Keywords: nerve regeneration, white matter, prevalence, severity, age, risk factor, retrospective analysis, cerebrovascular, neural regeneration
Abstract
White matter hyperintensities (WMHs) that arise with age and/or atherosclerosis constitute a heterogeneous disorder in the white matter of the brain. However, the relationship between age-related risk factors and the prevalence of WMHs is still obscure. More clinical data is needed to confirm the relationship between age and the prevalence of WMHs. We collected 836 patients, who were treated in the Renmin Hospital, Hubei University of Medicine, China from January 2015 to February 2016, for a case-controlled retrospective analysis. According to T2-weighted magnetic resonance imaging results, all patients were divided into a WMHs group (n = 333) and a non-WMHs group (n = 503). The WMHs group contained 159 males and 174 females. The prevalence of WMHs increased with age and was associated with age-related risk factors, such as cardiovascular diseases, smoking, drinking, diabetes, hypertension and history of cerebral infarction. There was no significant difference in sex, education level, hyperlipidemia and hyperhomocysteinemia among the different age ranges. These findings confirm that age is an independent risk factor for the prevalence and severity of WMHs. The age-related risk factors enhance the occurrence of WMHs.
Introduction
White matter hyperintensities (WMHs) of presumed vascular origin are commonly referred to as white matter changes or WMHs on T2-weighted magnetic resonance imaging (T2-MRI) and hypointensities on computed tomography (CT) in cerebral white matter areas (Williams et al., 2010; Seneviratne et al., 2013; Aribisala et al., 2014; Shim et al., 2015). The clinical manifestations of WMHs that arise with age and/or atherosclerosis often present with subtle functional impairment, cognitive impairment, dementia and with a high prevalence of neuropsychiatric disorders, such as major depressive disorder, bipolar disorder, schizophrenia, multiple sclerosis and normal pressure hydrocephalus (Grangeon et al., 2010; Yoon et al., 2014; Benedictus et al., 2015; Chang et al., 2015; Smith et al., 2016). A large volume of WMHs is a risk factor for cerebrovascular events and vascular cognitive impairment (Akisaki et al., 2006; Smith et al., 2008; Debette and Markus, 2010).
Cerebrovascular risk factors such as age, hypertension, diabetes mellitus, hyperhomocysteinemia, hyperlipidemia, and hypersensitive C-reactive protein are known risk factors for WMHs (Avci et al., 2015). WMHs are frequent findings on T2-MRI in the brains of elderly individuals but are also seen in middle-aged individuals (Williams et al., 2010). The relationship between the prevalence of WMHs and age remains obscure; therefore, it is essential to clarify this relationship with clinical data. This retrospective analysis investigated clinical data of WMHs patients attending our hospital in Central China from January 2015 to February 2016 to clarify the relationship between the prevalence of WMHs and age. This retrospective analysis also analyzed the relationship between the prevalence of WMHs and risk factors on the basis of age.
Subjects and Methods
Study design
This retrospective cohort analysis was conducted at the Renmin Hospital, Hubei University of Medicine, China. WMHs patients were diagnosed by MRI, and WMH was confirmed in T2-MRI. Control subjects did not present WMH in T2-MRI. The trial was approved by the Institutional Medical Ethics Committee of the Renmin Hospital, Hubei University of Medicine.
Subjects
The flow chart of this study is shown in Figure 1. We selected 836 Han Chinese subjects, including WMHs patients (n = 333) and non-WMHs subjects (n = 503). The MRI data of patients (≥ 16 years old) were obtained from the MRI Examination Center and the Department of Neurology. All collected cases were examined with conventional MRI sequences (including GRE sequence T1 and T2, T2 FLAIR, or diffusion weighted imaging). WMHs were diagnosed as areas of bright, high signal intensities noted on MRI T2-weighted and proton density sequences. All collected WMHs were a consequence of age and/or arteriosclerosis. Cases were excluded based on the following criteria: an ethnic origin other than Han, WMHs resulting from brain injury, infectious brain diseases, immunological brain diseases, congenital brain diseases or other brain disorders.
Figure 1.
