Abstract
Glucose is the main energy substrate for neurons, however, at certain conditions, e.g. in starvation, these cells could also use ketone bodies. This approach is used in clinical conditions as the ketogenic diet. The ketogenic diet is actually a biochemical model of fasting. It includes replacing carbohydrates by fats in daily meal. Synthesis of ketone bodies β-hydroxubutirate, acetoacetate and acetone begins once glycogen stores have depleted in the liver. The ketogenic diet can be used to treat clinical conditions, primarily epilepsy. The mechanism of neuroprotective action of ketogenic diet is not very clear. It is shown that ketone bodies influence neurons at three different levels, namely, metabolic, signaling and epigenetic levels. Ketone bodies are not always neuroprotective. Sometimes they can be toxic for the brain. Ketoacidosis which is a very dangerous complication of diabetes mellitus or alcoholism can be taken as an example. The exact mechanism of how neuroprotective properties of ketone bodies reverse to neurotoxic is yet to be established.
Keywords: β-hydroxybutirate, epilepsy, diabetes mellitus, alcoholism, metabolism, hydroxyl-carboxylic acid receptor, epigenetics, acidosis
Ketogenic Diet
Glucose is the main energy substrate for neurons, however, at certain conditions, e.g. in starvation, these cells could also use ketone bodies (Izumi et al., 1998; Fedorovich and Waseem, 2018). Furthermore, neurons in vivo would rather utilize lactate than glucose according to the astrocyte-neuron lactate shuttle hypothesis. Glucose is taken up by astrocytes which metabolize it into lactate. Lactate is then transported to neurons where it undergoes oxidation in mitochondria (Pellerin and Magistretti, 2012). Monocarboxylates are metabolized directly in Krebs cycle in mitochondria, therefore, they can be considered as non-glycolytic energy substrates. Transition to non-glycolytic energy substrates could remodel neuron functions. The ketogenic diet is based on this approach and may be used to treat clinical conditions (Gano et al., 2014).
The ketogenic diet is actually a biochemical model of fasting. It includes replacing carbohydrates by fats in daily meal. Synthesis of ketone bodies begins once glycogen stores have depleted in the liver. The term ‘ketone bodies’ is historical rather than exact chemical name. β-Hydroxybutirate, acetoacetate and acetone belong to ketone bodies. β-Hydroxybutirate and acetoacetate can be metabolized in mitochondria but not acetone. Interestingly, acetone possesses an anticonvulsive activity at certain conditions (Gasior et al., 2007; McNally and Hartman, 2012). Mechanism of acetone anticonvulsive properties is unknown (Gasior et al., 2007). Furthermore, in the case of ketogenic diet, the level of acetone in the brain appeared to be lower than in experiments where antiepileptic action was demonstrated (McNally and Hartman, 2012). The main ketone body is β-hydroxybutirate. Its concentration reaches 5–6 mM during starvation (Achanta and Rae, 2017). It is reported that in the case of ketogenic diet β-hydroxybutirate plasma level could be about 4–5 mM (Neal et al., 2009). It is suggested that 4–6 mM of β-hydroxybutirate could be considered as neuroprotective.
The ketogenic diet is used in clinic primarily for treatment of epilepsy (Stafstrom and Rho, 2012; Gano et al., 2014). Furthermore different studies have shown it could be advantageous in several neurodegenerative diseases, for instance, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis (Stafstrom and Rho, 2012). Application of the ketogenic diet to treat tumors and reverse cognitive decline in aging seems to be very promising option (Woolf et al., 2016).
The mechanism of neuroprotective action of ketogenic diet is not very clear. It is shown that ketone bodies influence neurons at three different levels (Fedorovich and Waseem, 2018).
1) Bioenergetics and metabolic level. Ketone bodies can serve as more (Holmgren et al., 2010) or less effective energy substrates compared to glucose. Inhibition of endocytosis in rat brain synaptosomes after glucose replacement by β-hydroxybutirate in incubation medium is the example of less effective energy substrate (Hrynevich et al., 2016). In addition, it is worth noting that glycolysis is bypassed in monocarboxylate-fueled neurons and consequently adenosine triphosphate (ATP) microdomains, which are generated by glycolytic enzymes, are dissipated. Ketone bodies can also influence a balance between glutamate and γ-aminbutiric acid (GABA) synthesis. This leads to excessive accumulation of GABA in central nervous system and prevalence of inhibitory synaptic transmission (Gano et al., 2014).
