Alzheimer's disease (AD) is a fatal progressive neurodegenerative disorder characterized by loss in memory, cognition, and executive function and activities of daily living. AD pathogenesis has been shown to involve loss of neurons and synapses, cholinergic deficits, amyloid-beta protein (Aβ) deposition, tau protein hyperphosphorylation, and neuroinflammation. Among the various cell pathologic events observed in AD, changes in Aβ metabolism proceed fastest, often preceding clinical symptoms. Since the detection of immunoreactive and enzymatically active cathepsin B (cathB; EC 3.4.22.1) in association with amyloid deposits (Bernstein et al., 1990; Cataldo and Nixon, 1990) there has been ongoing interest in possible functions of this lysosomal cysteine protease in AD, and intense research has been done during the past decades to learn more about the possible place of the enzyme in disease pathophysiology. Soon it became clear that cathB is not a bystander but an active player, which is prominently and in many ways involved in AD pathology. However, findings from different groups are controversial and confusing. We herein try to draw attention to the Janus-faced nature of cathB in AD, in that the enzyme may contribute to both neuroprotection and neurodegeneration (Figure 1). Although we have to acknowledge that human morally (good, bad) and aesthetic (ugly) categories are not really suitable to describe cell pathologic processes, we will use these terms to clearly separate studies showing “positive” (in the sense of lowering AD pathology) from those demonstrating “negative” (increasing AD pathology) properties of cathB in AD.
Figure 1.

Cathepsin B: a Janus-faced molecule in Alzheimer's disease.
Abeta: Amyloid-beta.
The good one: cathB may be neuroprotective by lowering Aβ levels and improving neuronal dysfunction: In search of the “amyloid precursor protein (APP) secretase” (i.e., an enzyme which cuts inside the beta/A4 portion of the APP, thus preventing Aβ formation), cathB was identified as a possible enzyme candidate (Tagawa et al., 1991; Schönlein et al., 1993). Mueller-Steiner et al. (2006) demonstrated that cathB may contribute to the reduction of Aβ peptides by cleaving Aβ1–42. Moreover, genetic inactivation of cathB increased Aβ1–42 concentrations and amyloid deposits in mice expressing the familial AD-mutant human APP, while lentivirus-mediated expression of cathB in aged mutant mice counteracted AD neuropathology by reducing pre-existing amyloid plaques (Mueller-Steiner et al., 2006). In addition, cathB was found to degrade Aβ in mice expressing the wild-type (WT) human APP (Wang et al., 2012). Recently, it was revealed that in APP/PS1 mice (containing human transgenes for both APP bearing the Swedish mutation and PSEN1 containing an L166P mutation), hippocampal injections of adeno-virus expressing cath B attenuate Aβ levels and improve learning and memory in radial arm water maze (Embury et al., 2017), while loss of cathB in human neuroblastoma cells and mouse fibroblasts leads to lysosomal dysfunction and accumulation of key AD proteins (Cermak et al., 2016). From these findings it was concluded that positive lysosomal modulation, and in particular enhancing activity or levels of cathB, would be a promising strategy to prevent or treat AD (Cermak et al., 2016).
The bad one: cathB may contribute to AD pathology by acting as a beta-secretase: There is consensus that use of beta-secretase inhibitors that lower brain Aβproteins is a promising option for AD treatment. Normally, the aspartic acid protease, beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) is regarded “the” beta secretase, because it cleaves APP at the beta-cleavage site, creating a soluble extracellular fragment and a cell membrane-bound fragment referred to as C99. However, BACE1 splits the WT beta-site (which is expressed by a vast majority of AD patients) very slowly (kcat/km: 46.6 m–1 s–1), while some cathepsins cleave the WT beta-site much faster (cath B: kcat/km: 20,470 m–1 s–1; Schechter and Ziv, 2011). In a series of elegant experiments Hook´s group has demonstrated that (1) cathB selectively splits the APP WT beta-secretase site but not the rare Swedish mutant beta-secretase site, (2) injections of cysteine protease inhibitors (including the only cathB specific inhibitor, CA-074Me) into brains of guinea pigs expressing APP with a WT beta-secretase cleavage site strongly reduce in vivo amyloid levels and (3) deletion of cathB gene improves memory deficits in a transgenic AD mouse model expressing APP containing the WT beta-secretase site sequence. Moreover, the cysteine protease inhibitor E64d reduces brain amyloid and improves memory deficits in AD animal models by inhibiting cathB, but not BACE1, beta-secretase activity (findings summarized in Hook et al. (2011)). Thus, cathB should be regarded as an additional beta-secretase in the regulated secretory pathway of brain neurons. Overall, cathB is approximately 75-fold more abundant than BACE1 in human brain (Schechter and Ziv, 2011). The recent observation that in presynaptic dystrophic neurites surrounding amyloid plaques of transgenic mice the concentration of the “established” beta-secretase BACE1 is considerably higher than the concentration of cathB (Gowrishankar et al., 2015) does not necessarily contradict a possible role of cath B as beta-secretase, since the enzyme is about 400 times more effective than BACE1 in cleaving the WT beta-site of APP (Schechter and Ziv, 2011).