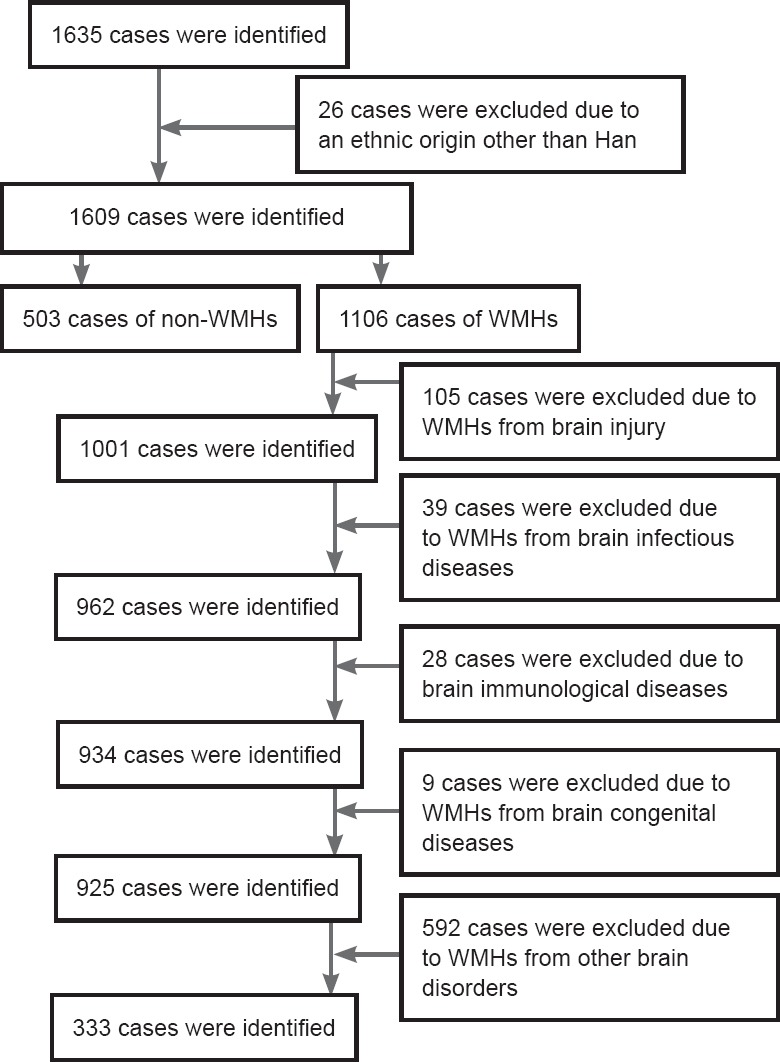
Study flow chart.
WMHs: White matter hyperintensities.
Data collection
The clinical and demographic characteristics of all patients were obtained from the MRI Examination Center and Hospital Medical Records Information Management System of Renmin Hospital, Hubei University of Medicine, including age, gender, risk factors, initial vital symptoms, laboratory parameters, and clinical outcomes.
The volume burden of WMHs was investigated with the Fazekas Scale (Fazekas et al., 1987). The Fazekas Scale is a simple visual rating scale used to rate the degree of WMHs, which contain periventricular hyperintensities (PVH) and deep white-matter hyperintensities (DWMH). PVH and DWMH are considered separately and rated from 0–3 on a four-point scale. Each case in this study has a score of at least 2 for either PVH or DWMH [PVH: 0 = absent, 1 = caps or pencil-thin lining around ventricles, 2 = smooth halo around ventricles (6–10 mm regular margins), 3 = irregular halo > 10 mm; DWMH: 0 = absent, 1 = punctate foci, 2 = beginning confluence, 3 = large confluent areas].
Statistical analysis
Statistical analysis was performed using SPSS for Windows Version 18.0 (SPSS, Chicago, IL, USA) and Microsoft Excel 2010 (Microsoft, Seattle, WA, USA). Significance of differences between the mean values in the two groups was verified using Student's t-test. A chi-square test was introduced to analyze the associations between two different variables. A P-value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics of WMHs patients
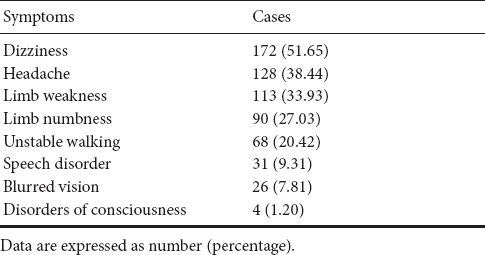
The clinical characteristics of WMHs patients are listed in Table 1. These mainly included dizziness, headache, limb weakness, limb numbness, unstable walking, speech disorder, blurred vision, and disorders of consciousness. The most common clinical manifestation was dizziness.
