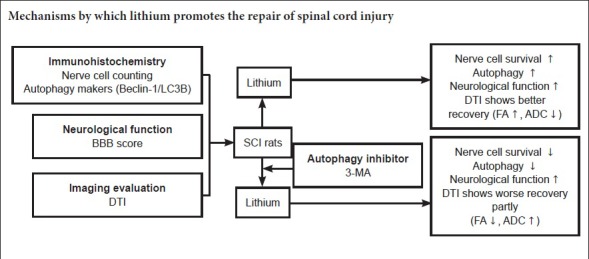
Keywords: nerve regeneration, spinal cord injury, lithium, secondary injury, autophagy, diffusion tensor imaging, neuroprotection, functional recovery, immunohistochemistry, Beclin-1, light-chain 3B, neural regeneration
Abstract
Lithium promotes autophagy and has a neuroprotective effect on spinal cord injury (SCI); however, the underlying mechanisms remain unclear. Therefore, in this study, we investigated the effects of lithium and the autophagy inhibitor 3-methyladenine (3-MA) in a rat model of SCI. The rats were randomly assigned to the SCI, lithium, 3-MA and sham groups. In the 3-MA group, rats were intraperitoneally injected with 3-MA (3 mg/kg) 2 hours before SCI. In the lithium and 3-MA groups, rats were intraperitoneally injected with lithium (LiCl; 30 mg/kg) 6 hours after SCI and thereafter once daily until sacrifice. At 2, 3 and 4 weeks after SCI, neurological function and diffusion tensor imaging indicators were remarkably improved in the lithium group compared with the SCI and 3-MA groups. The Basso, Beattie and Bresnahan locomotor rating scale score and fractional anisotropy values were increased, and the apparent diffusion coefficient value was decreased. Immunohistochemical staining showed that immunoreactivities for Beclin-1 and light-chain 3B peaked 1 day after SCI in the lithium and SCI groups. Immunoreactivities for Beclin-1 and light-chain 3B were weaker in the 3-MA group than in the SCI group, indicating that 3-MA inhibits lithium-induced autophagy. Furthermore, NeuN+ neurons were more numerous in the lithium group than in the SCI and 3-MA groups, with the fewest in the latter. Our findings show that lithium reduces neuronal damage after acute SCI and promotes neurological recovery by inducing autophagy. The neuroprotective mechanism of action may not be entirely dependent on the enhancement of autophagy, and furthermore, 3-MA might not completely inhibit all autophagy pathways.
Introduction
Spinal cord injury (SCI) is the direct or indirect damage to any part of the spinal cord that results in permanent impairment in strength, sensation or other body function below the injury site (Wyndaele and Wyndaele, 2006; Krause et al., 2017). Secondary injury mechanisms play important roles in the acute, sub-acute and chronic phases and lead to vasospasm, ischemia, inflammation, and free radical production (Oyinbo, 2011). These events result in neuronal loss, which is the key cause of permanent neurological dysfunction (Kanno et al., 2009; Tang et al., 2014; Li et al., 2015a; Kwan et al., 2017).
Cell autophagy or type II programmed cell death is an intracellular catabolic mechanism for recycling damaged organelles and senescent proteins and plays a very important role in cell survival, differentiation and homeostasis (Erlich et al., 2007; Mizushima et al., 2010). It was reported that enhancing autophagy promotes the recovery of neurological functions by inhibiting apoptosis (Sekiguchi et al., 2012; Liu et al., 2015; Colón and Miranda, 2016; Dai et al., 2017). Several studies have reported that autophagy is involved in SCI. Lysosomal dysfunction and the disruption of autophagy, as well as increased neuronal apoptosis, have been observed in SCI, suggesting that autophagy is involved in SCI (Silva et al., 2008; Liu et al., 2015). Accordingly, an increasing number of studies have focused on the therapeutic effect of modulating autophagy in SCI.
Lithium is the first line drug for treating bipolar disorder, and provides neuroprotection in multiple neurologic diseases (Young, 2009; Huo et al., 2012). Accumulating evidence suggests that lithium has numerous actions, including neuroprotection, inflammation inhibition, induction of neurotrophic factor secretion, and the enhancement of neurogenesis (Son et al., 2003; Senatorov et al., 2004; Su et al., 2007; Yasuda et al., 2009; Yuskaitis and Jope, 2009; Chi-Tso and Chuang, 2011; De Meyer et al., 2011; Li et al., 2011; Lauterbach, 2016). However, it remains unclear whether autophagy plays a positive or negative role in SCI (O’Donovan et al., 2015; Del Grosso et al., 2016; Fabrizi et al., 2017).
Multiple signaling pathways are involved in autophagy, including the PI3K/Akt/mTOR, AMPK/TSC/mTOR and eIF2α/Atg12 pathways (Periyasamy-Thandavan et al., 2009). It was reported that lithium affects several signaling pathways, including the PI3K/Akt and IP3 pathways, which are involved in autophagy. Therefore, it is reasonable to speculate that lithium promotes functional recovery by inducing autophagy in rat models of SCI, which has not yet been studied (Periyasamy-Thandavan et al., 2009; Chi-Tso and Chuang, 2011; Li et al., 2015b).
In the present study, we investigate the neuroprotective effect of lithium and the role of autophagy in SCI using the autophagy inhibitor 3-methyladenine (3-MA). To objectively and accurately evaluate recovery following SCI, we performed diffusion tensor imaging (DTI), which is an effective method of assessing neurological recovery, in addition to the Basso, Beattie, Bresnahan (BBB) locomotor rating scale (Zhang et al., 2015).
