Abstract
Background
Emerging studies reported that preoperative albumin-to-globulin ratio (AGR) correlated with tumor progression and prognosis in several types of cancer. The aim of this study was to systematically explore the association between preoperative AGR and clinical outcomes in cancers of the urinary system.
Methods
Relevant articles were searched in PubMed, Embase and Web of Science by two independent investigators from inception to June 1, 2018. Eligible studies were selected based on predetermined selection criteria. Summarized HRs or ORs and 95% CIs were calculated for prognosis and clinicopathologic features with the fixed-effects or random-effects models.
Results
Eight cohort studies comprising 2,668 patients were included for analysis. The pooled results showed that a low AGR significantly correlated with poor OS (HR: 0.38, 95% CI: 0.27–0.48, P<0.001), worse cancer-specific survival (CSS) (HR: 0.36, 95% CI: 0.22–0.50, P<0.001) and inferior event-free survival (EFS) (HR: 0.36, 95% CI: 0.25–0.48, P<0.001) in urologic cancers. In addition, patients in low and high AGR groups showed significant differences in lymphovascular invasion (P<0.001), pT status (P<0.001) and pN status (P<0.001).
Conclusion
Preoperative AGR might be a valuable, cheap and reproducible prognostic bio-marker in urologic cancers following surgical resection.
Keywords: albumin-to-globulin ratio, urologic cancer, prognosis, clinical features
Introduction
Albumin (ALB) and globulin (GLB) are two major abundant proteins in human serum. Increasing evidence has shown that albumin levels reflect nutritional status and also correlate with systemic inflammatory response.1–3 Furthermore, albumin could be used as a useful prognostic marker in cancers, such as ovarian cancer, gastric cancer, lung cancer and colorectal cancer.4–7 On the other hand, globulin, another major component of serum protein, has been reported to be involved in a series of immune and chronic inflammation responses,8–11 and might serve as a predictor of tumor progression and survival.12–15
However, the levels of albumin and globulin in serum could be easily influenced by other confounding factors, and this could affect the efficiency and accuracy in prognosis detection. To overcome the deficiency, a novel prognostic index, the albumin-to-globulin ratio (AGR), was identified and reported.16,17 AGR is a combination of serum albumin and globulin. It has been suggested that the AGR might be a more stable and reliable indicator than serum albumin or globulin alone in prognostication.18,19 It has been demonstrated to be a valuable and promising prognostic tumor marker in various types of cancers.18–22
Recently, several studies have reported the relationship between AGR and prognosis in urologic cancers, such as bladder cancer, renal cell carcinoma and upper tract urothelial carcinoma.23–26 However, reports on the prognostic effect of AGR in urologic cancers are inconsistent and debatable, and most studies published to date also have been restricted with small samples. Therefore, in order to provide clear evidence in favor of the prognostic significance of the AGR, it is necessary to conduct a meta-analysis to synthetically investigate the association between the AGR and clinical outcomes in patients with urinary system cancer.
Methods
Literature retrieval and study selection
A systematic literature search was performed in PubMed, Embase and Web of Science for eligible studies assessing the prognostic significances of the AGR in cancers of the urinary system until June 1, 2018. The search strategy combined the following terms: “albumin/globulin,” “albumin to globulin,” “albumin and globulin,” “albumin to globulin ratio,” or “AGR,” and “bladder,” “kidney,” “prostate,” “testicular,” “renal,” or “urothelial,” and “cancer,” “carcinoma,” “adenocarcinoma,” “tumor,” or “malignancy.”
Inclusion and exclusion criteria
Studies were identified eligible and included if they met the following inclusion criteria:
All patients enrolled were histologically confirmed to be primary urologic cancers;
The study reported the association between the preoperative albumin-to-globulin ratio (AGR) and OS/CSS/DFS/ PFS/RFS;
Cases were divided into two groups according to the cutoff value of AGR;
Full-text studies were published in English.
The exclusion criteria were as follows:
Nonoriginal studies, such as letters, reviews, meta-analysis, poster session or abstracts;
Studies on cancers that not derived from urinary system;
Insufficient data for calculating the HR and 95% CI.