The ugly one: cathB generates pyroglutamate Aβ: Pyroglutamate Aβ peptides (pGlu-Aβ) are N-terminally truncated forms of full-length Aβ peptides (1–40/1–42), in which the N-terminal glutamate is cyclized by the enzyme glutamyl cyclase to yield pyroglutamate-Aβ (3–40/3–42). N-terminally truncated pGlu-Aβ peptides starting at position 3 are prominently localized in amyloid plaques of postmortem brains from AD patients and in transgenic mouse models of the disease. They are “particularly pernicious” forms of Aβ peptides, because pGlu-Aβ peptides are more stable, neurotoxic, and causes more aggregation of Aβ than does full length Aβ (1–40/1–42) (Hook et al., 2014). Since beta-secretase cleavage of APP generates full length Aβ species (1–40/42), Hook et al. (2014) tested whether the beta-secretase BACE1 or the alternative beta-secretase cathB participate in the production of pGlu-Aβ. It was revealed that deletion or overexpression of the cathB gene decreased or increased, respectively, the levels of the molecular species pGlu-Aβ (3–40/42), full length Aβ (1–40/42), as well as pGlu-Aβ plaque load, whereas knocking out the BACE1 gene had not effect. Moreover, the application of the cathB inhibitor E64d also reduced brain pGlu-Aβ (3–42), full length Aβ(1–40/42), and pGlu-Aβ plaque load. Thus, cathB seems to be one of two known enzymes involved in N-terminal truncation of Aβ (the other one is most probably dipeptidyl peptidase IV). For further details on the experimental procedure and finding of the cited papers, see Table 1.
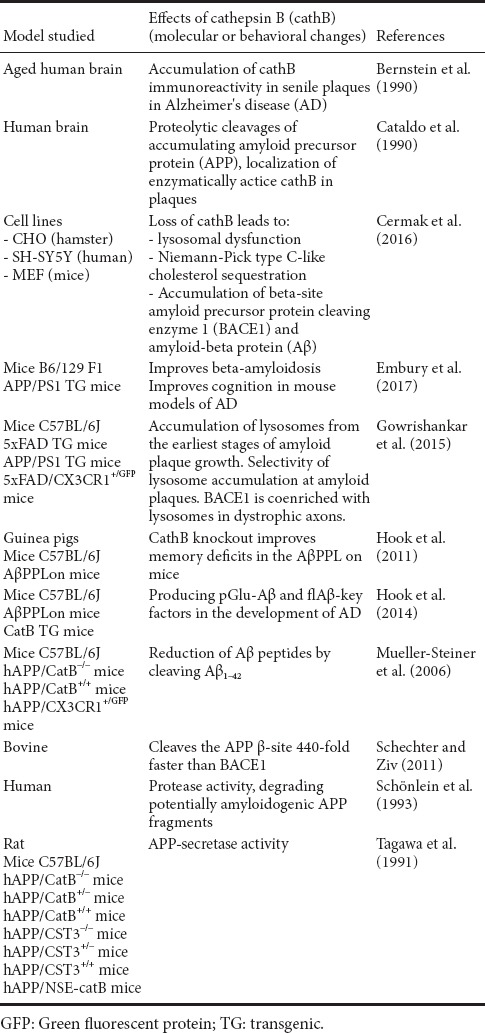
Table 1.