Materials and Methods
Animal care and groups
A total of 72 specific-pathogen-free adult healthy male Sprague-Dawley rats weighing 230–270 g were provided by the Experimental Animal Center of Xi’an Jiaotong University of China (production license No. SCXK (Shaan) 2007-001; user license No. SYXK (Shaan) 2007-003). All rats were housed under a 12-hour light/dark cycle. All experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The protocols were approved by the Animal Ethics Committee of Xi’an Jiaotong University of China. All efforts were made to minimize distress to the rats.
The rats were randomly separated into sham, SCI, lithium and 3-MA groups (n = 18 per group). Six rats from each group were randomly selected for BBB scoring and DTI examination, and three rats from each group were sacrificed for immunohistochemical staining at each time point.
SCI model
Rats in the SCI, lithium and 3-MA groups received SCI operation as previously described (Basso et al., 1995). Briefly, the rat was anesthetized with intraperitoneal injection of chloral hydrate (300 mg/kg) and placed in the prone position with a heating pad to maintain body temperature. After shaving and aseptic preparation, the spinal cord was exposed. Dorsal laminectomy was performed at T9–11. The T10 segment of the spinal cord was impacted with an NYU weight-drop impactor (10 g rod dropped from a height of 25 mm; Rutgers University, USA), which led to hemorrhage and edema at the site of impact, wagging tail reflex and lower limb spasm. All manifestations indicated the success of the injury model. The rats in the sham group underwent laminectomy alone. The tissue was sutured layer by layer, with a piece of fat sutured under the skin at the T10 level. After SCI surgery, manual bladder massage was performed three times, and intraperitoneal injection of penicillin 20 U/kg was given once daily until bladder function was reestablished.
Lithium and 3-MA treatments
Lithium chloride (LiCl; Kemiou, Tianjin, China) and 3-MA (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 0.9% NaCl. Rats in the 3-MA group were given intraperitoneal injection of 1 mL 3-MA (3 mg/kg) 2 hours before SCI (Chen et al., 2013; Tang et al., 2014). Rats in the lithium and 3-MA groups were administered 1 mL lithium by intraperitoneal injection (LiCl, 30 mg/kg) 6 hours after SCI and then once daily until sacrifice (Yick et al., 2004; Zakeri et al., 2014). The sham and SCI groups received 1 mL 0.9% NaCl via intraperitoneal injection.
Neurological function assessment
The BBB locomotor rating scale was used to assess neurological function after SCI (Zhang et al., 2015). The BBB score ranges from 0 (complete paralysis) to 21 (normal), based on the range and extent of motion, weight loading, coordination of the forelimbs and hindlimbs, and motion of the forepaw, hindpaw and tail. Three independent examiners blindly assessed the BBB score before operation, and at 6 hours and 1, 2, 3 and 4 weeks after SCI. The average score was taken as the final score for each rat at each time point.
DTI examination
A DTI scan was performed 24 hours before SCI and at 6 hours and 1, 2, 3 and 4 weeks after SCI using a 3.0 T SIGNA MRI scanner (GE Medical Systems, Milwaukee, WI, USA) at the same loci as the conventional MRI scan. The scanning parameters were as follows: diffusion-weighted coefficient (b-value) = 1000 s/mm2; diffusion-sensitive gradient = 15 different directions; repetition time = 3500 ms; echo time = 87.5 ms; thickness = 2.4 mm; space = 0; field of view = 10; acquisition matrix = 64 × 64. All data were input into a workstation running Advantage Windows 4.2 (GE Healthcare). The region of interest (ROI) was identified by the fat under the skin, which was displayed as a high signal on conventional T2WI MRI (Yan et al., 2007). Based on the fractional anisotropy (FA) map, the ROI was placed in the inferior medulla and the inferior oblongata. The ROI was selected by two independent testers, and apparent diffusion coefficient (ADC) and FA values were obtained. FA values reflect the degree of spatial displacement of water molecules, and higher FA values indicate stronger anisotropy. ADC values are independent of the diffusion directions, and indicate the diffusional displacement of water molecules.
Immunohistochemical staining
Three rats were randomly selected from the sham group, while three rats were randomly selected from the other groups at 6 hours and at 1, 3 and 7 days for immunohistochemistry. The rats were anesthetized with chloral hydrate (300 mg/kg) and received aortic cannulation through the apex of the left ventricle. The rat was then perfused with 4% paraformaldehyde. A 2.0-cm spinal cord tissue segment centered at the injury site (T10 segment) was harvested and immersed in 4% paraformaldehyde at 4°C for 12 hours, and thereafter, 6 µm-thick coronal paraffin sections were prepared.
Three slices from each rat were selected for immunohistochemical staining for the neuronal nuclear antigen NeuN and the autophagy markers Beclin-1 and microtubule-associated proteins 1A/1B light-chain 3B (LC3B). Briefly, the sections were deparaffinized, rehydrated in distilled water, placed in 3% H2O2 to remove residual peroxidase, and then rinsed with phosphate-buffered saline (PBS). The slices were blocked with 10% normal goat serum for 2 hours following permeabilization with 0.1% Triton X-100. Afterwards, the sections were incubated with anti-NeuN antibody (rabbit anti-rat IgG; 1:1000; Abcam, Cambridge, UK), anti-Beclin-1 antibody (mouse anti-rat IgG; 1:400; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-LC3B antibody (mouse anti-rat IgG; 1:400; Santa Cruz Biotechnology) at 4°C for 24 hours. The sections were then incubated with the corresponding horseradish peroxidase-labeled antibody (goat anti-mouse; 1:100; Beyotime, Shanghai, China) at 37°C for 30 minutes, followed by three washes with PBS. Specific staining was visualized with diaminobenzidine according to the supplier's instructions (Beyotime), followed by counterstaining with hematoxylin. Finally, sections were washed with PBS, dehydrated through a graded alcohol series (50%, 75%, 95%, 100%), cleared with dimethylbenzene, and mounted using a coverslip.
