Abstract
Apomorphine is a non-specific dopamine receptor agonist that has been used in the treatment of some diseases and mental disorders. Its use has particularly well documented in Parkinson's disease (PD). The dopaminergic agonists like apomorphine are related to oxidative processes that could induce cell damage and the functional impairment of some structures in the brain. However, most information about apomorphine in literature is focused on the improvement of the motor problems characteristic of PD, but little is known about the effects on cognitive behaviors and brain structures indirectly related to motor function. The presence of dopaminergic receptors in the hippocampus has recently been discovered, in connection with cognitive behaviors like learning and memory, these receptors are needed in neuronal plasticity. There has been a growing interest to know if this structure could be compromised by the effect of apomorphine and elucidate if part of the cognitive impairment present in the PD is due to the effect of apomorphine. In this mini-review, we summarized how apomorphine has been used since its creation, we discuss the latest information about its effect on the hippocampus and also the future perspectives to fully understand the effects of this compound.
Keywords: dopamine receptor, apomorphine, hippocampus, learning and memory, plasticity, dendritic length, oxidative stress, Parkinson's disease
The compound apomorphine (also known as apomorphia and sulfomorphide), at present known as a non-selective dopamine receptor agonist, was first synthesized in 1845 by the Finnish scientist Arppe (reviewed in Auffret et al. (2018a)). The earliest pharmacological studies using this compound showed physiological effects on the brain and in the cardiovascular system and gastric and genitourinary tracts [reviewed in Jenner and Katzenschlager (2016), and Auffret et al. (2018a)]. In the brain, apomorphine produced stereotyped motor behaviors in dogs, epileptiform convulsions in cats and sedation in humans (Auffret et al., 2018a, b). In addition, in the rat, Ungerstedt et al. (1969) reported that the intrastriatal microinjection of apomorphine provoked stereotyped motor behavior and blocked the parkinsonian symptoms caused by the administration of the dopamine receptor antagonist chlorpromazine. These data supported the hypothesis that apomorphine acts as a dopaminergic agonist on dopaminergic receptors located in the dorsal striatum, also called the caudate-putamen nucleus (Ungerstedt et al., 1969; Auffret et al., 2018a, b). In addition, several reports have shown that apomorphine does not activate one specifically receptor but functions as a non-specific dopamine receptor agonist, activating not only pre- but also post-synaptic dopaminergic receptors (Arroyo-García et al., 2018).
Dopamine (DA) receptors are divided in two families (D1-like and D2-like receptors). D1-like receptors include D1 and D5 dopamine receptors, while the D2-like family includes D2, D3 and D4 receptors (Flores et al., 1999; Jenner and Katzenschlager, 2016). Dopamine receptors are widely distributed in the brain with D3 receptors found at higher levels in the Islands of Calleja (considered part of the limbic system) while they are scarce in the caudate putamen nucleus (Flores et al., 1996, 1999) and D1 receptors found at high levels in the caudate putamen nucleus, but not reported in the Islands of Calleja (Flores et al., 1996, 1999; Zamudio et al., 2005). Nowadays, it is well known that apomorphine activates all the DA receptors, and recent reports suggest that apomorphine may also have an effect on serotoninergic and adrenergic receptors (Ribarič, 2012; Auffret et al., 2018a, b).
The effects of apomorphine are clearly dose-dependent, emesis and motor behavior being the best-studied effects (Ribarič, 2012; Jenner and Katzenschlager, 2016; Auffret et al., 2018a). Other well-documented apomorphine effects are sedation, hypnosis, sexual dysfunctions and neuroprotection (Ribarič, 2012; Jenner and Katzenschlager, 2016; Auffret et al., 2018a).
The therapeutic use of apomorphine has also been very broad, from its application in the treatment of erectile dysfunction or a mental disorder such as alcoholism to its current use in the treatment of Parkinson's disease (PD) (Ribarič, 2012; Jenner and Katzenschlager, 2016; Auffret et al., 2018a). Interestingly, while its efficacy in PD was reported more than six decades ago (Schwab et al., 1951), its use in the treatment of PD began only three decades ago by two research groups, one Spanish (Obeso et al., 1987) and the other one British (Stibe et al., 1987). PD occurs mainly in old people with a slightly higher incidence and prevalence in males compared to females (reviewed in Georgiev et al. (2017)). It is well known that PD is due to a reduction of dopaminergic transmission to the basal ganglia, especially to the caudate-putamen nucleus (reviewed in Jenner and Katzenschlager (2016), and Auffret et al. (2018b)) and consequently, apomorphine, as a non-specific dopamine receptor agonist, is a dopaminergic stimulant that, activating DA receptors, reduces the symptoms of PD (tremors, hypokinemia o akinesia and muscular rigidity) and is useful in the early stages of the disease to reduce resulting motor deficits.