Data extraction and quality assessment
The following data were extracted by two independent researchers: the surname of the first author, publication year, country of research, cancer type, included period, number of cases, study type, number of male and female, age distribution, survival type, cutoff value of AGR, cutoff selection, treatment method, follow-up time, overall survival (OS), CSS, disease-free survival (DFS), progression-free survival (PFS) and recurrence-free survival (RFS). The HRs and the 95% CI for cancer survival were extracted from the multivariate analysis, since they balanced many confounding factors. Additionally, the number of the patients for the clinicopathologic characteristics (tumor grade, lymphovascular invasion, pT status, pN status, pM status and pTNM stage) was extracted from the eligible studies. The Newcastle–Ottawa Scale (NOS) was utilized to assess the quality of the included studies.27,28 The scores according to NOS varied from 0 to 9. A score of 6 or more was identified as high quality.
Statistical analysis
Pooled HRs or ORs and their 95% CIs for cancer prognosis and clinical relevance were evaluated by Stata version 12.0 (Stata Corporation, College Station, TX, USA). DFS/PFS/ RFS values were merged into one survival outcome described as EFS.29,30 The heterogeneity among studies was tested by Cochran’s Q and Higgins I2 statistics; in the presence of significant heterogeneity (Phet <0.1 and/or I2 >50%), the random-effect model was employed; otherwise, the fixed-effect model was applied. Publication bias was evaluated by Begg’s test and funnel plot analysis. The sensitivity analysis was carried out by sequentially omitting individual studies to assess the robustness of the pooled results. A two-sided P<0.05 was considered statistically significant.
Results
The detailed process of study selection is shown in Figure 1. According to the inclusion and exclusion criteria, eight full-texts were considered eligible and included in the meta-analysis, with a total of 2,668 cases.23–26,31–34 In these eight articles, three different kinds of urinary system cancers were included as follows: renal cell carcinoma (RCC, two articles), upper urinary tract urothelial carcinoma (UTUC, two articles) and bladder cancer (BC, four articles). All the included studies were prospective cohort trials. These studies were all published in English and released in the year of 2015 (one study), 2016 (one study), 2017 (two studies) and 2018 (four studies). These studies were carried out in three countries, namely, Turkey (one study), Japan (one study), P. R. China (six studies). All cases in the eligible studies were classified into two groups (low and high AGR groups), and the clinicopathologic characteristics of the patient data are shown in Table S1. Among these studies, six studies reported the association between AGR and OS, four studies reported the relationship between AGR and cancer-specific survival (CSS), and two studies for PFS, RFS, DFS, respectively. The main characteristics of all included studies are presented in Table 1.
Figure 1.
Flow diagram of the meta-analysis.
Table 1.
Main characteristics of eligible studies in the meta-analysis
