Alzheimer's disease (AD): AD, a neurodegenerative disorder and a significant cause of dementia throughout the world mostly affects the older adults but sometimes also seen in young age (early state AD) (Agrawal et al., 2017). As per the Alzheimer Association report 2017, AD remains to be considered as the 6th leading cause of death which nearly touches around 5.5 million people only in the USA and the data is similar throughout the world (approximately 37 million people suffers from AD) (Alzheimer's Association, 2017). On the other hand, the treatments are insufficient, and the available therapies do not claim the complete cure of disease. Instead, they relieve the symptoms in the preliminary stage. Also, AD therapies are very costly, mostly unaffordable for an average income population. Such constraint is due to the inaccessibility of the brain. The human brain is the most complex and delicate organ, highly protected by various physiological shields primarily the blood-brain-barrier (BBB). The BBB upholds the homeostasis of the brain and restricts the entry of most of the foreign components including lipids, peptides, and essential nutrients. Thus, the entry of almost all the drugs and bioactive to the brain remains complicated (Agrawal et al., 2018b). The intranasal (IN) route offers an alternative approach for drug delivery to the brain without the interference of the BBB. In our previous article (Agrawal et al., 2018a), we have discussed various innovative approaches, describing the novel techniques, efficiency, limitations, clinical investigations, patents, FDA status, current scenario and future possibilities of direct nose-to-brain drug delivery.
Intranasal route: The current AD therapy mainly concerns about the systemic delivery of anti-AD drug to the brain via the oral route and intravenous injection. The major drawback of these approaches is poor drug concentration, reduced therapeutic efficacy, and greater peripheral side effects. Also, other common strategies utilize surgical procedures and intracerebroventricular injection which seem highly invasive, painful and inconvenient for the patients. In this context, the IN route provides a direct and non-invasive route which by-passes the BBB, increases the drug concentration in the brain, reduces the systemic side effect, painless, patient-friendly and improves the drug performance (Agrawal et al., 2018a). The first concept of IN drug delivery to the brain was introduced in 1989, by William H. Fery II. They discovered that IN administration of drugs could achieve the active targeting of therapeutic agents to the brain for the treatment of neurodegenerative disorders (like an AD, Parkinson's disease (PD)) and stroke. However, various successful attempts made by researchers throughout the world. These researchers designate the IN route, a suitable route for delivery of various therapeutic agents like insulin, piperine, quercetin, curcumin, H102-peptide, nerve growth factor (NGF), essential fibroblast growth factor (bFGF), S14G-humanin, oxytocin, melatonin, vitamin B-12 and many more (Frey et al., 1997).
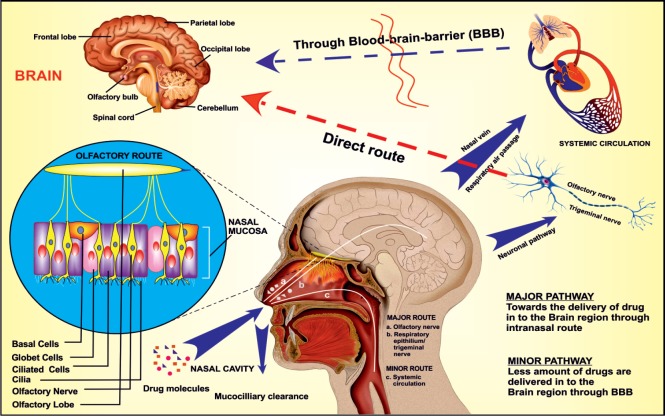
Drug transport mechanism from nose to the brain: The upper region of the nasal cavity (known as an olfactory region) remains directly connected to the brain (frontal cortex; especially olfactory bulb) via olfactory nerves. Along with this, the middle and the largest region of the nasal cavity (the respiratory region) remain supplied with the trigeminal sensory neurons and blood vessels. When the drug administered into the nasal cavity, firstly it has to pass the mucociliary clearance in the vestibular region. Further, the drug molecule reaches to the internal portion of the nasal cavity where it comes in contact with the blood vessels (respiratory epithelium) and neuronal network (olfactory and respiratory epithelium) (Crowe et al., 2018). From the blood vessels, the drug entered into the systemic circulation and distributed throughout the body as per the relative volume of distribution. This systemic bioavailability remains as the minor route of drug transport to the brain in which the drug entered the brain via BBB. While another route (primary route) of brain drug delivery is the direct neuronal pathway, in which the drug follow intracellular and extracellular transport mechanism to enter into the different regions of the brain via olfactory and trigeminal sensory neurons (Figure 1) (Lochhead and Thorne, 2012). The exact mechanism of drug transport from the nasal cavity to the brain is still a different issue among scientists although; it was supposed to follow the combined way to enter into the brain. Moreover, the drug transport mechanism depends upon various factors such as nature of the drug, type of delivery system or dosage form, a device used for IN application, formulation parameters, experimental and physiological conditions. It remained observed that the excipients added to the formulation to improve the drug retention time (like a gelling agent, and mucoadhesive polymers), a permeation enhancer, and the drug carrier system significantly affect the drug concentration in the brain. Additionally, the drug absorption through the neuronal pathway more promptly happens if the formulation remains targeted to the posterior upper region (olfactory region) of the nasal cavity (Dhuria et al., 2010).