For analysis, four randomly selected fields were photographed at 400× magnification on a microscope (Olympus, Tokyo, Japan). NeuN-positive cells at the injury site were counted manually and blindly by three examiners. Images of Beclin-1 and LC3B-stained sections were imported into Image Plus Pro 6.0 software (Media Cybernetics, Rockville, MD, USA) to quantify the positively-stained area. Relative area, which was defined as the ratio of the average area in the experimental group to that in the sham group, was analyzed to compare autophagy levels among the groups.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analysis was performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). One-way analysis of variance followed by the least significant difference post hoc test was used to compare differences in intergroup data at each time point. Pearson correlation analysis was used to analyze FA and ADC values and BBB scores. A value of P < 0.05 was considered statistically significant.
Results
General condition of the experimental animals
All 72 rats recovered from anesthesia within 2 hours of surgery and survived over the course of the experimental period. All 72 rats were included in the final analysis.
Neurological function assessment
Lower hindlimb function was assessed with the BBB scale 24 hours before SCI, and at 6 hours and 1, 2, 3 and 4 weeks after SCI. All rats were evaluated on schedule and received 21 points before SCI. The rats in the SCI, lithium and 3-MA groups displayed flaccid paralysis and a failure of autonomic urination. Neurological function in the sham group was the same as in the pre-operative period at all time points after laminectomy. The graph shows the BBB scores at the different time points. Locomotor function was dramatically reduced after SCI and gradually improved with time in the SCI, lithium and 3-MA groups (P < 0.05; Figure 1). Recovery was significantly better in the lithium group than in the SCI and 3-MA groups at 1, 2, 3 and 4 weeks after SCI (P < 0.05). There was no difference in BBB scores between the 3-MA and SCI groups at 1 and 2 weeks after SCI (P > 0.05), but BBB scores were higher in the 3-MA group than in the SCI group at 3 and 4 weeks (P < 0.05).
Figure 1.
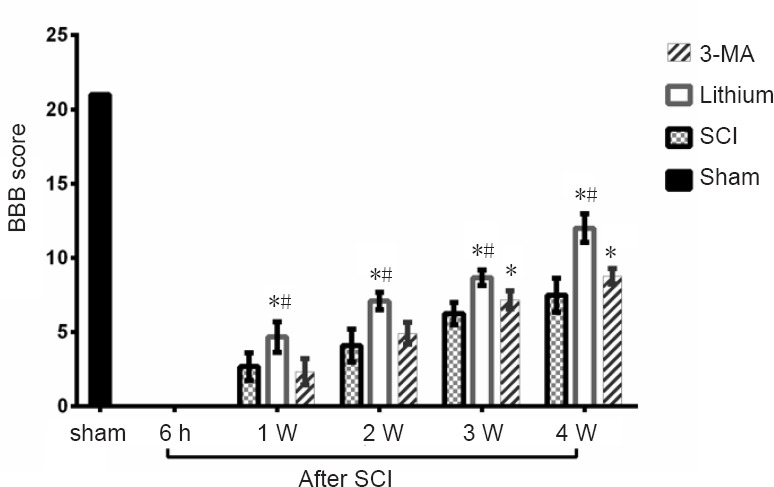
Effects of lithium and 3-MA on motor function in rats with SCI.
The BBB locomotor rating scale scores ranged from 0 to 21 points. Lower scores indicate poorer motor function. BBB scores were significantly higher in the lithium group than in the SCI and 3-MA groups at 1, 2, 3 and 4 weeks after SCI (P < 0.05). There was no difference in BBB scores between the 3-MA group and the SCI group at 1 or 2 weeks after SCI, while BBB scores were higher in the 3-MA group than in the SCI group (P < 0.05). *P < 0.05, vs. SCI group; #P < 0.05, vs. 3-MA group (mean ± SD, n = 6; one-way analysis of variance followed by the least significant difference post hoc test). BBB: Basso, Beattie & Bresnahan; 3-MA: 3-methyladenine; SCI: spinal cord injury; h: hours; W: week(s).
Changes in FA and ADC values at the injury site
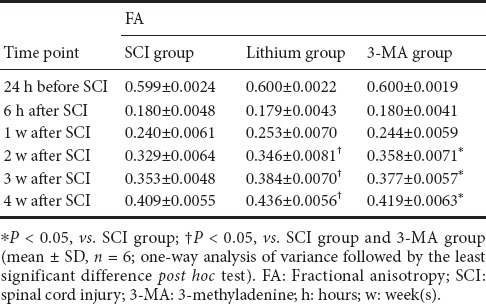
FA values significantly decreased (P < 0.05), while ADC values increased significantly (P < 0.05) after SCI. FA values gradually increased over time in all groups (P < 0.05), and there was no difference among the three groups at 6 hours after SCI (P > 0.05). At 1 week after SCI, the FA value was higher in the lithium group than in the SCI and 3-MA groups (P < 0.05), and there was no difference between the SCI and 3-MA groups (P > 0.05). At 2, 3 and 4 weeks after SCI, the FA value was higher in the lithium group than in the SCI and 3-MA groups (P < 0.05), and higher in the 3-MA group than in the SCI group (P < 0.05; Table 1).
Table 1.