2) Signaling level. Recently it was shown that a ketone body can function as a ligand for G-protein linked receptor hydroxyl-carboxylic acid (HCA)2 (Blad et al., 2012). Ketogenic diet is able to inhibit activation of microglial cells, promotes a neuroprotective phenotype in microglia and decreases interleukin level that provides anti-inflammatory action in brain (Yang and Cheng, 2010; Ghosh et al., 2018). At least partially, these effects are mediated by HCA2 located in microglial cells (Ghosh et al., 2018). HCA2 belongs to G-protein linked receptors which decrease cyclic adenosine monophosphate (cAMP) level (Blad et al., 2012), however, how they regulate microglial cells is still unknown in details.
3) Epigenetic level. Epigenetic mechanisms provide an adaptive layer of control in the regulation of gene expression that enables an organism to adjust to a changing environment (Stephens et al., 2013). Epigenetic regulation is functionally relevant changes in the genome that do not involve a change in a nucleotide sequence. Examples of mechanisms leading to such changes are DNA methylation and histone modification. β-Hydroxybutirate similarly to butyrate is an inhibitor of histone deacetylase. Inhibition of histone deacetylase leads to the changes in histones folding and increase in synthesis of antioxidants enzymes (Shimazu et al., 2013).
Finally, neuroprotective properties of the ketogenic diet might be explained by rather indirect effect at the whole organism level than direct action on neurons. Changes in microbiome during ketogenic diet followed by involvement of gut-brain axis which has recently been demonstrated can be taken as an example (Olson et al., 2018). Authors showed that the gut microbiota is altered by the ketogenic diet and required for protection against several kinds of seizures. Antibiotics blocked this effect. Furthermore, anticonvulsive strains of microbes can be transferred from one animal to others (Olson et al., 2018).
In summary, there is no single target for the ketogenic diet. It is suggested that different targets or group of targets, which interact with each other, are involved depending on a disease.
Ketone bodies are transported through blood brain barrier and neuronal plasma membrane by monocarboxylate transporters (MCT). The expression of MCT in rats is variable increasing during starvation or in a ketogenic diet and decreasing with age (Leino et al., 1999; Vannucci and Simpson, 2003). This is explained by the fact that suckling is actually a certain type of natural ketogenic diet because a suckling rodent consumes ‘high fat’ maternal milk. Age dependency of MCT expression underlies the efficacy of clinical application of ketogenic diet in childhood epilepsy compared to adults.
The classical ketogenic diet is 4:1 diet. This means combining 4 parts of fats with 1 part of carbohydrates and proteins in food. However, other modifications of ketogenic diet exist as well.
Middle chain triglyceride diet. In this diet, daily meal is enriched by middle chain triglyceride. Generally there are derivatives of coconut oil. It is believed that middle chain fatty acids are more effective precursors for ketone bodies compared to other lipids. Moreover, middle chain fatty acids may have intrinsic anticonvulsive properties.
In modified Atkins diet, the significant part of calories comes not only from fats. Proteins are also significant contributors.
The low-glycemic index treatment is based on sophisticated calculation of glycemic index for different kinds of meal. While it is generally based on restriction of carbohydrates in daily meal similarly to the ketogenic diet.
Intermittent fasting. This treatment is most similar to the main principle of the ketogenic diet-based fasting. This type of therapy includes days when individuals do not consume any food. Human body starts to use fats from own deposits with the following ketosis (Gano et al., 2014).
Ketoacidosis
Ketone bodies are not always neuroprotective. Sometimes they can be toxic for the brain. Ketoacidosis which is a very dangerous complication of diabetes mellitus can be taken as an example.
Insulin deficit leads to the significant increase in glucagon plasma concentration. Glucagon is an antagonist of insulin. Glucose synthesis from glycogen by gluconeogenesis in liver significantly increases when insulin is unable to inhibit glucagon effects. At the same time, utilization of glucose by liver, muscles and adipose tissue decreases. Ultimately, these processes result in hyperglycemia. Hyperglycemia is further progressed due to activity of other hormones having antagonistic action to insulin. They include cortisol, epinephrine, somatotropin.
