Traumatic brain injury (TBI) represents a global pandemic and is currently a leading cause of injury related death worldwide. Unfortunately, those who survive initial injury often suffer devastating functional, social, and economic consequences.
Among those most affected by the sequelae of TBI is the geriatric population, who suffer mortality at significantly higher rates than other population cohorts. Furthermore, this is particularly unfortunate given the increased incidence of head trauma among the elderly when compared to their younger peers (Taylor et al., 2017). Advanced age is correlated with greater rates of pre-existing medical co-morbidities, long term use of anticoagulant and antiplatelet medications, and deconditioning. Overall, the frailty syndrome is used to describe this progressive loss of function and debility in the geriatric population. Frailty is significantly associated with poor outcomes in both the elective and emergency surgery populations (Makary et al., 2010). Identification of frailty among the geriatric trauma population may assist with risk stratification and prognostication following TBI. However, frailty is difficult to diagnose in the injured population, given the need to perform functional assessments of strength and cognition that may not be feasible in the immediately injured population.
However, there is one factor of the frailty syndrome may be of particular use in this population. Sarcopenia, which refers to a loss of muscle mass and strength, is strongly correlated with frailty and is significantly associated with poor outcomes following surgery (Rangel et al., 2017). Increasingly prevalent with age, sarcopenia results in changes in gait, strength, motility, nutrition, and functional independence due to skeletal muscle depletion. This leads to a predisposition towards falls and injury, which, when combined with their pre-existing functional deficits, predisposes patients to poor outcomes such as increased mortality, lengths of stay, and complications (Leeper et al., 2016).
The causes of sarcopenia are incompletely understood and suspected to be multifactorial. At a macroscopic level, lack of physical activity and malnutrition with associated decreased protein intake contribute to loss of muscle mass. Systemic changes further result in alterations in hormone production and affect the production of insulin growth factor 1 (IGF-1), resulting in muscular atrophy (Marty et al., 2017). Increased muscular catabolic activity may also result from enhanced oxidative stress and chronic low grade inflammation that are associated with aging and sarcopenia, as evidenced by elevated levels of interleukin-6 and tumor necrosis factor-α (Budui et al., 2015). Increased levels of pro-inflammatory cytokines may further lead to neural dysfunction and degradation, potentiating the development of sarcopenia. IGF-1, which is involved in neuronal myelination, axonal sprouting, and neural repair, is blunted by increased levels of tumor necrosis factor-α (Grounds, 2002; Kwon and Yoon, 2017). Motor neuron dysfunction may result in diminished trophic factor deliver, decreased sarcomere activation, and subsequent atrophy (Kwon and Yoon, 2017).
Regardless of its causes, the pre-dominant advantage of using sarcopenia for prognostication is the ability to objectively identify decreased muscle volumes with widely available imaging modalities (Leeper et al., 2016). Unlike other methods of frailty assessment in the elderly, which require use of either subjective scoring systems, assessments of patient function, or advanced imaging techniques better suited for the outpatient setting, sarcopenia is most commonly diagnosed using computerized tomography (CT) to determine muscle area in the axial place (Makary et al., 2010). This makes is use highly advantageous in the trauma population, who are likely to be unable to perform subjective or physical assessments and in whom imaging techniques like dual-energy X-ray absorptiometry (DEXA) scan may not be readily available. Furthermore, CT scan is a commonly used modality in the initial evaluation of trauma patients upon presentation, providing readily accessible imaging without the need for additional testing.
Most commonly, sarcopenia is diagnosed using the cross-sectional area of the psoas muscle. However, geriatric patients suffer a significant proportion of severe TBI from low velocity accidents, such as falls from standing (Taylor et al., 2017). Routinely, these patients are not evaluated with whole body CT given the lack of physical injury to the torso, preventing psoas muscle measurement. Recently though, a novel method for sarcopenia assessment was described using the cross-sectional area of the masseter muscle (Wallace et al., 2017).
