4 treatment allowed for savings of $112,602 for each QALY gained and $1,554,198 for each case of CP prevented. In light of these analyses, MgSO4 intervention seems highly cost-effective.
International Recommendations
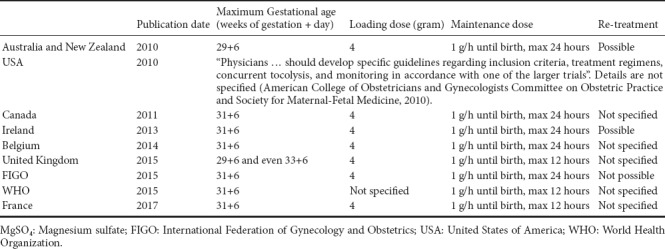
Since 2010, many national obstetrical societies have recommended the use of MgSO4 before preterm birth as a means of providing neuroprotection to preterm neonates (Table 1). MgSO4 use, in terms of the maximum term of administration (from 29 + 6 WG to 33 + 6 WG), duration of the maintenance dose (from 12–24 hours), and possibility of re-treatment (mentioned in the Australian, New Zealand, Irish, and FIGO recommendations) (Table 1), varies among countries. Finally, because preterm birth is a major health issue, the World Health Organization strongly recommends the use of MgSO4 for fetal protection against neurological complications before 32 WG.
Table 1.
Protocols for the administration of MgSO4 for neuroprotection according to international recommendations
Conclusions
Antenatal MgSO4 administration is a key intervention for preventing CP in preterm neonates. MgSO4 infusion is associated with minor transient maternal side effects and but is well tolerated by neonates. Maternal weight and preeclamptic status affect maternal and neonatal magnesium levels, which could in turn influence the neuroprotective effects of MgSO4. The standardization of an acceptable range of maternal magnesium levels that elicit neuroprotective effects may optimize the beneficial impact of MgSO4 on neonatal neurological outcomes. This strategy would require monitoring of the maternal magnesium level and, if necessary, dose adjustments.
Considering the beneficial effects of antenatal MgSO4 administration on CP, postnatal administration of MgSO4 in preterm infants should be considered and its effects should be assessed via RCTs to obtain the optimal magnesium serum levels for neuroprotection.
Additional file: Open peer review reports 1 (108.1KB, pdf) and 2 (100.5KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Creed Stary, Stanford University School of Medicine, USA; Han Zhang, University of North Carolina at Chapel Hill, USA.
P-Reviewer: Stary C, Zhang H; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Ali A, Walentik C, Mantych GJ, Sadiq HF, Keenan WJ, Noguchi A. Iatrogenic acute hypermagnesemia after total parenteral nutrition infusion mimicking septic shock syndrome: two case reports. Pediatrics. 2003;112:e70–72. doi: 10.1542/peds.112.1.e70. [DOI] [PubMed] [Google Scholar]
- 2.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, Smith D Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists Committee on Obstetric Practice; Society for Maternal-Fetal Medicine. Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010;115:669–671. doi: 10.1097/AOG.0b013e3181d4ffa5. [DOI] [PubMed] [Google Scholar]
- 4.Bain E, Bubner T, Ashwood P, Crowther CA, Middleton P WISH Project Team. Implementation of a clinical practice guideline for antenatal magnesium sulphate for neuroprotection in Australia and New Zealand. Aust N Z J Obstet Gynaecol. 2013a;53:86–89. doi: 10.1111/ajo.12008. [DOI] [PubMed] [Google Scholar]
- 5.Bain E, Bubner T, Ashwood P, Van Ryswyk E, Simmonds L, Reid S, Middleton P, Crowther CA. Barriers and enablers to implementing antenatal magnesium sulphate for fetal neuroprotection guidelines: a study using the theoretical domains framework. BMC Pregnancy Childbirth. 2015;15:176. doi: 10.1186/s12884-015-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bain E, Middleton P, Crowther CA. Different magnesium sulphate regimens for neuroprotection of the fetus for women at risk of preterm birth. Cochrane Database Syst Rev:CD009302. 2012 doi: 10.1002/14651858.CD009302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain ES, Middleton PF, Crowther CA. Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review. BMC Pregnancy Childbirth. 2013b;13:195. doi: 10.1186/1471-2393-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain ES, Middleton PF, Yelland LN, Ashwood PJ, Crowther CA. Maternal adverse effects with different loading infusion rates of antenatal magnesium sulphate for preterm fetal neuroprotection: the IRIS randomised trial. BJOG. 2014;121:595–603. doi: 10.1111/1471-0528.12535. [DOI] [PubMed] [Google Scholar]