Apomorphine was used unsuccessfully in the treatment of addictions (such as heroin or alcohol; Ribarič, 2012). However, recently, due to its agonist actions on dopamine receptors, apomorphine is used to produce behavioral sensitization in rodents as an animal model for the study of addictions (Jenner and Katzenschlager, 2016; Auffret et al., 2018a). The use of an animal model of addiction is a useful tool to understand the morphological changes produced by some addictive drugs with an effect on dopaminergic transmission, such as cocaine and amphetamines.
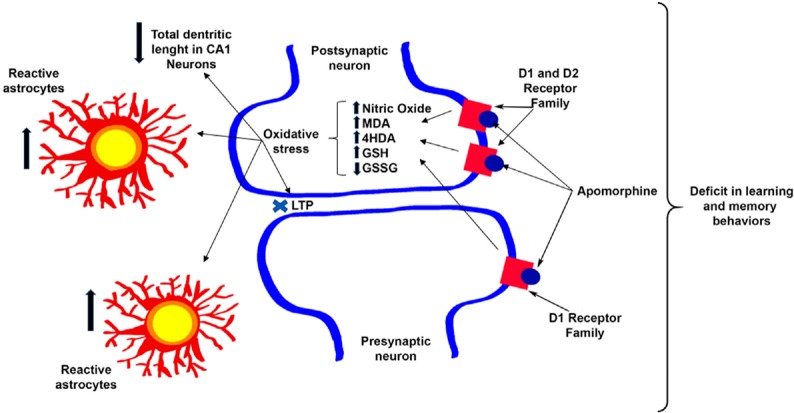
It has recently been shown that the hippocampus receives dopaminergic innervation from the ventral tegmental area (VTA) and has a high dopamine receptor density (Kramar et al., 2014; Rocchetti et al., 2015; Rosen et al., 2015). This fact prompted our group to perform research on this structure, where the effects of non-selective and simultaneous activation of dopamine receptors are almost unknown, unlike the prefrontal cortex (PFC) or nucleus accumbens (NAcc). Different studies have shown that apomorphine affects brain functions and cognitive processes like learning and memory, which are linked to the hippocampus (Gourgiotis et al., 2012; McNamara et al., 2014; Haleem and Farhan, 2015). We showed that the activation of dopamine receptors in the hippocampus by 20 µM apomorphine prevented long-term potentiation (LTP) induction at CA3-CA1 synapses, which gave us the first evidence of an apomorphine effect on the hippocampus and, which has an effect on learning and memory behaviors (Arroyo-García et al., 2018). To confirm this, we administrated 1 mg/kg of apomorphine for 15 days to a group of mice and found that in the Water Morris Test, the learning and memory decreased in apomorphine-treated mice (Arroyo-García et al., 2018). Additionally, we studied the effects of apomorphine on oxidative stress and morphology and found that oxidative stress biomarkers as well as the inflammatory response increased, and that the dendritic length in the CA1 pyramidal neurons decreased in apomorphine-treated animals (Arroyo-García et al., 2018) (Figure 1).
Figure 1.
Apomorphine effects on the hippocampus.
The scheme represents the effect of 1 mg/kg apomorphine administration for 15 days. It causes an increase of the oxidative state and in the number of reactive astrocytes and a decrease in the dendritic length of CA1 pyramidal neurons. Furthermore, apomorphine impairs plasticity (LTP) at CA3–CA1 synapses and produces a deficit in learning and memory.
While more research is necessary to complete these studies, these new results highlight that while the simultaneous and non-selective activation of dopamine receptors by psycho-stimulants (as apomorphine) may be helpful for the treatment of motor diseases, at the same time, there may be negative side effects such as brain damage and impairments in learning and memory.
Additional file: Open peer review report 1 (116.7KB, pdf) .
Acknowledgments
Thank to Miguel Angel Dominguez of Benemérita Universidad Autónoma de Puebla for his help in the design of the figure. The author LEAG acknowledge CONACYT for the fellowship. The author GF acknowledges the “Sistema Nacional de Investigadores” of Mexico for membership. Thanks to Professor Robert Simpson of Connectivity for editing the English language text.
Footnotes
Conflicts of interest: Authors have no conflicts of interest to declare.
Financial support: This work was supported by grants from the PRODEP, No. CA-BUAP-120 and the CONACYT grant, No. 252808 (to GF) and MINECO/FEDER grant, No. BFU2012-38208 and the Junta de Andalucía, No. P11-CVI-7290 (to ARM).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Francesco Mattia Rossi, Universidad de la Republica Uruguay Facultad de Ciencias, Uruguay.