In their recent study, Wallace et al. (2017) performed a retrospective analysis of all geriatric blunt trauma patients admitted to their trauma service to using the cross sectional area of the masseter to diagnose sarcopenia. Cross-sectional measurements were performed in the axial plane 2-cm below the zygomatic arch. Standard measurements of psoas cross-sectional area were concurrently performed at the 4th vertebral body. Both masseter and psoas muscle areas were significantly correlated (r = 0.38). However, masseter area was significantly associated with 2-year mortality in both unadjusted (hazard ratio (HR) 0.78, P = 0.019) and multivariate Cox proportional hazard models (HR 0.76, P = 0.76), whereas psoas area was not (HR 0.80, P = 0.098; HR 0.68, P = 0.051). Additionally, while Kaplan-Meier analysis of both models demonstrated significant rates of mortality in patients with the smallest muscle during the first 30 days, only decreased masseter area was associated with significant decrease in 2-year survival.
Given these exciting results, we sought to measure masseter area in geriatric patients with severe TBI to identify the association of sarcopenia with short term mortality (Hu et al., 2018). We performed a retrospective review of all trauma patients admitted to the University of Alabama at Birmingham (UAB), an American College of Surgeons verified level 1 trauma center from 2011–2016. UAB is a major urban tertiary referral center with approximately 4500 trauma activations annually. All patients ≥ 55 years old with TBI were included, with TBI severity stratified using admission Glasgow Coma Score (GCS). An admission GCS ≤ 8 was classified as severe. Patients without imaging of the masseter muscle or with death within 24 hours due to a cause not related to the TBI were excluded.
Cross sectional axial area of both masseter muscles was measured 2 cm below the zygomatic arch, as previously described. Mean area was used for analysis. Sarcopenia was defined as one standard deviation from the gender-based mean of the study population. Our primary outcome for comparison was 30-day mortality.
During the study period, we identified 600 patients with TBI, 424 of which were classified as severe. One hundred and eight were age ≥ 55 years and included for analysis, with a mean age of 67.4 ± 10.6 years. Twenty-five patients were identified with sarcopenia compared to 83 without. Mean masseter area among patients with sarcopenia was 4.55 ± 1.25 cm2 for males and 3.37 ± 1.03 cm2 for females, compared to the normal averages of 5.54 ± 1.38 cm2 and 4.41 ± 1.29 cm2, respectively.
Patients with sarcopenia were demographically matched with those without, sharing similar median injury severity scores (26 [20–36] vs. 26 [24–34]; P = 0.82). However, sarcopenia was associated with significantly worse outcomes. Functional outcomes appear worse in patients with sarcopenia. Those patients without sarcopenia were significantly more likely to be discharge to home (13.3% vs. 0%) or inpatient rehab (18.1% vs. 8.0%) (P = 0.04). With regard to the primary objective, mortality was significantly elevated at 30-days (80.0% vs. 50.6%, P = 0.01) and increased at 48-hours, although not significantly (60.0% vs. 41.0%, P = 0.11). These findings were consistent on multivariate regression analysis, with increased risk of 30-day mortality in patients with sarcopenia (odds ratio (OR) 2.95, P = 0.045; 95% confidence interval (CI) 1.03–8.48). Similarly, increasing masseter area was significantly associated with decreased risk of 30-day mortality on Cox proportional hazard analysis (HR 0.78, P = 0.04; 95% CI 0.62–0.97). Kaplan-Meier analysis demonstrated significantly decreased 30-day survival (P = 0.09) among patients with sarcopenia compared to those without (Figure 1).
Figure 1.