- 9.Bickford CD, Magee LA, Mitton C, Kruse M, Synnes AR, Sawchuck D, Basso M, Senikas VM, von Dadelszen P MAG-CP Working Group. Magnesium sulphate for fetal neuroprotection: a cost-effectiveness analysis. BMC Health Serv Res. 2013;13:527. doi: 10.1186/1472-6963-13-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borja-Del-Rosario P, Basu SK, Haberman S, Bhutada A, Rastogi S. Neonatal serum magnesium concentrations are determined by total maternal dose of magnesium sulfate administered for neuroprotection. J Perinat Med. 2014;42:207–211. doi: 10.1515/jpm-2013-0151. [DOI] [PubMed] [Google Scholar]
- 11.Bouet PE, Brun S, Madar H, Baisson AL, Courtay V, Gascoin-Lachambre G, Lasocki S, Sentilhes L. Implementation of an antenatal magnesium sulfate protocol for fetal neuroprotection in preterm infants. Sci Rep. 2015;5:14732. doi: 10.1038/srep14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookfield KF, Su F, Elkomy MH, Drover DR, Lyell DJ, Carvalho B. Pharmacokinetics and placental transfer of magnesium sulfate in pregnant women. Am J Obstet Gynecol. 2016;214:737.e1–9. doi: 10.1016/j.ajog.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol. 2010;202:292.e1–9. doi: 10.1016/j.ajog.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill AG, Odibo AO, Stout MJ, Grobman WA, Macones GA, Caughey AB. Magnesium sulfate therapy for the prevention of cerebral palsy in preterm infants: a decision-analytic and economic analysis. Am J Obstet Gynecol. 2011;205:542.e1–7. doi: 10.1016/j.ajog.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Chollat C, Le Doussal L, de la Villéon G, Provost D, Marret S. Antenatal magnesium sulphate administration for fetal neuroprotection: a French national survey. BMC Pregnancy Childbirth. 2017;17:304. doi: 10.1186/s12884-017-1489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chollat C, Sentilhes L, Marret S. Fetal neuroprotection by magnesium sulfate: from translational research to clinical application. Front Neurol. 2018;9:247. doi: 10.3389/fneur.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol. 2009;200:595–609. doi: 10.1016/j.ajog.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costantine MM, Weiner SJ. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network (2009) Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol. 114:354–364. doi: 10.1097/AOG.0b013e3181ae98c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y, Van Horn LV. Perspective: The case for an evidence-based reference interval for serum magnesium: the time has come. Adv Nutr. 2016;7:977–993. doi: 10.3945/an.116.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 21.Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, Marret S, Doyle LW AMICABLE Group. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med. 2017;14:e1002398. doi: 10.1371/journal.pmed.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Silva DA, Sawchuck D, von Dadelszen P, Basso M, Synnes AR, Liston RM, Magee LA. Magnesium sulphate for eclampsia and fetal neuroprotection: a comparative analysis of protocols across canadian tertiary perinatal centres. J Obstet Gynaecol Can. 2015;37:975–987. doi: 10.1016/s1701-2163(16)30047-0. [DOI] [PubMed] [Google Scholar]
- 23.De Silva DA, Synnes AR, von Dadelszen P, Lee T, Bone JN, Magee LA MAG-CP; CPN and CNN collaborative groups. MAGnesium sulphate for fetal neuroprotection to prevent Cerebral Palsy (MAG-CP)-implementation of a national guideline in Canada. Implement Sci. 2018;13:8. doi: 10.1186/s13012-017-0702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doll E, Wilkes J, Cook LJ, Korgenski EK, Faix RG, Yoder BA, Srivastava R, Sherwin CMT, Spigarelli MG, Clark EAS, Bonkowsky JL. Neonatal magnesium levels correlate with motor outcomes in premature infants: a long-term retrospective cohort study. Front Pediatr. 2014;2:120. doi: 10.3389/fped.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev:CD004661. 