| Study, year | Disease type | Country | Study type | Included period | No. of cases | Male/ female | Age (years) | Survival type | Cutoff value | Cutoff selection | Follow- up | MVA | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Zhang et al 201524 | UTCC | China | R | 2006–2008 | 187 | 85/102 | median: 70 | OS, CSS | 1.45 | ROC | ≥5 years | yes | 7 |
| Liu et al 201625 | BC | China | R | 2000–2013 | 296 | 250/46 | mean: 61.71 | CSS, RFS | 1.6 | ROC | ≥5 years | yes | 7 |
| He et al 201733 | RCC | China | R | 2000–2012 | 895 | 600/295 | mean: 51.44 | OS | 1.47 | ROC | ≥5 years | yes | 8 |
| Liu et al 201726 | BC | China | R | 2009–2013 | 189 | 165/24 | NA | OS, PFS, CSS | 1.55 | ROC | ≥5 years | yes | 7 |
| Koparal et al 201823 | RCC | Turkey | R | 2010–2016 | 162 | 102/60 | mean:56.5 | OS, DFS | 1.4 | ROC | ≥5 years | yes | 6 |
| Niwa et al 201832 | BC | China | R | 2000–2015 | 364 | 294/70 | median: 71 | RFS, PFS | 1.6 | NA | ≥5 years | yes | 8 |
| Shang et al 201834 | BC | China | R | 2004–2011 | 470 | 354/188 | median: 70 | OS, CSS | 1.68 | ROC | ≥5 years | yes | 8 |
| Fukushima et al 201831 | UTCC | Japan | R | 2003–2016 | 105 | 74/31 | median: 74 | OS, DFS | 1.24 | ROC | ≥5 years | yes | 6 |
Abbreviations: BC, bladder carcinoma; CSS, cancer-specific survival; DFS, disease-free survival; MVA, multivariate analysis; NA, not available; NOS, Newcastle–Ottawa Scale; OS, overall survival; PFS, progression-free survival; R, retrospectively; RCC, renal cell carcinoma; RFS, recurrence-free survival; ROC, receiver operating characteristic curve; UTCC, upper tract urothelial carcinoma
AGR and prognosis
Overall survival
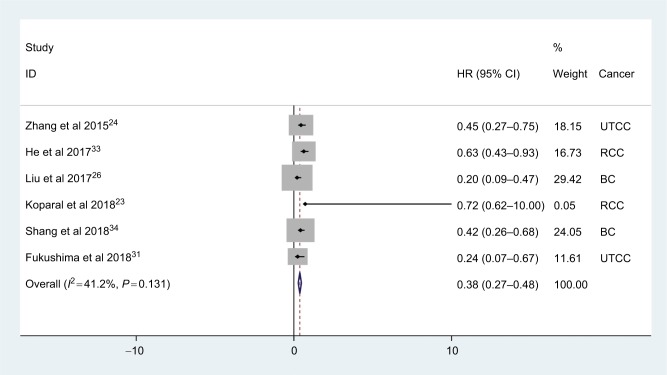
A total of six studies involving 2008 patients reported the effect of AGR on OS in urologic cancer. The pooled results showed that low AGR was significantly related with poor OS (HR: 0.38, 95% CI: 0.27–0.48, P<0.001) with no significant heterogeneity (I2=41.2%, Phet =0.132; Figure 2).
Figure 2.
Meta-analysis of the relationship between AGR and OS.
Abbreviations: AGR, albumin-to-globulin ratio; BC, bladder carcinoma; OS, overall survival; RCC, renal cell carcinoma; UTCC, upper tract urothelial carcinoma.
As shown in Table 2, when stratified by the cancer type, low AGR had significantly worse OS in UTCC (HR: 0.37, 95% CI: 0.18–0.56, P<0.001), RCC (HR: 0.63, 95% CI: 0.38–0.88, P<0.001) and BC (HR: 0.30, 95% CI: 0.16–0.44, P<0.001). In addition, the significant differences were also consistently observed in subgroup meta-analysis stratified by the sample size and cutoff value (Table 2).
Table 2.
Subgroup analysis of the association between AGR and OS
| Subgroup factor | Divided standard | No. of studies | Pooled HR (95% CI) | P-value | Heterogeneity
|
|
|---|---|---|---|---|---|---|
| I2 (%) | Phet | |||||
|
| ||||||
| Cancer type | UTCC24,31 | 2 | 0.37 (0.18–0.56) | <0.001 | 14.5 | 0.279 |
| RCC23,33 | 2 | 0.63 (0.38–0.88) | <0.001 | 0.0 | 0.970 | |
| BC26,34 | 2 | 0.30 (0.16–0.44) | <0.001 | 55.9 | 0.132 | |
| Sample size | ≥30033,34 | 2 | 0.51 (0.35–0.67) | <0.001 | 36.8 | 0.208 |
| <30023,24,26,31 | 4 | 0.29 (0.16–0.42) | <0.001 | 0.0 | 0.445 | |
| Cutoff value | ≥1.4726,33,34 | 3 | 0.38 (0.26–0.50) | <0.001 | 72.6 | 0.026 |
| <1.4723,24,31 | 3 | 0.37 (0.18–0.56) | <0.001 | 0.0 | 0.551 | |
Abbreviations: BC, bladder carcinoma; RCC, renal cell carcinoma; UTCC, upper tract urothelial carcinoma.