Figure 1.
Drug transport from the nasal cavity to the brain primarily through the neuronal pathway via olfactory and trigeminal sensory neurons and secondly through the systemic circulation.
The neuronal pathway is a direct route of drug transfer to the brain while the drug entered into the systemic circulation needs to cross the blood-brain barrier (BBB) (Adapted with permission from Agrawal et al., 2018a).
Current research and available therapies: Nowadays, the direct nose-to-brain drug delivery appears as an innovative, convenient and effective strategy for the treatment of various brain disorders. A considerable research database (including on-going research, preclinical investigations, clinical studies and various patents) promises the potency of the IN approach for the treatment of AD and other brain disorders (Dhuria et al., 2010). Presently, the AD therapy is limited to only few FDA approved conventional symptomatic drugs like galantamine, donepezil, rivastigmine, memantine and tacrine (tacrine: discontinued in the USA due to side effects) (Agrawal et al., 2018a, b). The available therapy is very costly and effective only in mild to the moderate AD. Also, the patient experience severe side effects. Thus various researches are carried out to deliver the therapeutic agents directly from nose-to-brain (Khan et al., 2017). Mostly, the research strategy utilizes a suitable carrier system including polymeric nanoparticles (Alexander et al., 2016), liposomes, lipid nanocarriers (solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), microemulsion, nanoemulsion), hydrogel, in-situ gelation (Alexander et al., 2015), etc., for the treatment of the AD. These carrier systems enhance the nasomucosal permeability of the drug, protects the drug from enzymatic degradation in nasal cavity; reduces the nasomucosal clearance; improves the drug retention time; increases the bioavailability in the brain, drug targeting to a specific region of the brain to reduce the side effects. Sometimes, nasal solution or powder or mucoadhesive formulation (Alexander et al., 2011) seems suitable for effective nose-to-brain drug delivery. In the present scenario, scientists have explored a lot on galantamine, donepezil, rivastigmine, risperidone, tacrine, deferoxamine, tarenflurbil, insulin, insulin-like growth factor, curcumin, piperine, quercetin, NGF, bFGF, wheat germ agglutinin, H102 peptide, erythropoietin, vasoactive intestinal peptide (VIP), V24P, melanocortins, various anti-amyloid β antibodies, stem cells for nerve regeneration and various genes or genetic factors for the treatment of the AD.
From the full range of bioactive or drug molecules (with anti-AD activity), the extensive research using the IN route limited to only 19 molecules among these, only two drug substances (insulin and rivastigmine) are under clinical investigation rest are still under preclinical examination. Although, the preclinical studies of various drugs (galantamine, donepezil, tacrine, memantine, risperidone, curcumin, and quercetin) and peptides (NGF, S14G-humanin, VIP, bFGF, and melanocortins) on different AD animal models demonstrated improved memory, cognition and behavioral functions of the animal. Along with this (Agrawal et al., 2018a), histochemical analysis and fluorescence, as well as radio imaging of the extracted brain, show a decrease in amyloid β plaque in the brain. However, after all these successful preclinical investigations very few studies reproduce satisfactory results in clinical investigations. The obtained results may occur due to various limitations of IN route (limited drug permeability, nasomucosal clearance, and low retention time) and lack of a suitable device to deliver the drug at the particular region (olfactory region) in the nose (Dhuria et al., 2010). Scientists are working to resolve this issue and hopeful for the success in the development of IN drug delivery system for the treatment of AD soon. In our previous review work, we have described the detailed insights of on-going IN researches for the treatment of AD (Agrawal et al., 2018a).