FA values at the injury site at different time points after SCI
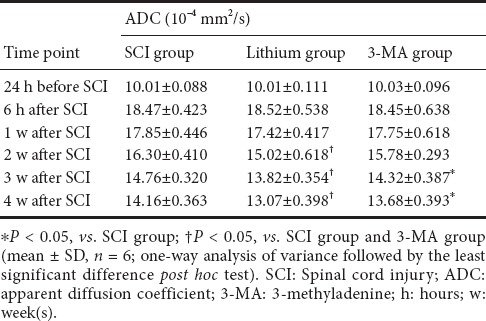
ADC values gradually decreased with time in all groups (P < 0.05), and there was no difference among the three groups at 6 hours or 1 week after SCI (P > 0.05). At 2 weeks after SCI, the ADC value was lower in the lithium group than in the SCI and 3-MA groups (P < 0.05), and there was no difference between the SCI and 3-MA groups (P > 0.05). At 3 and 4 weeks after SCI, the ADC value was again lower in the lithium group than in the SCI and 3-MA groups (P < 0.05), and was lower in the 3-MA group than in the SCI group (P < 0.05; Table 2).
Table 2.
ADC values at the injury site at different time points after SCI
Correlation between DTI and neurological function
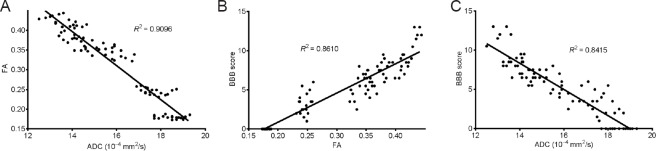
Pearson correlation analysis showed that FA values were negatively correlated with ADC values in the rat model of spinal cord contusion injury (r = −0.9537, P < 0.05; (Figure 2A), consistent with our previous observations (Zhang et al., 2015; He, 2015). FA values were positively and linearly correlated with BBB scores (r = 0.9279, P < 0.05; Figure 2B). ADC values were negatively correlated with BBB scores, and the correlation was linear (r = −0.9173, P < 0.05; Figure 2C).
Figure 2.
Correlation between diffusion tensor imaging and neurological function assessment (Pearson correlation analysis).
(A) FA values were negatively correlated with ADC values, and the correlation was linear (r = –0.9537, P < 0.05). (B) FA values were positively correlated with BBB locomotor rating scale scores, and the correlation was linear (r = 0.9279, P < 0.05). (C) ADC values were negatively and linearly correlated with the BBB scores (r = –0.9173, P < 0.05). SCI: Spinal cord injury; FA: fractional anisotropy; ADC: apparent diffusion coefficient; BBB: Basso, Beattie & Bresnahan.
Immunolabeling for neurons
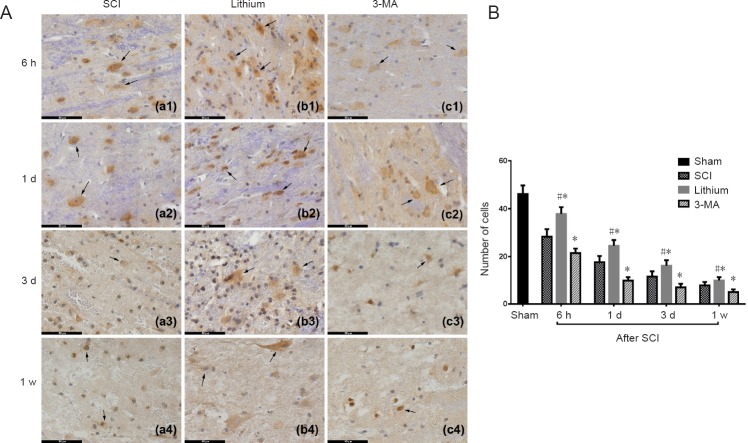
Immunohistochemical staining showed that the number of neurons (NeuN+ cells) at the site of injury was reduced in the SCI group at 6 hours after SCI, and continued to diminish with time compared with the sham group. In the lithium group, neurons were similarly reduced at 6 hours after SCI but were more numerous than in the SCI and 3-MA groups at 1 and 3 days and 1 week after SCI. In the 3-MA group, neurons were greatly reduced in number compared with the SCI group at 6 hours after SCI and compared with the SCI and lithium groups at 1 and 3 days and 1 week after SCI (Figure 3A). Furthermore, these neurons had an abnormal morphology.
Figure 3.
Immunohistochemical staining and counting of neurons (NeuN+ cells) in the injured rat spinal cord at different time points.
(A) Neurons were more numerous in the lithium group than in the other groups at 6 hours, 1 and 3 days, and 1 week. Arrows point to neurons. Scale bars: 50 μm. (B) Neurons in each experimental group were significantly decreased at 1 and 3 days and 1 week after SCI compared with the previous time point. More neurons survived in the lithium group than in the SCI group, while more neurons survived in the SCI group than in the 3-MA group. All data are expressed as the mean ± SD (n = 3; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. SCI group; #P < 0.05, vs. 3-MA group. SCI: Spinal cord injury; 3-MA: 3-methyladenine; h: hours; d: day(s); w: week.
Cell counting showed that the number of neurons in all three experimental groups decreased significantly at 6 hours after SCI compared with the sham group (P < 0.05). The number of neurons in the experimental groups continued to decrease at 1 and 3 days and 1 week after SCI, compared with the previous time point (P < 0.05). More neuronal cells survived in the lithium group than in the SCI group, and more neuronal cells survived in the SCI group than in the 3-MA group (P < 0.05; Figure 3B).