Table 1.
Clinical characteristics of 333 patients with white matter hyperintensities
Spatial distribution of WMH features
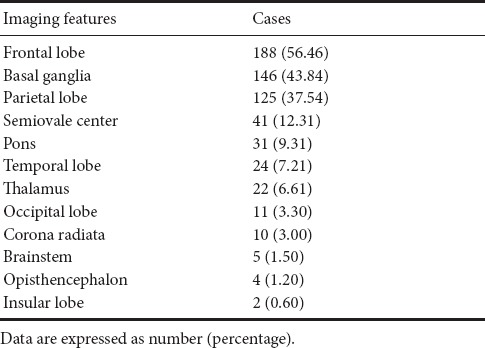
The distribution of WMH features according to MRI examination is listed in Table 2. The most common location of WMHs was the frontal lobe. Thereafter, the order for the most common location of WMHs is: basal ganglia, parietal lobe, semiovale center, pons, temporal lobe, thalamus, occipital lobe, corona radiate, brainstem, opisthencephalon and insular lobe.
Table 2.
Distribution of white matter hyperintensity features according to magnetic resonance imaging in 333 patients with white matter hyperintensities
Age distribution for WMHs patients
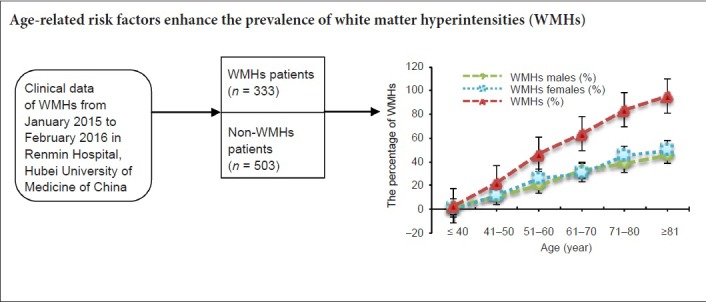
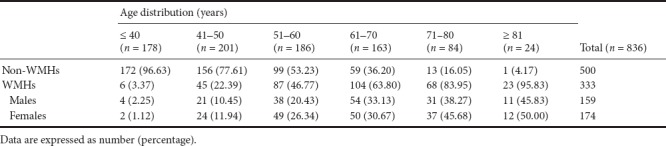
We collected 836 cases, including 333 WMHs patients and 503 non-WMHs subjects. There were six age-range groups (less than 40, 41–50, 51–60, 61–70, 71–80 and more than 80). The 333 WMHs patients contained 159 males and 174 females.
Table 3 shows that the prevalence of WMHs increases with age. The same results were found in male and female subjects (Table 3). The chi-square test showed that the prevalence of WMHs increased with age (P < 0.0001). Fisher's exact test showed that the prevalence of WMHs was higher in the 41 to 50 age-range group than in the less-than-40 group (P < 0.0001), and higher in the 51 to 60 age-range group than in the 41 to 50 age-range group (P < 0.0001), and higher in the 61 to 70 age-range group than in the 51 to 60 age-range group (P = 0.0018), and higher in the 71 to 80 age-range group than in the 61 to 70 age-range group (P = 0.0010). These results indicate that the prevalence of WMHs increases with age.
Table 3.