2009 doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Drassinower D, Friedman AM, Levin H, Običan SG, Gyamfi-Bannerman C. Does magnesium exposure affect neonatal resuscitation? Am J Obstet Gynecol. 2015;213:424. doi: 10.1016/j.ajog.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 27.García Alonso L, Pumarada Prieto M, González Colmenero E, Concheiro Guisán A, Suárez Albo M, Durán Fernández-Feijoo C, González Durán L, Fernández Lorenzo JR. Prenatal therapy with magnesium sulfate and its correlation with neonatal serum magnesium concentration. Am J Perinatol. 2018;35:170–176. doi: 10.1055/s-0037-1606358. [DOI] [PubMed] [Google Scholar]
- 28.Huey CG, Chan KM, Wong ET, Nelson JM, Durand M. Los Angeles County-University of Southern California Medical Center clinical pathology case conference: extreme hypermagnesemia in a neonate. Clin Chem. 1995;41:615–618. [PubMed] [Google Scholar]
- 29.Hyun HS, Choi HS, Kim JK, Ahn SY, Yoo HS, Kim ES, Chang YS, Park WS. Idiopathic severe hypermagnesemia in an extremely low birth weight infant on the first day of life. Korean J Pediatr. 2011;54:310–312. doi: 10.3345/kjp.2011.54.7.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James AT, Corcoran JD, Hayes B, Franklin O, El-Khuffash A. The effect of antenatal magnesium sulfate on left ventricular afterload and myocardial function measured using deformation and rotational mechanics imaging. J Perinatol. 2015;35:913–918. doi: 10.1038/jp.2015.104. [DOI] [PubMed] [Google Scholar]
- 31.Kang SW, Choi SK, Park E, Chae SJ, Choi S, Jin Joo H, Lee GJ, Park HK. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. 2011;1371:121–128. doi: 10.1016/j.brainres.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 32.Malapaka SVN, Ballal PK. Low-dose magnesium sulfate versus Pritchard regimen for the treatment of eclampsia imminent eclampsia. Int J Gynaecol Obstet. 2011;115:70–72. doi: 10.1016/j.ijgo.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Marret S, Gressens P, Gadisseux JF, Evrard P. Prevention by magnesium of excitotoxic neuronal death in the developing brain: an animal model for clinical intervention studies. Dev Med Child Neurol. 1995;37:473–484. doi: 10.1111/j.1469-8749.1995.tb12035.x. [DOI] [PubMed] [Google Scholar]
- 34.Marret S, Marpeau L, Bénichou J. Benefit of magnesium sulfate given before very preterm birth to protect infant brain. Pediatrics. 2008;121:225–226. doi: 10.1542/peds.2007-2971. [DOI] [PubMed] [Google Scholar]
- 35.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, Bénichou J. Magnesium sulphate given before verypreterm birth to protect infant brain: the randomised controlled PREMAG trial. BJOG. 2007;114:310–318. doi: 10.1111/j.1471-0528.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 36.Mazur A, Maier JAM, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 37.McPherson JA, Rouse DJ, Grobman WA, Palatnik A, Stamilio DM. Association of duration of neuroprotective magnesium sulfate infusion with neonatal and maternal outcomes. Obstet Gynecol. 2014;124:749–755. doi: 10.1097/AOG.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 38.Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, Tomich PG. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- 39.Morag I, Rotem I, Frisch M, Hendler I, Simchen MJ, Leibovitz L, Maayan-Metzger A, Strauss T. Cumulative pain-related stress and developmental outcomes among low-risk preterm infants at one year corrected age. Early Hum Dev. 2017;109:1–5. doi: 10.1016/j.earlhumdev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Narasimhulu D, Brown A, Egbert NM, Rojas M, Haberman S, Bhutada A, Minkoff H, Rastogi S. Maternal magnesium therapy, neonatal serum magnesium concentration and immediate neonatal outcomes. J Perinatol. 2017;37:1297–1303. doi: 10.1038/jp.2017.132. [DOI] [PubMed] [Google Scholar]
- 41.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 42.Nunes RD, Schutz FD, Traebert JL. Association between the use of magnesium sulfate as neuroprotector in prematurity and the neonatal hemodynamic effects. J Matern Fetal Neonatal Med. 2017:1–6. doi: 10.1080/14767058.2017.1332033. [DOI] [PubMed] [Google Scholar]