Cancer-specific survival
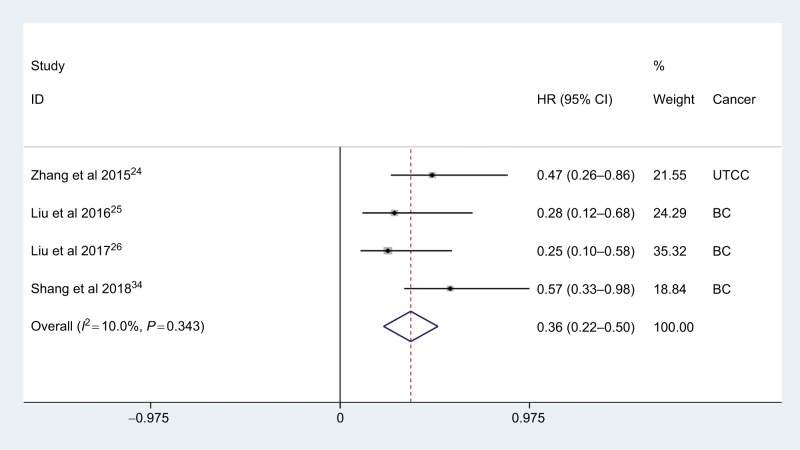
Four studies with 1,142 cases reported data on the relationship between AGR and CSS in urologic cancer. The meta-analysis suggested that low AGR significantly correlated with worse CSS (HR: 0.36, 95% CI: 0.22–0.50, P<0.001), with no significant heterogeneity across studies (I2=10.0%, Phet =0.343; Figure 3). Notably, the negative effect of high AGR on CSS was also observed in patients with bladder carcinoma (HR: 0.33, 95% CI: 0.18–0.49, P<0.001).
Figure 3.
Meta-analysis of the relationship between AGR and CSS.
Abbreviations: AGR, albumin-to-globulin ratio; BC, bladder carcinoma; CSS, cancer-specific survival; UTCC, upper tract urothelial carcinoma.
Event-free survival
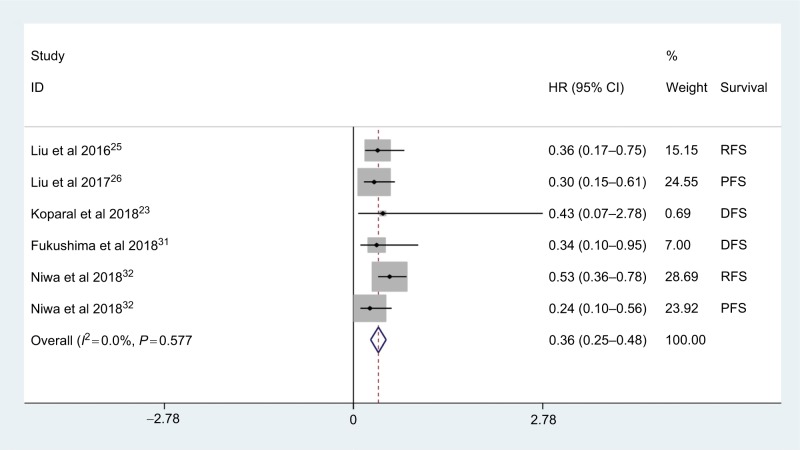
Six studies reported the association between AGR and EFS in urologic cancer; there were two studies focusing on PFS, DFS and RFS. As shown in Figure 4, the pooled results indicated that the patients with low AGR had an inferior EFS in urologic cancer (HR: 0.36, 95% CI: 0.25–0.48, P<0.001) with no obvious heterogeneity among studies (I2=0.0%, Phet =0.577). And significant associations were also found between AGR and RFS (HR: 0.47, 95% CI: 0.30–0.64, P<0.001), PFS (HR: 0.27, 95% CI: 0.11–0.43, P<0.001) and DFS (HR: 0.35, 95% CI: 0.06–0.75, P<0.001).
Figure 4.
Meta-analysis of the relationship between AGR and EFS.
Abbreviations: AGR, albumin-to-globulin ratio; DFS, disease-free survival; EFS, event-free survival; PFS, progression-free survival; RFS, recurrence-free survival.
AGR and clinicopathologic characteristics
Tumor grade
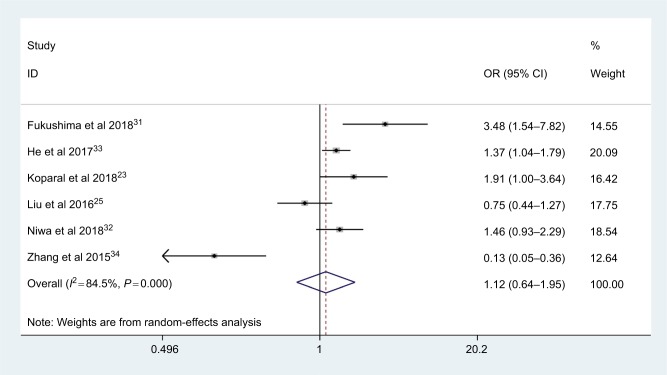
A total of six studies with 2,009 patients reported the relationship between AGR and tumor grade. As an obvious heterogeneity existed among studies (I2=84.5%, Phet =0.000), the random-effect model was used. The pooled results indicated that there was no significant association between AGR and tumor grade (OR: 1.12, 95% CI: 0.64–1.95, P=0.69; Figure 5)
Figure 5.