Distribution of white matter hyperintensities (WMHs) by age range and sex
Association between risk factors and the prevalence of WMHs
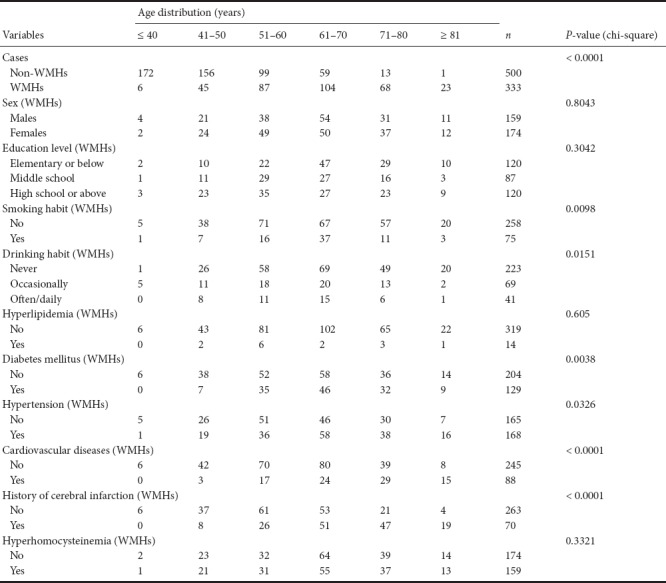
Table 4 shows that the prevalence of WMHs is associated with age-related risk factors: age (P < 0.0001), cardiovascular diseases (P < 0.0001), smoking (P = 0.0098), drinking (P = 0.0151), diabetes (P = 0.0038), hypertension (P = 0.0326), and history of cerebral infarction (P < 0.0001). There was no significant difference in sex (P = 0.8043), education level (P = 0.3042), hyperlipidemia (P = 0.605) and hyperhomocysteinemia (P = 0.3321) among different age ranges.
Table 4.
Association between risk factors and the prevalence of white matter hyperintensities (WMHs) on the basis of age
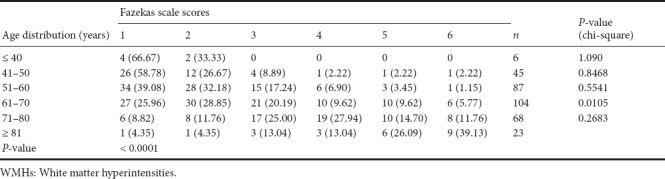
The volume burden of WMHs assessed by the Fazekas scale is shown in Table 5. Chi-square analysis indicated that there was a significant difference between the 61 to 70 age-range group and the 71 to 80 age-range group (P = 0.0105). The Fazekas scale scores were associated with increasing age (Table 5) (P < 0.0001). The trend of the increasing WMHs volume burden (Fazekas scale scores) with advancing age is shown in Table 5.
Table 5.
Association between age distribution and Fazekas scale scores (WMHs volume burden)
Discussion
WMHs are often referred to as being areas in cerebral white matter that appear hyperintense on T2-MRI and hypointense on CT. WMHs are a common pathophysiological feature that influence cognitive, mental and physical function in the adult and elderly population. WMHs are associated with various geriatric disorders, including cerebrovascular diseases (Poggesi et al., 2008; Pu et al., 2009), cardiovascular diseases (Te et al., 2015), hypertension (Henry Feugeas et al., 2005), diabetes (Poggesi et al., 2008), obesity, cognitive impairment and dementia (Mok et al., 2011; Raji et al., 2012; Doi et al., 2015; Cooke et al., 2016), and psychiatric disorders (Thomas et al., 2004; Meurs et al., 2016; Murray et al., 2016). This one-hospital, clinical, retrospective analysis demonstrated that the prevalence of WMHs increases with age. This clinical investigation of Han Chinese individuals identified a relationship between the prevalence of WMHs and age-related risk factors (age, cardiovascular diseases, smoking, drinking, diabetes mellitus, hypertension and history of cerebral infarction) on the basis of different age groups. Moreover, the severity of WMHs (the WMHs volume burden measured with Fazekas scale scores) also increased with age. Thus, this analysis indicates that aging is an independent contributor to the prevalence and severity of WMHs. The age-related risk factors enhance the occurrence of WMHs.
Compelling imaging results have revealed that WMHs is an age-related chronic ischemia process that is a common occurrence in the elderly. Major risk factors for WMHs are age and hypertension (Miki and Sakamoto, 2013). The pathological changes of WMHs are characterized by demyelination, loss of glial cells, spongy appearance, and age-related vascular pathology (occlusion of small vessels by venous collagenosis and arteriolar tortuosity) (Brown et al., 2002a, b), which are all involved in the process of aging. WMHs are common in the world's population, such as in Japanese (Honda et al., 2015; Yamawaki et al., 2015), Caucasian (Corbin et al., 2004), African-American (Nyquist et al., 2014), Caribbean black (Corbin et al., 2004), and Chinese populations (Tsai et al., 2013, 2015). Numerous clinical studies have shown that the prevalence of WMHs increases with age (Pantoni et al., 2005; van Straaten et al., 2006). Our investigation further confirmed that the prevalence and progression of WMHs is an independent contributor to disability in the elderly and increases with age, indicating that age is an independent contributor to the prevalence and progression of WMHs. The implications of these findings indicate that WMHs are a biomarker of aging that may help to develop preventive strategies to decrease the burden of disability from WMHs in later life.