Research boundaries: After the entire successful lab (preclinical and clinical) results, it remained observed that not even one IN therapy is available in the market for the treatment of AD. This shortcoming may be due to a lack of successful clinical investigations particularly the human trials. Most of the time, the results of preclinical stages and clinical studies are contradictory or does not found significantly useful in human trials. The contradiction may be due to the variable physiological responses and in vivo pharmacokinetic and pharmacodynamic behaviors. The use of various additives, the nature of the drug, formulation parameters and experimental conditions also affects the drug bioavailability in the brain. Therefore, a suitable delivery device is required to directly target the drug to the olfactory region of the nasal cavity which further facilitates the absorption of the drug through neuronal channels. Moreover, there are some incidences of contradictory results of the similar studies conducted in different laboratories by a different group of researchers. The reason behind these contradictory results is the non-uniformity of the experimental methodology (Agrawal et al., 2018a). Furthermore, the pathophysiology and target site of various brain disorders is not exactly clear, and the movement of the drug inside the brain is also not confirmed which affect the development of a promising system. Together, all these data suggest the need for a more precise study to understand the brain responses and pathologies of brain disorders and development of standard experimental procedures for reproducibility of the results (Dhuria et al., 2010).
Conclusion and future perspective: The healthcare societies throughout the world considered AD as a primary cause of death in elderly as well as young age population which may get more severe in future. Hence, a promising, non-invasive and cost-effective therapy is highly desirable. The IN drug delivery present a potential approach and is supposed to be the next generation therapy for brain disorders. Apart from the treatment, the prevention remains always considered as a better way to have a healthy life. As the AD is a multifactorial disorder, depends on various pathological factors (like genetic modifications, mutation, aging, and associated lifestyle factors). Instead, it remained observed that a healthy lifestyle and food habits reduce the risk of the AD. The incidences of the AD is very high in most of the developed countries like the USA, and Europe. However, in some country like India remains less prone to the AD. The reason is probably the lifestyle and food habits which involve the daily intake of various antioxidants and neuroprotectants (like curcumin, piperine, and quercetin) as spices in food and the exposure to nature instead of a machine lifestyle. To sum up, the healthy lifestyle helps to prevent the disease, but for treatment perspective, the IN route is a direct, potential and convenient approach to treat the brain disorders with proper clinical investigations.
The direct nose-to-brain drug delivery offers a potential strategy for the treatment of AD. The strategy is still under investigations with various successful lab scale studies and numerous challenges to reach the pharmacy counter. The researchers working in this stream have an excellent opportunity to explore the field and work in-depth to get the better understanding of drug disposition in the brain, disease pathology, pharmacokinetic and pharmacodynamic parameters, and experimental procedures. We suppose that a complete head to toe study considering and resolving the pitfalls will lead to the development of successful IN formulation.
Additional file:Open peer review report 1 (110.1KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Salvatore Guarini, University of Modena and Reggio Emilia, Italy.
P-Reviewer: Guarini S; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, Alexander A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018a;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal M, Ajazuddin, Tripathi DK, Saraf S, Saraf S, Antimisiaris SG, Mourtas S, Hammarlund-Udenaes M, Alexander A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer's disease. J Control Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Hamano N, Li SD, Chougule M, Shoyele SA, Gupta U, Ajazuddin, Alexander A. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin Drug Deliv. 2018b;15:589–617. doi: 10.1080/17425247.2018.1471058. [DOI] [PubMed] [Google Scholar]
- 4.Alexander A, Saraf S, Saraf S. A comparative study of chitosan and poloxamer based thermosensitive hydrogel for the delivery of PEGylated melphalan conjugates. Drug Dev Ind Pharm. 2015;41:1954–1961. doi: 10.3109/03639045.2015.1011167. [DOI] [PubMed] [Google Scholar]
- 5.Alexander A, Ajazuddin, Patel RJ, Saraf S, Saraf S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J Control Release. 2016;241:110–124. doi: 10.1016/j.jconrel.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Alexander A, Ajazuddin M, Swarna M, Sharma M, Tripathi DK. Polymers and permeation enhancers: specialized components of mucoadhesives. S J Pharm Sci. 2011;4:91–95. [Google Scholar]
- 7.Alzheimer's Association. 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 8.Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 10.Frey WH, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA, Rahman YE. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- 11.Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268:364–389. doi: 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.