Meta-analysis of the association between AGR and tumor grade.
Abbreviation: AGR, albumin-to-globulin ratio.
Lymphovascular invasion (LVI)
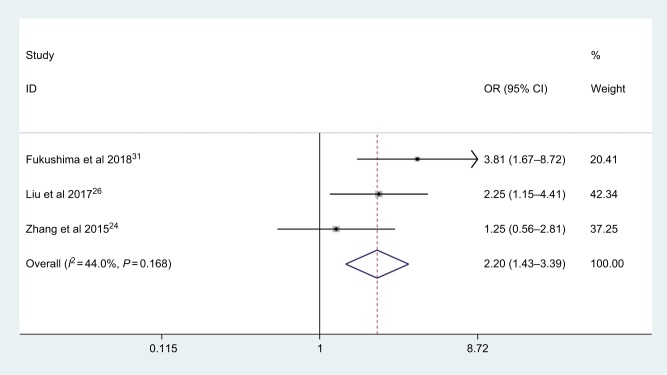
Only three studies with 481 patients explored the correlation between AGR and lymphovascular invasion. As shown in Figure 6, no significant heterogeneity was observed (I2=44.0%, Phet =0.168), and the patients with low AGR were more likely to have lymphovascular invasion (OR: 2.20, 95% CI: 1.43–3.39, P<0.001)
Figure 6.
Meta-analysis of the association between AGR and lymphovascular invasion.
Abbreviation: AGR, albumin-to-globulin ratio.
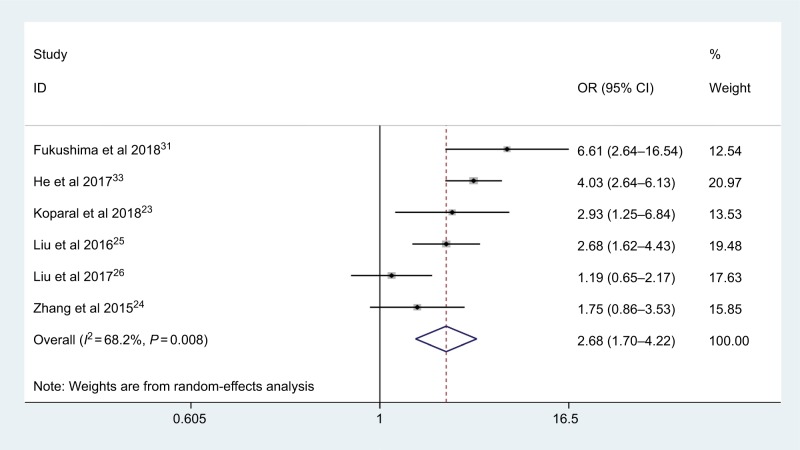
pT status
Six articles with 1,834 cases coved the effect of low AGR on pT status. The random-effects model was employed (I2=68.2%, Phet =0.008); the combined results indicated that low AGR was significantly associated with deeper depth of tumor invasion (OR: 2.68, 95% CI: 1.70–4.22, P<0.001; Figure 7).
Figure 7.
Meta-analysis of the association between AGR and pT status.
Abbreviation: AGR, albumin-to-globulin ratio.