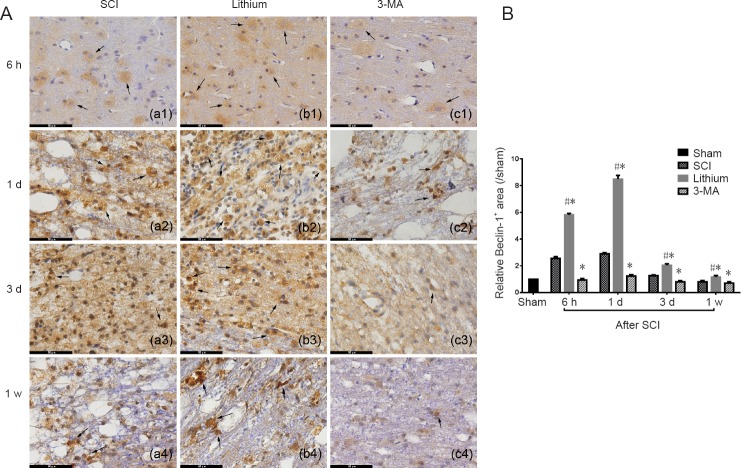
Beclin-1 immunohistochemistry
The Beclin-1+ area was larger and more strongly stained, and Beclin-1+ cells were more numerous in the SCI group at 6 hours after SCI compared with the sham group. Further expansion of the Beclin-1+ area was found at 1 day, but it diminished from 3 days after SCI. In the lithium group, the Beclin-1+ area was larger and more intensely stained, and Beclin-1+ cells were more numerous at 6 hours after SCI compared with the SCI group. The Beclin-1+ area was even larger at 1 day, but it started to diminish from 3 days after SCI, although the staining was still more intense than in the SCI group at 1 week. In the 3-MA group, the staining was slightly more intense than in sham group 1 day after SCI, while it was weaker than in the SCI group at 3 days and 1 week after SCI (Figure 4A).
Figure 4.
Immunohistochemical staining for Beclin-1 and the relative Beclin-1+ area in the injured spinal cord at different time points.
(A) The number of Beclin-1+ cells and the intensity of staining were higher in the lithium group than in the other groups at 6 hours, 1 and 3 days, and 1 week. Arrows point to Beclin-1 protein. Scale bars: 50 μm. (B) The relative area of Beclin-1+ staining was greater in the lithium group than in the SCI and 3-MA groups, while it was lower in the 3-MA group than in the SCI group. All data are expressed as the mean ± SD (n = 3; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. SCI group; #P < 0.05, vs. 3-MA group. SCI: Spinal cord injury; 3-MA: 3-methyladenine; h: hours; d: day(s); w: week.
The relative Beclin-1+ area in the SCI group at 6 hours after SCI was significantly greater than 1 (P < 0.05), indicating that it was larger than in the sham group and that the level of autophagy increased after SCI. The relative Beclin-1+ area reached a peak at 1 day after SCI and decreased from 3 days after SCI. There was a similar trend in the lithium group, with the relative area increasing from 6 hours after SCI, peaking at 1 day, and decreasing significantly from 3 days (P < 0.05). Compared with the SCI group, the relative Beclin-1+ area in the lithium group was greater at 6 hours, 1 and 3 days and 1 week after SCI (P < 0.05). In comparison, the relative Beclin-1+ area was significantly smaller in the 3-MA group than in the SCI group at 6 hours and 1 and 3 days after SCI (P < 0.05; Figure 4B).
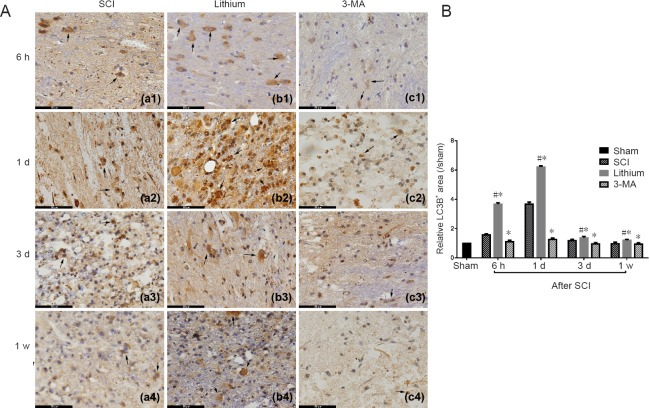
LC3B immunohistochemistry
The LC3B+ area was expanded, the staining intensity was greater, and positive cells were increased in the SCI group at 6 hours after SCI compared with the sham group. Further expansion of the LC3B+ area was found at 1 day, but it decreased from 3 days after SCI. The LC3B+ area was expanded, the staining intensity was greater, and positive cells were increased in the lithium group at 6 hours after SCI compared with the SCI group. Further expansion of the LC3B+ area was found at 1 day, and it shrank from 3 days after SCI, although the staining was still more intense than in the SCI group at 1 week after SCI. In the 3-MA group, 1 day after SCI, the staining was slightly stronger than in the sham group, while it was weaker than in the SCI group at 3 days and 1 week after SCI (Figure 5A).
Figure 5.
Immunohistochemical staining for LC3B and the relative LC3B+ area in the injured spinal cord at different time points.
(A) Number of LC3B+ cells and the intensity of staining were greater in the lithium group than in the other groups at 6 hours, 1 and 3 days, and 1 week. Arrows point to LC3B protein. Scale bars: 50 μm. (B) The relative LC3B+ area was larger in the lithium group than in the SCI and 3-MA groups, while it was lower in the 3-MA group than in the SCI group. All data are expressed as the mean ± SD (n = 3; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. SCI group; #P < 0.05, vs. the 3-MA group. SCI: Spinal cord injury; 3-MA: 3-methyladenine; h: hours; d: day(s); w: week.