Insulin deficit accelerates protein catabolism. Amino acids formed in this process can also contribute to gluconeogenesis in liver resulting in deterioration of hyperglycemia severity. The massive breakdown of lipids in adipose tissue caused by insulin deficit leads to the strong increase in free fatty acid plasma levels. About 80% of energy is produced by free fatty acid oxidation in the case of insulin deficit. This in turn leads to accumulation of ketone bodies which are products of free fatty acid degradation. Their accumulation is significantly faster than utilization and/or renal elimination. This process results in elevation in ketone bodies plasma levels up to 20–25 mM (Adrogué et al., 1982; Kanikarla-Marie and Jain, 2016; Achanta and Rae, 2017). Buffer capacity of kidneys declines causing metabolic acidosis. Lastly, patients with diabetic ketoacidosis have blood glucose levels in the range between 11 and 55 mM (the normal range is between 4 and 6.1), with arterial pH ranging between 7.35 and 7.20. Diabetic ketoacidosis could lead to coma and death if acute drop in ketone body plasma levels has not been controlled (Kanikarla-Marie and Jain, 2016).
Alcoholism can also induce pathological ketoacidosis (McGuire et al., 2006). There are at least three reasons for excessive accumulation of ketone bodies in the case of this disease.
- First, acetoacetate and β-hydroxybutirate can be synthesized from acetaldehyde which is ethanol metabolite.
- Alcoholism leads to diabetes mellitus-like hormonal disturbance. Decreasing insulin synthesis and rising glucagon concentration lead to ketone bodies accumulation by mechanism which is similar to diabetic ketoacidosis.
- Patients typically receive calories mainly from alcohol in the case of chronic alcoholism. This causes malnutrition, decrease in carbohydrates intake and depletion of glycogen stores. Long-lasting consumption of alcohol at the advanced stage of disease could lead to a state similar to that during the extreme ketogenic diet.
Ketone body plasma levels can reach 15 mM in the case of alcoholic ketoacidosis. Unlike diabetic ketoacidosis, a decrease in plasma pH is not always detectable. Even alkaline shift has sometimes been observed (McGuire et al., 2006).
The most fascinating question is why ketoacidosis might induce coma? Whether this is explained by events at tissue level or high levels of ketone bodies directly affect neurons?
Ketogenic Diet versus Ketoacidosis: Neuroprotection versus Neurotoxicity
It is still unclear which condition, ketosis or acidosis, is the most damaging for brain cells. Actually, ketoacidosis does not produce very pronounced acidification. In contrast, in certain types of brain ischemia pHout can drop to 5.5 units, for instance, in brain ischemia accompanied by hyperglycemia. Shift of pH in ketoacidosis is far away from neuronal death threshold. Furthermore, this value apparently does not reach the threshold for opening acid-sensitive ion channels, which are able to induce calcium-dependent neuronal damage. Previously we have demonstrated that acidic shift of pHout by several tenths of a unit, but not pHin, leads to mitochondria depolarization and oxidative stress in synaptosomes (Pekun et al., 2013). These effects could potentially result in neurodegenerative changes, however, they unlikely could lead to coma. Therefore, high levels of ketone bodies would likely have neurotoxic properties.
Advancements in out knowledge of how low and intermediate concentrations of ketone bodies influences neurons has helped to develop the concept of neuroprotective ketosis. However, this problem is far away from to be completely resolved. Our knowledge regarding effect of high ketone body levels which occurs during ketoacidosis are still very limited. Therefore, the exact mechanism of how neuroprotective properties of ketone bodies reverse to neurotoxic is yet to be established (Figures 1 and 2).
Figure 1.
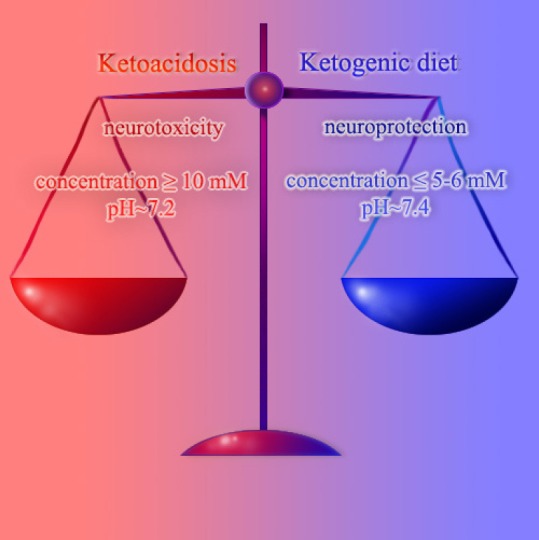
Scheme of difference between the ketogenic diet and ketoacidosis.