A number of studies have noted that the etiology of WMHs is heterogeneous, but is most likely ischemic and neurodegenerative in nature, resulting from cortico-subcortical disconnections and loss of brain cells. All these vascular risk factors will promote previous pathogenesis, and are important in the occurrence of WMHs. These clinical results revealed that age-related risk factors (such as age, cardiovascular diseases, smoking, drinking, diabetes, hypertension and history of cerebral infarction) increase the prevalence of WMHs. However, the prevalence of WMHs is unrelated to gender, education, hyperlipidemia or hyperhomocysteinemia, although several studies support the notion that hyperlipidemia and hyperhomocysteinemia are risk factors for WMHs (Uehara et al., 1999; Wong et al., 2006; Kuo et al., 2010; Marseglia et al., 2015; Shang et al., 2015). These studies reported that patients with a history of hyperlipidemia had less severe WMHs at the time of stroke and that hyperlipidemia may play a relatively protective role in cerebral small-vessel diseases (Jimenez-Conde et al., 2010). Therefore, the role of hyperhomocysteinemia and hyperlipidemia in the progress of WMHs remains unclear.
Overall, age is an independent contributor to the prevalence and severity of WMHs. The age-related risk factors increase the occurrence of WMHs. However, this study is not a randomized controlled trial, and further studies and longitudinal data are needed to confirm the temporal sequence of events and these conclusions, and to discover the exact role of age-related risk factors in the occurrence of WMHs. However, as this is a retrospective analysis, there may be bias in subject selection and stratification.
Additional file: Open peer review report 1 (8.1KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the Natural Science Foundation of Hubei Province of China, No. 2015CFB260; the Health and Family Planning Scientific Research Project of Hubei Province of China, No. WJ2015MB219 (to WBH and ZYC). The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: The experiments were approved by the Institutional Medical Ethics Committee of Renmin Hospital, Hubei University of Medicine, China.
Reporting statement: The study complied with the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guideline.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Renmin Hospital, Hubei University of Medicine, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The requirement for informed consent was waived due to the retrospective nature of this study.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Daoli Zhu, Nantong University, China; Haiqiang Qin, Capital Medical University, China.
Funding: This study was supported by the Natural Science Foundation of Hubei Province of China, No. 2015CFB260; the Health and Family Planning Scientific Research Project of Hubei Province of China, No. WJ2015MB219 (to WBH and ZYC).
P-Reviewer: Zhu D, Qiu H; C-Editor: Zhao M; S-Editor: Yu J, Li CH; L-Editor: Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, Tanaka S, Ohashi Y, Iguchi A, Yokono K, Ito H. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT) Diabetes Metab Res Rev. 2006;22:376–384. doi: 10.1002/dmrr.632. [DOI] [PubMed] [Google Scholar]
- 2.Aribisala BS, Wiseman S, Morris Z, Valdes-Hernandez MC, Royle NA, Maniega SM, Gow AJ, Corley J, Bastin ME, Starr J, Deary IJ, Wardlaw JM. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke. 2014;45:605–607. doi: 10.1161/STROKEAHA.113.004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avci AY, Lakadamyali H, Arikan S, Benli US, Kilinc M. High sensitivity C-reactive protein and cerebral white matter hyperintensities on magnetic resonance imaging in migraine patients. J Headache Pain. 2015;16:9. doi: 10.1186/1129-2377-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedictus MR, van Harten AC, Leeuwis AE, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46:2661–2664. doi: 10.1161/STROKEAHA.115.009475. [DOI] [PubMed] [Google Scholar]
- 5.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002a;203(204):159–163. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 6.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Apoptosis in leukoaraiosis lesions. J Neurol Sci. 2002b;203(204):169–171. doi: 10.1016/s0022-510x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 7.Chang KJ, Lee S, Lee Y, Lee KS, Back JH, Jung YK, Lim KY, Noh JS, Kim HC, Roh HW, Choi SH, Kim SY, Joon Son S, Hong CH. Severity of white matter hyperintensities and length of hospital stay in patients with cognitive impairment: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Alzheimers Dis. 2015;46:719–726. doi: 10.3233/JAD-142823. [DOI] [PubMed] [Google Scholar]