The quantitative analysis revealed that the relative LC3B+ area in the SCI group at 6 hours after SCI was significantly greater than 1 (P < 0.05), indicating that the LC3B+ area was larger than in the sham group, and suggesting that the level of autophagy increased after SCI. The relative LC3B+ area peaked at 1 day after SCI and decreased from 3 days after SCI. There was a similar trend in the lithium group, with the relative area increasing from 6 hours after SCI, peaking at 1 day, and significantly decreasing from 3 days (P < 0.05). Compared with the SCI group, the relative LC3B+ area in the lithium group was greater at 6 hours, 1 and 3 days and 1 week after SCI (P < 0.05). However, the relative LC3B+ area was significantly smaller in the 3-MA group than in the SCI group at 6 hours and 1 and 3 days after SCI (P < 0.05; Figure 5B).
Discussion
Advanced evaluation of SCI
Conventional MRI is widely used for patients with SCI. However, it fails to clearly display the degree of injury or the recovery and regeneration of neuronal fibers in the spinal cord after injury. Therefore, in the present study, we used DTI for the three-dimensional reconstruction of white matter fiber bundles.
Based on our previous study, DTI is an objective and accurate method for evaluating recovery following SCI and the effect of therapeutic interventions in complete transection SCI models (Zhang et al., 2015). SCI causes damage to cell membranes and myelin sheaths, leading to the destruction of the molecular diffusion barrier and the unrestricted movement of water (Li et al., 2016). This occurred immediately after SCI. Subsequently, along with glial scar formation, the displacement of water molecules was reduced, and the regeneration of axons forced the water molecules to diffuse primarily in one direction, which was reflected as a gradual increase in the FA value and a decrease in the ADC value. The DTI outcomes we observed in this study were consistent with the pathological changes. The FA and ADC values correlated well with the BBB scores. Lithium promoted recovery following SCI, while 3-MA reduced the therapeutic effectiveness of lithium. Therefore, DTI can accurately reflect axonal necrosis and degeneration, glial cell regeneration and demyelination after SCI, and display changes in the microstructure of the spinal cord in vivo (Zhang et al., 2015; Jirjis et al., 2017).
Autophagy in lithium treatment for SCI
Autophagy is an evolutionarily conserved process, and over 30 autophagy-related genes (Atgs) have been identified, of which LC3B (or LC3II) and Beclin-1 are standard markers (Kirisako et al., 1999; Ohsumi, 2001; Mizushima and Yoshimori, 2007; Periyasamy-Thandavan et al., 2009). Accumulating evidence suggests that lithium has neuroprotective properties, suggesting that it may have potential as a new therapy for SCI (Sarkar et al., 2005; Wada et al., 2005; Yan et al., 2007; Pasquali et al., 2009). Although lithium has been widely used for safely and effectively treating neuropsychiatric disorders, it is rarely used for acute SCI (Ohsumi, 2001; Chang et al., 2011; Chen et al., 2013; Kim et al., 2013; Duo and He, 2015; Hou et al., 2015; Seo et al., 2015; Quartini et al., 2016; Zhou et al., 2016; Wu et al., 2018). Therefore, the effectiveness of lithium treatment for acute SCI remained unclear.
The role of autophagy in recovery following injury is still controversial. While some studies have reported that enhanced autophagy improves neuroprotection, others have suggested that the suppression of autophagy is beneficial to recovery (Li et al., 2010; Shimada et al., 2012; O’Donovan et al., 2015; Del Grosso et al., 2016). Previous studies in other fields have demonstrated that lithium can enhance autophagy, or in contrast, reduce apoptosis and autophagy (Wong et al., 2011; Raja et al., 2015; Guttuso, 2016). Our results show that lithium promotes neurological functional recovery and neural cell survival, which supports the notion that lithium has neuroprotective properties. Furthermore, we observed that these neuroprotective effects were inhibited by 3-MA, which downregulated the autophagy induced by lithium. This implies that lithium reduces neuronal damage and promotes functional recovery by inducing autophagy. Nevertheless, BBB scores were still higher in the 3-MA group than in the SCI group from 3 weeks after SCI, in accordance with the neural cell counting results. This suggests that the mechanisms of autophagy are complex and that other signaling pathways that are not inhibited by 3-MA or activated by lithium play a role in the process (Galluzzi et al., 2017) Furthermore, lithium may also promote neurotrophin secretion, inhibit inflammation or enhance the proliferation of neural progenitor cells (Son et al., 2003; Senatorov et al., 2004; Su et al., 2007; Yasuda et al., 2009; Chi-Tso and Chuang, 2011; Li et al., 2011).
The opposing concept that autophagy aggravates injury may be explained by differences in lithium concentrations and target cells in previous studies. Discrepancies may also be caused by differences in animal models, the therapeutic window and the treatment period. Fang et al. (2016) found that early activated autophagy alleviates spinal cord ischemia/reperfusion injury, while later excessively elevated autophagy aggravates the injury. Therefore, autophagy appears to be a dynamic process with differential effects, depending on the time frame and model. Further study is needed to examine the signaling pathways affected by lithium and the dynamic changes in the autophagy pathway.
Summary
Further clinical trials are required to explore the effect of lithium therapy in acute SCI patients. In addition, studies are needed to optimize the time window of treatment, the treatment dose and protocol and to reduce the side effects of lithium.
In conclusion, our findings demonstrate that lithium protects neurons and promotes autophagy in a rat model of acute SCI. DTI is an effective method for evaluating recovery following SCI, and correlates well with neurological functional scores in our rat model of spinal cord contusion injury. The dynamic changes in autophagy after SCI and the effects of lithium on this process need to be investigated further.
Additional file: Open peer review report 1 (6.6KB, pdf) .