An overview on models studied, details of the experimental procedures and results of the cited papers
CathB as a possible target for AD treatment: The correctness of all aforementioned findings provided: what would be the lesson to be learned for AD treatment? Almost all authors dealing with the “dark side” of cathB in AD pathology recommend to depress the enzyme by using synthetic inhibitors (the naturally occurring cysteine protease inhibitors, cystatins, show by the way their “own” pathologic changes in AD, which were not discussed here). Since in AD lysosome-associated pathologic changes occur very early (already being detectable at the age of 3 months in the brains of transgenic mice (Gowrishankar et al., 2015), an intervention would have to be started before this age in order to prevent lysosome accumulation and to inhibit cathB. In terms of “human” AD this would mean: such a treatment has to be initiated “on mere suspicion” decades before the clinical manifestation of the disease, which is ethically unacceptable. And to depress cathB would also reduce the positive effects of the enzyme on AD pathology. For all these reasons we see currently only little chance to lower AD pathology via cathB modulation.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Bernstein HG, Kirschke H, Wiederanders B, Schmidt D, Rinne A. Antigenic expression of cathepsin B in aged human brain. Brain Res Bull. 1990;24:543–549. doi: 10.1016/0361-9230(90)90157-u. [DOI] [PubMed] [Google Scholar]
- 2.Cataldo AM, Nixon RA. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990;87:3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cermak S, Kosicek M, Mladenovic-Djordjevic A, Smiljanic K, Kanazir S, Hecimovic S. Loss of Cathepsin B and l leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer's proteins. PLoS One. 2016;11:e0167428. doi: 10.1371/journal.pone.0167428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embury CM, Dyavarshetty B, Lu Y, Wiederin JL, Ciborowski P, Gendelman HE, Kiyota T. Cathepsin B improves ß-amyloidosis and learning and memory in models of Alzheimer's disease. J Neuroimmune Pharmacol. 2017;12:340–352. doi: 10.1007/s11481-016-9721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, Ferguson SM. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer's disease amyloid plaques. Proc Natl Acad Sci U S A. 2015;112:E3699–3708. doi: 10.1073/pnas.1510329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-beta and improves memory deficits in Alzheimer's disease animal models by inhibiting cathepsin B, but not BACE1, beta-secretase activity. J Alzheimers Dis. 2011;26:387–408. doi: 10.3233/JAD-2011-110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hook G, Yu J, Toneff T, Kindy M, Hook V. Brain pyroglutamate amyloid-beta is produced by cathepsin B and is reduced by the cysteine protease inhibitor E64d, representing a potential Alzheimer's disease therapeutic. J Alzheimers Dis. 2014;41:129–149. doi: 10.3233/JAD-131370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Schönlein C, Probst A, Huber G. Characterization of proteases with the specificity to cleave at the secretase-site of beta-APP. Neurosci Lett. 1993;161:33–36. doi: 10.1016/0304-3940(93)90133-6. [DOI] [PubMed] [Google Scholar]
- 10.Schechter I, Ziv E. Cathepsins S, B and L with aminopeptidases display beta-secretase activity associated with the pathogenesis of Alzheimer's disease. Biol Chem. 2011;392:555–569. doi: 10.1515/BC.2011.054. [DOI] [PubMed] [Google Scholar]
- 11.Tagawa K, Kunishita T, Maruyama K, Yoshikawa K, Kominami E, Tsuchiya T, Suzuki K, Tabira T, Sugita H, Ishiura S. Alzheimer's disease amyloid beta-clipping enzyme (APP secretase): identification, purification, and characterization of the enzyme. Biochem Biophys Res Commun. 1991;177:377–387. doi: 10.1016/0006-291x(91)91994-n. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Sun B, Zhou Y, Grubb A, Gan L. Cathepsin B degrades amyloid-beta in mice expressing wild-type human amyloid precursor protein. J Biol Chem. 2012;287:39834–39841. doi: 10.1074/jbc.M112.371641. [DOI] [PMC free article] [PubMed] [Google Scholar]