- 8.Cooke GE, Wetter NC, Banducci SE, Mackenzie MJ, Zuniga KE, Awick EA, Roberts SA, Sutton BP, McAuley E, Kramer AF. Moderate physical activity mediates the association between white matter lesion volume and memory recall in breast cancer survivors. PLoS One. 2016;11:e0149552. doi: 10.1371/journal.pone.0149552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbin DO, Poddar V, Hennis A, Gaskin A, Rambarat C, Wilks R, Wolfe CD, Fraser HS. Incidence and case fatality rates of first-ever stroke in a black Caribbean population: the Barbados Register of Strokes. Stroke. 2004;35:1254–1258. doi: 10.1161/01.STR.0000127371.24658.df. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T. Effects of white matter lesions on trunk stability during dual-task walking among older adults with mild cognitive impairment. Age (Dordr) 2015;37:120. doi: 10.1007/s11357-015-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 13.Grangeon MC, Seixas C, Quarantini LC, Miranda-Scippa A, Pompili M, Steffens DC, Wenzel A, Lacerda AL, de Oliveira IR. White matter hyperintensities and their association with suicidality in major affective disorders: a meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010;15:375–381. doi: 10.1017/s1092852900029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry Feugeas MC, De Marco G, Peretti, Godon-Hardy S, Fredy D, Claeys ES. Age-related cerebral white matter changes and pulse-wave encephalopathy: observations with three-dimensional MRI. Magn Reson Imaging. 2005;23:929–937. doi: 10.1016/j.mri.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Honda Y, Noguchi A, Maruyama K, Tamura A, Saito I, Sei K, Soga T, Ushiba K, Hirano T, Sakurai T, Shiokawa Y. Volumetric analyses of cerebral white matter hyperintensity lesions on magnetic resonance imaging in a Japanese population undergoing medical check-up. Geriatrics & gerontology international. 2015;15(Suppl 1):43–47. doi: 10.1111/ggi.12672. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, Massasa E, Cloonan L, Gilson A, Capozzo K, Cortellini L, Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Furie KL, Roquer J, Rosand J, Rost NS. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41:437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo HK, Chen CY, Liu HM, Yen CJ, Chang KJ, Chang CC, Yu YH, Lin LY, Hwang JJ. Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int J Cardiol. 2010;143:184–191. doi: 10.1016/j.ijcard.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Marseglia LM, Nicotera A, Salpietro V, Giaimo E, Cardile G, Bonsignore M, Alibrandi A, Caccamo D, Manti S, D’Angelo G, Mami C, Di Rosa G. Hyperhomocysteinemia and MTHFR polymorphisms as antenatal risk factors of white matter abnormalities in two cohorts of late preterm and full term newborns. Oxid Med Cell Longev. 2015;2015:543134. doi: 10.1155/2015/543134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meurs M, Roest AM, Groenewold NA, Franssen CF, Westerhuis R, Kloppenburg WD, Doornbos B, Beukema L, Lindmae H, de Groot JC, van Tol MJ, de Jonge P. Gray matter volume and white matter lesions in chronic kidney disease: exploring the association with depressive symptoms. Gen Hosp Psychiatry. 2016;40:18–24. doi: 10.1016/j.genhosppsych.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Miki Y, Sakamoto S. Age-related white matter lesions (leukoaraiosis): an update. Brain Nerve. 2013;65:789–799. [PubMed] [Google Scholar]
- 21.Mok V, Wong KK, Xiong Y, Wong A, Schmidt R, Chu W, Hu X, Leung EY, Chen S, Chen Y, Tang WK, Chen X, Ho CL, Wong KS, Wong ST. Cortical and frontal atrophy are associated with cognitive impairment in age-related confluent white-matter lesion. J Neurol Neurosurg Psychiatry. 2011;82:52–57. doi: 10.1136/jnnp.2009.201665. [DOI] [PubMed] [Google Scholar]