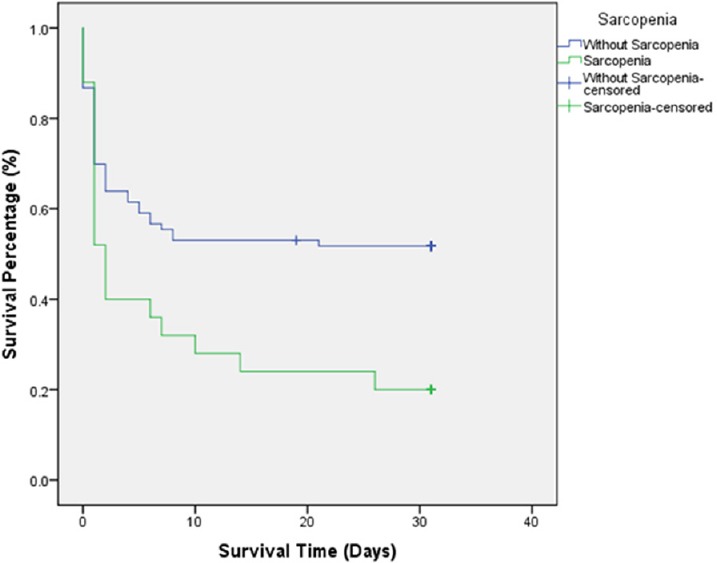
Unadjusted Kaplan-Meier 30-day survival curve for trauma patients with and without sarcopenia.
Kaplan-Meier survival curves comparing patients with severe traumatic brain injury and sarcopenia to those without sarcopenia. Survival time censored at 30 days following injury. Analysis by log rank revealed significantly decreased survival among patients with sarcopenia (P = 0.09).
Our study demonstrates that measurement of masseter area is a simple and effective tool to assess for sarcopenia in the elderly population. Sarcopenia is significantly associated with poor outcomes and increased short term mortality following severe TBI. Use of masseter based measurements to identify sarcopenia is advantageous in the geriatric patient with TBI as these patients may not undergo whole body CT scan following low velocity injury. We feel that masseter measurements should expand to all geriatric trauma patients as a simple and available method to identify sarcopenia in the trauma population. Temporomandibular joint dysfunction should be investigated as a potential confounder that may cause masseter hypertrophy. Further study should focus on the application of aggressive treatment strategies in patients identified with sarcopenia to determine if early, targeted intervention can improve outcomes for this at risk population.
This work was presented at the annual 2017 meeting of The American Association for the Surgery of Trauma.
Additional file: Open peer review report 1 (96.1KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Renata Ciccarelli, University of Chieti-Pescara, Italy.
P-Reviewer: Ciccarelli R; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015;12:22–26. doi: 10.11138/ccmbm/2015.12.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grounds MD. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19–24. doi: 10.1023/a:1015234709314. [DOI] [PubMed] [Google Scholar]
- 3.Hu P, Uhlich R, White J, Kerby J, Bosarge P. Sarcopenia measured using masseter area predicts early mortality following severe traumatic brain injury. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5422. doi: 10.1089/neu20175422. [DOI] [PubMed] [Google Scholar]
- 4.Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeper CM, Lin E, Hoffman M, Fombona A, Zhou T, Kutcher M, Rosengart M, Watson G, Billiar T, Peitzman A, Zuckerbraun B, Sperry J. Computed tomography abbreviated assessment of sarcopenia following trauma: The CAAST measurement predicts 6-month mortality in older adult trauma patients. J Trauma Acute Care Surg. 2016;80:805–811. doi: 10.1097/TA.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Marty E, Liu Y, Samuel A, Or O, Lane J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone. 2017;105:276–286. doi: 10.1016/j.bone.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Rangel EL, Rios-Diaz AJ, Uyeda JW, Castillo-Angeles M, Cooper Z, Olufajo OA, Salim A, Sodickson AD. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg. 2017;83:1179–1186. doi: 10.1097/TA.0000000000001657. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace JD, Calvo RY, Lewis PR, Brill JB, Shackford SR, Sise MJ, Sise CB, Bansal V. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: Comparing the masseter to the psoas using computed tomography. J Trauma Acute Care Surg. 2017;82:65–72. doi: 10.1097/TA.0000000000001297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