- 43.Ow LL, Kennedy A, McCarthy EA, Walker SP. Feasibility of implementing magnesium sulphate for neuroprotection in a tertiary obstetric unit. Aust N Z J Obstet Gynaecol. 2012;52:356–360. doi: 10.1111/j.1479-828X.2012.01434.x. [DOI] [PubMed] [Google Scholar]
- 44.Rattray BN, Kraus DM, Drinker LR, Goldberg RN, Tanaka DT, Cotten CM. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J Perinatol. 2014;34:819–822. doi: 10.1038/jp.2014.106. [DOI] [PubMed] [Google Scholar]
- 45.Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23:73–80. doi: 10.1684/mrh.2010.0208. [DOI] [PubMed] [Google Scholar]
- 46.Rigo J, Pieltain C, Christmann V, Bonsante F, Moltu SJ, Iacobelli S, Marret S. Serum magnesium levels in preterm infants are higher than adult levels: a systematic literature review and meta-analysis. Nutrients. 2017;9:E1125. doi: 10.3390/nu9101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, Harper M, Thorp JM Jr, Ramin SM, Malone FD, Carpenter M, Miodovnik M, Moawad A, O’Sullivan MJ, Peaceman AM, Hankins GD, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shih STF, Tonmukayakul U, Imms C, Reddihough D, Graham HK, Cox L, Carter R. Economic evaluation and cost of interventions for cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60:543–558. doi: 10.1111/dmcn.13653. [DOI] [PubMed] [Google Scholar]
- 49.Siwicki K, Bain E, Bubner T, Ashwood P, Middleton P, Crowther CA. Nonreceipt of antenatal magnesium sulphate for fetal neuroprotection at the Women's and Children's Hospital, Adelaide 2010-2013. Aust N Z J Obstet Gynaecol. 2015;55:233–238. doi: 10.1111/ajo.12334. [DOI] [PubMed] [Google Scholar]
- 50.Suply E, Leclair MD, Neunlist M, Roze JC, Flamant C. Spontaneous intestinal perforation and necrotizing enterocolitis: a 16-year retrospective study from a single center. Eur J Pediatr Surg. 2015;25:520–525. doi: 10.1055/s-0034-1396418. [DOI] [PubMed] [Google Scholar]
- 51.Tan YH, Groom KM. A prospective audit of the adherence to a new magnesium sulphate guideline for the neuroprotection of infants born less than 30 weeks’ gestation. Aust N Z J Obstet Gynaecol. 2015;55:90–93. doi: 10.1111/ajo.12271. [DOI] [PubMed] [Google Scholar]
- 52.Teela KC, De Silva DA, Chapman K, Synnes AR, Sawchuck D, Basso M, Liston RM, von Dadelszen P, Magee LA MAG-CP Collaborative Group. Magnesium sulphate for fetal neuroprotection: benefits and challenges of a systematic knowledge translation project in Canada. BMC Pregnancy Childbirth. 2015;15:347. doi: 10.1186/s12884-015-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tudela CM, McIntire DD, Alexander JM. Effect of maternal body mass index on serum magnesium levels given for seizure prophylaxis. Obstet Gynecol. 2013;121:314–320. doi: 10.1097/AOG.0b013e31827d90cc. [DOI] [PubMed] [Google Scholar]
- 54.Turitz AL, Too GT, Gyamfi-Bannerman C. Proximity of magnesium exposure to delivery and neonatal outcomes. Am J Obstet Gynecol. 2016;215:508.e1–6. doi: 10.1016/j.ajog.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Vilchez G, Dai J, Lagos M, Sokol RJ. Maternal side effects & fetal neuroprotection according to body mass index after magnesium sulfate in a multicenter randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31:178–183. doi: 10.1080/14767058.2017.1279143. [DOI] [PubMed] [Google Scholar]
- 56.Wolf HT, Hegaard HK, Greisen G, Huusom L, Hedegaard M. Treatment with magnesium sulphate in pre-term birth: a systematic review and meta-analysis of observational studies. J Obstet Gynaecol. 2012;32:135–140. doi: 10.3109/01443615.2011.638999. [DOI] [PubMed] [Google Scholar]
- 57.Wolf HT, Huusom L, Weber T, Piedvache A, Schmidt S, Norman M, Zeitlin J EPICE Research Group. Use of magnesium sulfate before 32 weeks of gestation: a European population-based cohort study. BMJ Open. 2017;7:e013952. doi: 10.1136/bmjopen-2016-013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng X, Xue Y, Tian Q, Sun R, An R. Effects and safety of magnesium sulfate on neuroprotection: a meta-analysis based on PRISMA guidelines. Medicine (Baltimore) 2016;95:e2451. doi: 10.1097/MD.0000000000002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