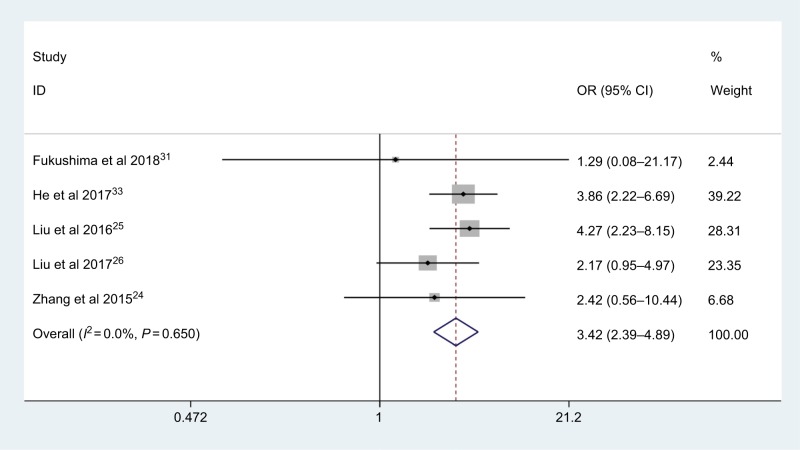
pN status
Five studies, consisting of 1,672 patients, explored the association between AGR and pN status. Analysis revealed the pooled OR of 3.42 with 95 % CI: 2.39–4.89 (P<0.001) (Figure 8) with no obvious heterogeneity (I2=0.0%, Phet =0.650). The pooled results showed that patients with low AGR were at significantly greater risk of lymph node metastasis.
Figure 8.
Meta-analysis of the association between AGR and pN status.
Abbreviation: AGR, albumin-to-globulin ratio.
pM status and pTNM stage
Only one study by He et al32 reported the associations of AGR with clinicopathologic features of the pM status and pTNM stage in RCC patients. Patients in low and high AGR groups showed significant differences in pM-stage (P<0.001) and pTNM stage (P<0.001).
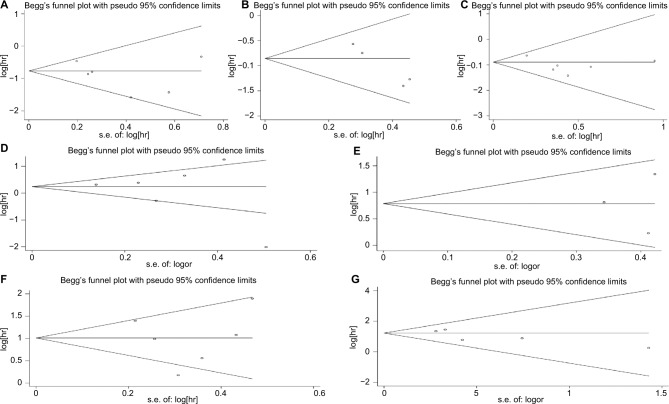
Publication bias
Funnel plot and Begg’s test were utilized to assess the publication bias. The result of Begg’s test confirmed that there was no evidence of significant publication bias among studies (POS =0.707; PCSS =0.308; PEFS =1.000; Ptumor grade = 1.000; PLVI =1.000; PpT = 1.000; PpN = 0.462; Figure 9A–G).
Figure 9.
Publication bias assessment for OS (A), CSS (B), EFS (C), tumor grade (D), LVI (E), pT (F) and pN (G).
Abbreviations: CSS, cancer-specific survival; EFS, event-free survival; LVI, lymphovascular invasion; OS, overall survival.
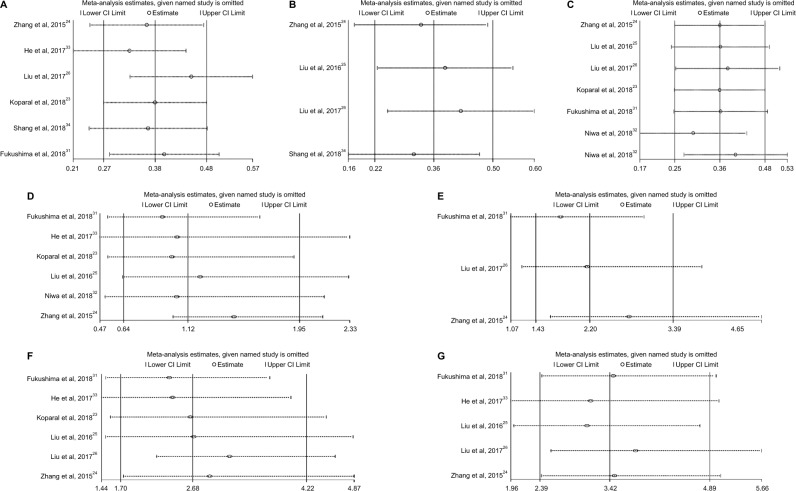
Sensitivity analysis
Sensitivity analysis was performed to determine whether the pooled data would be affected by any individual cohorts, and the answers were negative (Figure 10A–G).
Figure 10.
Sensitivity analysis for OS (A), CSS (B), EFS (C), tumor grade (D), LVI (E), pT (F) and pN (G).