Funding: This work was supported by grants from the PRODEP, No. CA-BUAP-120 and the CONACYT grant, No. 252808 (to GF) and MINECO/FEDER grant, No. BFU2012-38208 and the Junta de Andalucía, No. P11-CVI-7290 (to ARM).
P-Reviewer: Rosji FM; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Arroyo-García LE, Vázquez-Roque RA, Díaz A, Treviño S, De La Cruz F, Flores G, Rodríguez-Moreno A. The effects of non-selective dopamine receptor activation by apomorphine in the mouse hippocampus. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-0991-2. doi: 10.1007/s12035-018-0991-2. [DOI] [PubMed] [Google Scholar]
- 2.Auffret M, Drapier S, Vérin M. The many faces of apomorphine: lessons from the past and challenges for the future. Drugs R D. 2018a;18:91–107. doi: 10.1007/s40268-018-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffret M, Drapier S, Vérin M. Pharmacological insights into the use of apomorphine in Parkinson's disease: clinical relevance. Clin Drug Investig. 2018b;38:287–312. doi: 10.1007/s40261-018-0619-3. [DOI] [PubMed] [Google Scholar]
- 4.Flores G, Barbeau D, Quirion R, Srivastava LK. Decreased binding of dopamine D3 receptors in limbic subregions after neonatal bilateral lesion of rat hippocampus. J Neurosci. 1996;16:2020–2026. doi: 10.1523/JNEUROSCI.16-06-02020.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores G, Liang JJ, Sierra A, Martínez-Fong D, Quirion R, Aceves J, Srivastava LK. Expression of dopamine receptors in the subthalamic nucleus of the rat: characterization using reverse transcriptase-polymerase chain reaction and autoradiography. Neuroscience. 1999;91:549–556. doi: 10.1016/s0306-4522(98)00633-2. [DOI] [PubMed] [Google Scholar]
- 6.Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM. Gender differences in Parkinson's disease: A clinical perspective. Acta Neurol Scand. 2017;136:570–584. doi: 10.1111/ane.12796. [DOI] [PubMed] [Google Scholar]
- 7.Gourgiotis I, Kampouri NG, Koulouri V, Lempesis IG, Prasinou MD, Georgiadou G, Pitsikas N. Nitric oxide modulates apomorphine-induced recognition memory deficits in rats. Pharmacol Biochem Behav. 2012;102:507–514. doi: 10.1016/j.pbb.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Haleem DJ, Farhan M. Inhibition of apomorphine-induced behavioral sensitization in rats pretreated with fluoxetine. Behav Pharmacol. 2015;26:159–166. doi: 10.1097/FBP.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 9.Jenner P, Katzenschlager R. Apomorphine - pharmacological properties and clinical trials in Parkinson's disease. Parkinsonism Relat Disord 33 Suppl. 2016;1:S13–21. doi: 10.1016/j.parkreldis.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kramar CP, Barbano MF, Medina JH. Dopamine D1/D5 receptors in the dorsal hippocampus are required for the acquisition and expression of a single trial cocaine-associated memory. Neurobiol Learn Mem. 2014;116:172–180. doi: 10.1016/j.nlm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obeso JA, Grandas F, Vaamonde J, Rosario Luguin M, Martinez-Lage JM. Apomorphine infusion for motor fluctuations in Parkinson's disease. Lancet. 1987;1:1376–1377. doi: 10.1016/s0140-6736(87)90679-9. [DOI] [PubMed] [Google Scholar]
- 13.Ribarič S. The pharmacological properties and therapeutic use of apomorphine. Molecules. 2012;17:5289–5309. doi: 10.3390/molecules17055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, Tronche F, Levesque D, Moquin L, Gratton A, Wong TP, Rubinstein M, Giros B. Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol Psychiatry. 2015;77:513–525. doi: 10.1016/j.biopsych.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18:1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwab RS, Amador LV, Lettvin JY. Apomorphine in Parkinson's disease. Trans Am Neurol Assoc. 1951;56:251–253. [PubMed] [Google Scholar]
- 17.Stibe C, Lees A, Stern G. Subcutaneous infusion of apomorphine and lisuride in the treatment of parkinsonian on-off fluctuations. Lancet. 1987;1:871. doi: 10.1016/s0140-6736(87)91660-6. [DOI] [PubMed] [Google Scholar]
- 18.Ungerstedt U, Butcher LL, Butcher SG, Anden NE, Fuxe K. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 1969;14:461–471. doi: 10.1016/0006-8993(69)90122-x. [DOI] [PubMed] [Google Scholar]
- 19.Zamudio S, Fregoso T, Miranda A, De La Cruz F, Flores G. Strain differences of dopamine receptor levels and dopamine related behaviors in rats. Brain Res Bull. 2005;65:339–347. doi: 10.1016/j.brainresbull.2005.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.