Figure 2.
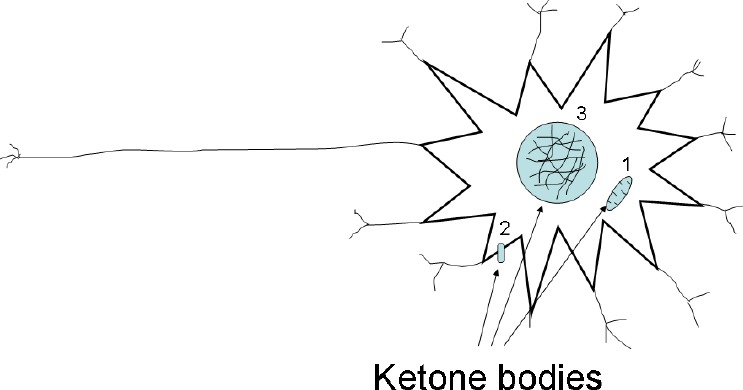
Three levels of ketone bodies influence on neurons.
There are metabolic (1), signaling (2) and epigenetic (3) levels. Ketone bodies able to modulate mitochondria (1), activate G-protein linked receptor hydroxyl-carboxylic acid 2 and inhibit histone deacetylase (3).
Additional file: Open peer review report 1 (93.9KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by the Belorussian Republican Foundation of Fundamental Research (grant No. B17-006).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Zhengshan Liu, University of Rochester Medical Center, USA.
Funding: This work was supported by the Belorussian Republican Foundation of Fundamental Research (grant No. B17-006).
P-Reviewer: Liu Z; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Achanta LB, Rae CD. Beta-hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 2.Adrogué HJ, Wilson H, Boyd AE, 3rd, Suki WN, Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N Engl J Med. 1982;307:1603–1610. doi: 10.1056/NEJM198212233072603. [DOI] [PubMed] [Google Scholar]
- 3.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 4.Fedorovich SV, Waseem TV. Metabolic regulation of synaptic activity. Rev Neurosci. 2018 doi: 10.1515/revneuro-2017-0090. doi: 10.1515/revneuro-2017-0090. [DOI] [PubMed] [Google Scholar]
- 5.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia. 2007;48:793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S, Castillo E, Frias ES, Swanson RA. Bioenergetic regulation of microglia. Glia. 2018;66:1200–1212. doi: 10.1002/glia.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmgren CD, Mukhtarov M, Malkov AE, Popova IY, Bregestovski P, Zilberter Y. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J Neurochem. 2010;112:900–912. doi: 10.1111/j.1471-4159.2009.06506.x. [DOI] [PubMed] [Google Scholar]
- 9.Hrynevich SV, Waseem TV, Hebert A, Pellerin L, Fedorovich SV. beta-Hydroxybutyrate supports synaptic vesicle cycling but reduces endocytosis and exocytosis in rat brain synaptosomes. Neurochem Int. 2016;93:73–81. doi: 10.1016/j.neuint.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Ishii K, Katsuki H, Benz AM, Zorumski CF. beta-Hydroxybutyrate fuels synaptic function during development. Histological and physiological evidence in rat hippocampal slices. J Clin Invest. 1998;101:1121–1132. doi: 10.1172/JCI1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016;95:268–277. doi: 10.1016/j.freeradbiomed.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res. 1999;113:47–54. doi: 10.1016/s0165-3806(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 13.McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J. 2006;23:417–420. doi: 10.1136/emj.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 16.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–1741.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pekun TG, Lemeshchenko VV, Lyskova TI, Waseem TV, Fedorovich SV. Influence of intra- and extracellular acidification on free radical formation and mitochondria membrane potential in rat brain synaptosomes. J Mol Neurosci. 2013;49:211–222. doi: 10.1007/s12031-012-9913-3. [DOI] [PubMed] [Google Scholar]
- 18.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens KE, Miaskowski CA, Levine JD, Pullinger CR, Aouizerat BE. Epigenetic regulation and measurement of epigenetic changes. Biol Res Nurs. 2013;15:373–381. doi: 10.1177/1099800412444785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- 23.Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and beta-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122. doi: 10.3389/fnmol.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010;42:145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