Abbreviations: CSS, cancer-specific survival; EFS, event-free survival; LVI, lymphovascular invasion; OS, overall survival.
Discussion
Recently, a series of scores/ratios based on hematological parameters have been reported, such as modified Glasgow prognostic score (mGPS) and C-reactive protein (CRP) and albumin ratio; they were found to be potential prognostic markers in various human cancers.35–38 Additionally, some other important systemic inflammatory (SIR) markers, including neutrophil-to-lymphocyte ratio (NLR), neutrophil-platelet score and lymphocyte-to-monocyte ratio were reported; their prognostic values have also been widely evaluated in multiple malignancies, including gastric cancer, Ewing sarcoma and urologic tumors.39–41
To the best of our knowledge, as yet, no meta-analyses have been performed to assess the prognostic and clinicopathologic relevance of the AGR in cancers of the urinary system. In this meta-analysis, a total of eight studies comprising 2,668 patients were included to explore the association between AGR and the prognosis of patients with urologic cancers. The pooled results indicated that a low pretreatment AGR was significantly associated with worse clinical outcomes in urologic cancers.
Albumin accounts for the largest proportion of serum albumin in the human body. It is synthesized by hepatocytes and could be used as one of the evaluation indexes of liver function and to assess subjects’ nutritional status.42–44 Besides, it plays multiple crucial roles in physiological activities, such as maintaining intravascular permeability stress and as a free radical scavenger.45,46 Many previous studies also investigated the association between ALB and cancer. Serum albumin levels in cancer patients were significantly lower than in those without cancer,46,47 and low albumin levels often mean poor nutritional status, and imply weakened several immune defense systems. Furthermore, albumin was considered as an important inflammatory response marker,48 low albumin level was related to enhanced inflammatory response to the tumor and increased cytokine release, including interleukin(IL)–6 and tumor necrosis factor(TNF)-α, all of these might be surrogates for more aggressive tumor behaviors.49,50 On the other hand, albumin has shown related antitumor and antioxidant effects; it could stabilize cell growth and DNA replication and inhibit the proliferation and growth of tumor cells.51–53 Low albumin levels could also decrease the response to treatment in cancer patients,54,55 and its clinical role as an unfavorable prognostic biomarker was also reported in various human malignancies.56,57
Referring to the nonalbumin proteins, they are also named as globulin (total protein – albumin). It comprises many different proinflammatory proteins, such as CRP, complement components, interleukins, immunoglobulins and so on. Prior studies had reported that inflammatory proteins were associated with tumor prognosis and progression, and displayed the predictive significance in different human cancers. For example, patients with elevated preoperative CRP had a poor survival in UTUC,58–60 and high complement 3 levels were shown to be related to poor prognosis in patients with colorectal cancer,61 and an increased preoperative gamma globulin levels predicted poor survival in lung cancer.62 In addition, the globulins were shown to be closely related with chronic inflammation. Some serum globulins, such as IL-6, IL-8, TNF-α, VEGF, they were all inflammation-related factors, and played important roles in the tumor occurrence and progression. These inflammatory factors could promote the proliferation, invasion, metastasis of tumor cell, as well as subvert the host immune response, and contribute to the tumor drug resistance.63,64 Overall, albumins and globulins are related with nutrition status, immunoinflammatory reactions in the human body as well as the tumor progression and development. And the AGR takes both the ALB and GLB levels into account, it may more precisely and comprehensively reflect the body’s nutritional and inflammatory states. It could be a significant prognostic biomarker that helpful to predict the clinical outcomes in cancers.
As far as we know, our study is the first meta-analysis to evaluate the prognostic significance of the AGR in patients with urologic cancers. We found that a low pretreatment AGR was closely related to worse clinical outcomes in cancers of the urinary system. A low pretreatment AGR was significantly related to shorter OS, worse CSS and inferior EFS in urologic cancers. And there were significant differences in the lymphovascular invasion, pT status and pN status among low and high AGR groups.