Acknowledgments
We are very grateful to Jia-Lin Zhu from Xi’an Jiaotong University of China for technical support.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the Beijing Excellent Talent Training Funding in China, No. 2017000021469G215 (to DZ); the Youth Science Foundation of Beijing Tiantan Hospital of China, No. 2016-YQN-14 (to DZ); the Natural Science Foundation of Capital Medical University of China, No. PYZ2017082 (to DZ); the Xi’an Science and Technology Project in China, No. 2016048SF/YX04(3) (to XHL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Animal Ethics Committee of Xi’an Jiaotong University of China. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Chang Ho Hwang, University of Ulsan, Republic of Korea.
Funding: This study was supported by the Beijing Excellent Talent Training Funding in China, No. 2017000021469G215 (to DZ); the Youth Science Foundation of Beijing Tiantan Hospital of China, No. 2016-YQN-14 (to DZ); the Natural Science Foundation of Capital Medical University of China, No. PYZ2017082 (to DZ); the Xi’an Science and Technology Project in China, No. 2016048SF/YX04(3) (to XHL).
P-Reviewer: Hwang CH; C-Editor: Zhao M; S-Editor: Wang J, Li CH; L-Editor: Patel B, Raye W, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating-scale for open-field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Chang JW, Choi H, Cotman SL, Jung YK. Lithium rescues the impaired autophagy process in CbCln3Δex7/8/Δex7/8 cerebellar cells and reduces neuronal vulnerability to cell death via IMPase inhibition. J Neurochem. 2011;116:659–668. doi: 10.1111/j.1471-4159.2010.07158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HC, Fong TH, Hsu PW, Chiu WT. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res. 2013;179:E203–210. doi: 10.1016/j.jss.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Chi-Tso C, Chuang DM. Neuroprotective action of lithium in disorders of the central nervous system. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:461. doi: 10.3969/j.issn.1672-7347.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colón JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11:1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai B, Yan T, Shen YX, Xu YJ, Shen HB, Chen D, Wang JR, He SH, Dong QR, Zhang AL. Edaravone protects against oxygen-glucose-serum deprivation/restoration-induced apoptosis in spinal cord astrocytes by inhibiting integrated stress response. Neural Regen Res. 2017;12:283–289. doi: 10.4103/1673-5374.199006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van Vaeck L, Fransen P, Bult H, De Meyer GRY. Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. Br J Pharmacol. 2011;162:1410–1423. doi: 10.1111/j.1476-5381.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Grosso A, Antonini S, Angella L, Tonazzini I, Signore G, Cecchini M. Lithium improves cell viability in psychosine-treated MO3. 13 human oligodendrocyte cell line via autophagy activation. J Neurosci Res. 2016;94:1246–1260. doi: 10.1002/jnr.23910. [DOI] [PubMed] [Google Scholar]
- 9.Duo Z, He XJ. Advances in mechanisms of treatment for spinal cord injury with lithium. Zhongguo Gushang. 2015;28:679–682. [PubMed] [Google Scholar]
- 10.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Fabrizi C, Pompili E, Somma F, De Vito S, Ciraci V, Artico M, Lenzi P, Fornai F, Fumagalli L. Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. J Appl Toxicol. 2017;37:207–213. doi: 10.1002/jat.3344. [DOI] [PubMed] [Google Scholar]
- 12.Fang B, Li XQ, Bao NR, Tan WF, Chen FS, Pi XL, Zhang Y, Ma H. Role of autophagy in the bimodal stage after spinal cord ischemia reperfusion injury in rats. Neuroscience. 2016;328:107–116. doi: 10.1016/j.neuroscience.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Guttuso T. Low-dose lithium adjunct therapy associated with reduced off-time in Parkinson's disease: a case series. J Neurol Sci. 2016;368:221–222. doi: 10.1016/j.jns.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Hou L, Xiong N, Liu L, Huang J, Han C, Zhang G, Li J, Xu X, Lin Z, Wang T. Lithium protects dopaminergic cells from rotenone toxicity via autophagy enhancement. BMC Neurosci. 2015;16:82. doi: 10.1186/s12868-015-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo K, Sun Y, Li H, Du X, Wang X, Karlsson N, Zhu C, Blomgren K. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol Cell Neurosci. 2012;51:32–42. doi: 10.1016/j.mcn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Jirjis MB, Valdez C, Vedantam A, Schmit BD, Kurpad SN. Diffusion tensor imaging as a biomarker for assessing neuronal stem cell treatments affecting areas distal to the site of spinal cord injury. J Neurosurg Spine. 2017;26:243–251. doi: 10.3171/2016.5.SPINE151319. [DOI] [PubMed] [Google Scholar]
- 17.Kanno H, Ozawa H, Seldguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–148. doi: 10.1016/j.nbd.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Kim EC, Meng H, Jun AS. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol. 2013;97:1068–1073. doi: 10.1136/bjophthalmol-2012-302881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause JS, Newman JC, Clark JMR, Dunn M. The natural course of spinal cord injury: changes over 40 years among those with exceptional survival. Spinal Cord. 2017;55:502–508. doi: 10.1038/sc.2016.159. [DOI] [PubMed] [Google Scholar]