- 22.Murray A, McNeil C, Salarirad S, Deary I, Phillips L, Whalley L, Staff R. Brain hyperintensity location determines outcome in the triad of impaired cognition, physical health and depressive symptoms: A cohort study in late life. Arch Gerontol Geriatr. 2016;63:49–54. doi: 10.1016/j.archger.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, Kral BG, Vaidya D. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis. 2014;37:244–250. doi: 10.1159/000358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, O’Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- 25.Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O’Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L Leukoaraiosis And DISability Study Group. Urinary complaints in nondisabled elderly people with age‐related white matter changes: the leukoaraiosis and disability (LADIS) study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 26.Pu Y, Liu L, Zou X, Chen P, Wang Y, Zhou Y, Dong K, Zhao X, Wang C, Wang Y. Relationship between leukoaraiosis and cerebral large artery stenosis. Neurol Res. 2009;31:376–380. doi: 10.1179/174313209X444071. [DOI] [PubMed] [Google Scholar]
- 27.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33:834.e837–834.e816. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seneviratne U, Chong W, Billimoria PH. Brain white matter hyperintensities in migraine: clinical and radiological correlates. Clin Neurol Neurosurg. 2013;115:1040–1043. doi: 10.1016/j.clineuro.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Shang D, Shi C, Qin J, Song B, Xu Y, Sun S. Mental retardation, hypogonadism, epilepsy, white matter lesions and hyperhomocysteinemia in a family. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2015;32:136–137. doi: 10.3760/cma.j.issn.1003-9406.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Shim YS, Yang DW, Roe CM, Coats MA, Benzinger TL, Xiong C, Galvin JE, Cairns NJ, Morris JC. Pathological correlates of white matter hyperintensities on magnetic resonance imaging. Dement Geriatr Cogn Disord. 2015;39:92–104. doi: 10.1159/000366411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CD, Johnson ES, Van Eldik LJ, Jicha GA, Schmitt FA, Nelson PT, Kryscio RJ, Murphy RR, Wellnitz CV. Peripheral (deep) but not periventricular MRI white matter hyperintensities are increased in clinical vascular dementia compared to Alzheimer's disease. Brain Behav. 2016;6:e00438. doi: 10.1002/brb3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 33.Te M, Zhao E, Zheng X, Sun Q, Qu C. Leukoaraiosis with mild cognitive impairment. Neurol Res. 2015;37:410–414. doi: 10.1179/1743132815Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 34.Thomas P, Hazif-Thomas C, Saccardy F, Vandermarq P. Loss of motivation and frontal dysfunction. Role of the white matter change. Encephale. 2004;30:52–59. doi: 10.1016/s0013-7006(04)95416-4. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai CF, Anderson N, Thomas B, Sudlow CL. Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: Systematic review and meta-analysis. Int J Stroke. 2015;10:485–493. doi: 10.1111/ijs.12508. [DOI] [PubMed] [Google Scholar]
- 37.Uehara T, Tabuchi M, Mori E. Risk factors for silent cerebral infarcts in subcortical white matter and basal ganglia. Stroke. 1999;30:378–382. doi: 10.1161/01.str.30.2.378. [DOI] [PubMed] [Google Scholar]
- 38.van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, Inzitari D, Waldemar G, Erkinjuntti T, Mäntylä R, Wahlund LO, Barkhof F LADIS Group. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- 39.Williams LR, Hutchinson CE, Jackson A, Horan MA, Jones M, McInnes L, Rabbitt PM, Pendleton N. Clinical correlates of cerebral white matter hyperintensities in cognitively normal older adults. Arch Gerontol Geriatr. 2010;50:127–131. doi: 10.1016/j.archger.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Wong A, Mok V, Fan YH, Lam WW, Liang KS, Wong KS. Hyperhomocysteinemia is associated with volumetric white matter change in patients with small vessel disease. J Neurol. 2006;253:441–447. doi: 10.1007/s00415-005-0022-x. [DOI] [PubMed] [Google Scholar]
- 41.Yamawaki M, Wada-Isoe K, Yamamoto M, Nakashita S, Uemura Y, Takahashi Y, Nakayama T, Nakashima K. Association of cerebral white matter lesions with cognitive function and mood in Japanese elderly people: a population-based study. Brain Behav. 2015;5:e00315. doi: 10.1002/brb3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon B, Shim YS, Cheong HK, Hong YJ, Lee KS, Park KH, Ahn KJ, Kim DJ, Kim YD, Choi SH, Yang DW. White matter hyperintensities in mild cognitive impairment: clinical impact of location and interaction with lacunes and medial temporal atrophy. J Stroke Cerebrovasc Dis. 2014;23:e365–372. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