- 21.Kwan T, Floyd CL, Kim S, King PH. RNA binding protein human antigen R is translocated in astrocytes following spinal cord injury and promotes the inflammatory response. J Neurotrauma. 2017;34:1249–1259. doi: 10.1089/neu.2016.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauterbach EC. Six psychotropics for pre-symptomatic & early Alzheimer's (MCI), Parkinson’s, and Huntington's disease modification. Neural Regen Res. 2016;11:1712–1726. doi: 10.4103/1673-5374.194708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Jia Z, Cao Y, Wang Y, Li H, Zhang Z, Bi J, Lv G, Fan Z. Mitochondrial division inhibitor 1 ameliorates mitochondrial injury, apoptosis, and motor dysfunction after acute spinal cord injury in rats. Neurochem Res. 2015a;40:1379–1392. doi: 10.1007/s11064-015-1604-3. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Li Q, Du X, Sun Y, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J Cereb Blood Flow Metab. 2011;31:2106–2115. doi: 10.1038/jcbfm.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JF, Yan JY, Xia RF, Zhang X, Tan XH, Guan J, Ye Z, Zhang SL. Glial scar formation and astrocyte role in spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5609–5616. [Google Scholar]
- 26.Li Q, Li H, Roughton K, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis. 2010;1:e56. doi: 10.1038/cddis.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XH, Li JB, He XJ, Wang F, Huang SL, Bai ZL. Timing of diffusion tensor imaging in the acute spinal cord injury of rats. Sci Rep. 2015b;5:12639. doi: 10.1038/srep12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6:e1582. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 30.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donovan TR, Rajendran S, O’Reilly S, O’Sullivan GC, McKenna SL. Lithium modulates autophagy in esophageal and colorectal cancer cells and enhances the efficacy of therapeutic agents in vitro and in vivo. PLoS One. 2015;10:e0134676. doi: 10.1371/journal.pone.0134676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 33.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 34.Pasquali L, Longone P, Isidoro C, Ruggieri S, Paparelli A, Fornai F. Autophagy, lithium, and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40:173–194. doi: 10.1002/mus.21423. [DOI] [PubMed] [Google Scholar]
- 35.Periyasamy-Thandavan S, Jiang M, Schoenlein P, Dong Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. Am J Physiol Renal Physiol. 2009;297:F244–F256. doi: 10.1152/ajprenal.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quartini A, Iannitelli A, Bersani G. Lithium: from mood stabilizer to putative cognitive enhancer. Neural Regen Res. 2016;11:1234–1235. doi: 10.4103/1673-5374.189175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raja M, Soleti F, Bentivoglio AR. Lithium treatment in patients with huntington's disease and suicidal behavior. Movement Disord. 2015;30:1438–1438. doi: 10.1002/mds.26260. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–956. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 40.Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington's disease. Mol Psychiatry. 2004;9:371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- 41.Seo JY, Kim YH, Kim JW, Kim SI, Ha KY. Effects of therapeutic hypothermia on apoptosis and autophagy after spinal cord injury in rats. Spine. 2015;40:883–890. doi: 10.1097/BRS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 42.Shimada K, Motoi Y, Ishiguro K, Kambe T, Matsumoto S, Itaya M, Kunichika M, Mori H, Shinohara A, Chiba M, Mizuno Y, Ueno T, Hattori N. Long-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotion. Neurobiol Dis. 2012;46:101–108. doi: 10.1016/j.nbd.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 43.Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OFX, Sousa N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3 beta. Neuroscience. 2008;152:656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus (vol 85, pg 872, 2003) J Neurochem. 2003;85:1624–1624. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- 45.Su H, Chu T-H, Wu W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp Neurol. 2007;206:296–307. doi: 10.1016/j.expneurol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 47.Wada A, Yokoo H, Yanagita T, Kobayashi H. Lithium: Potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci. 2005;99:307–321. doi: 10.1254/jphs.crj05009x. [DOI] [PubMed] [Google Scholar]
- 48.Wong YW, Tam S, So KF, Chen JYH, Cheng WS, Luk KDK, Tang SW, Young W. A three-month, open-label, single-arm trial evaluating the safety and pharmacokinetics of oral lithium in patients with chronic spinal cord injury. Spinal Cord. 2011;49:94–98. doi: 10.1038/sc.2010.69. [DOI] [PubMed] [Google Scholar]
- 49.Wu F, Wei X, Wu Y, Kong X, Hu A, Tong S, Liu Y, Gong F, Xie L, Zhang J. Chloroquine promotes the recovery of acute spinal cord injury by inhibiting autophagy-associated inflammation and endoplasmic reticulum stress. J Neurotrauma. 2018;35:1329–1344. doi: 10.1089/neu.2017.5414. [DOI] [PubMed] [Google Scholar]
- 50.Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 51.Yan XB, Wang SS, Hou HL, Ji R, Zhou JN. Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav Brain Res. 2007;177:282–289. doi: 10.1016/j.bbr.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 53.Yick LW, So KF, Cheung PT, Wu WT. Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury. J Neurotrauma. 2004;21:932–943. doi: 10.1089/neu.2004.21.932. [DOI] [PubMed] [Google Scholar]
- 54.Young W. Review of lithium effects on brain and blood. Cell Transplant. 2009;18:951–975. doi: 10.3727/096368909X471251. [DOI] [PubMed] [Google Scholar]
- 55.Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakeri M, Afshari K, Gharedaghi MH, Shahsiah R, Rahimian R, Maleki F, Dehpour AR, Javidan AN. Lithium protects against spinal cord injury in rats: role of nitric oxide. J Neurol Surg A Cent Eur Neurosurg. 2014;75:427–433. doi: 10.1055/s-0033-1345098. [DOI] [PubMed] [Google Scholar]
- 57.Zhang D, Li XH, Zhai X, He XJ. Feasibility of 3.0 T diffusion-weighted nuclear magnetic resonance imaging in the evaluation of functional recovery of rats with complete spinal cord injury. Neural Regen Res. 2015;10:412–418. doi: 10.4103/1673-5374.153689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou KL, Chen DH, Jin HM, Wu K, Wang XY, Xu HZ, Zhang XL. Effects of calcitriol on experimental spinal cord injury in rats. Spinal Cord. 2016;54:510–516. doi: 10.1038/sc.2015.217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.