However, several limitations in the present meta-analysis should be taken into consideration. First, all included studies were designed retrospectively, and the number of total sample size included was relatively small. Second, studies enrolled were all involved in Asian ethnicity groups; this might limit the generalization of our conclusions; the prognostic value of AGR in more populations are needed for further confirmation. Third, the cutoff values for low AGR were different in those studies with a slight range from 1.24 to 1.68, a unified cutoff value is necessary before it could be really applied in clinical practices. The heterogeneity observed may be the inclusion of a small number of studies covering three different types of urologic tumor, each with their own particular morphologic, pathologic and clinical characteristics. Fourth, negative results were usually harder to be published than positive results, this might lead to some data missing, the number of studies exploring the relationship between AGR and some clinical features was small or none. Finally, some other factors, such as age, treatment strategy or clinical stages, also could affect the patient survival, and medication used and accompanied diseases of the patients could also influence the level of albumin and globulin.
In conclusion, our meta-analysis synthetically established a connection between pretreatment AGR and patients with urinary system cancers. A low AGR was significantly related to worse long-term survival and advanced clinicopathologic features. AGR would serve as a valuable and noninvasive prognostic marker in urologic cancers. Nevertheless, larger and prospective multicenter research trials should be conducted to validate the clinical application of pretreatment AGR in urologic cancers.
Supplementary material
Table S1.
Relevant data for AGR and clinicopathologic features
| Tumor grade
| ||||
|---|---|---|---|---|
| High grade | Low grade | |||
|
| ||||
| Study | Low AGR | High AGR | Low AGR | High AGR |
| Fukushima et al 20181 | 31 | 22 | 15 | 37 |
| He et al 20172 | 166 | 195 | 205 | 329 |
| Koparal et al 20183 | 32 | 29 | 37 | 64 |
| Liu et al 20164 | 35 | 40 | 119 | 102 |
| Niwa et al 20185 | 53 | 96 | 59 | 156 |
| Zhang et al 20156 | 5 | 37 | 73 | 72 |
|
| ||||
| Lymphovascular invasion | ||||
|
| ||||
| Low AGR | High AGR | |||
|
| ||||
| Study | yes (+) | no (−) | yes (+) | no (−) |
| Fukushima et al 20181 | 26 | 20 | 15 | 44 |
| Liu et al 20177 | 40 | 70 | 16 | 63 |
| Zhang et al 20156 | 13 | 65 | 15 | 94 |
|
| ||||
| pT status | ||||
|
| ||||
| pT3/4 | pTa-2 | |||
|
| ||||
| Study | Low AGR | High AGR | Low AGR | High AGR |
| Fukushima et al 20181 | 25 | 9 | 21 | 50 |
| He et al 20172 | 83 | 35 | 288 | 489 |
| Koparal et al 20183 | 18 | 10 | 51 | 83 |
| Liu et al 20164 | 69 | 33 | 85 | 109 |
| Liu et al 20177 | 42 | 27 | 68 | 52 |
| Zhang et al 20156 | 21 | 19 | 57 | 90 |
|
| ||||
| pN status | ||||
|
| ||||
| Low AGR | High AGR | |||
|
| ||||
| Study | Positive (+) | Negative (−) | Positive (+) | Negative (−) |
| Fukushima et al 20181 | 1 | 45 | 1 | 58 |
| He et al 20172 | 47 | 324 | 19 | 505 |
| Liu et al 20164 | 49 | 105 | 14 | 128 |
| Liu et al 20177 | 24 | 86 | 9 | 70 |
| Zhang et al 20156 | 5 | 73 | 3 | 106 |
Abbreviation: AGR, albumin-to-globulin ratio.
References
- 1.Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of albumin/globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res. 2018;38(4):2329–2334. doi: 10.21873/anticanres.12478. [DOI] [PubMed] [Google Scholar]
- 2.He X, Guo S, Chen D, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer. 2017;8(2):258–265. doi: 10.7150/jca.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koparal MY, Polat F, Çetin S, Bulut EC, Sözen TS. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int Braz J Urol. 2018;44 doi: 10.1590/S1677-5538.IBJU.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):484.e1–48484. doi: 10.1016/j.urolonc.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment Albumin-to-Globulin ratio in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(3):e655–e661. doi: 10.1016/j.clgc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Yu W, Zhou LQ, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PLoS One. 2015;10(12):e0144961. doi: 10.1371/journal.pone.0144961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Huang H, Li S, et al. The prognostic value of preoperative serum albumin-globulin ratio for high-grade bladder urothelial carcinoma treated with radical cystectomy: A propensity score-matched analysis. J Cancer Res Ther. 2017;13(5):837–843. doi: 10.4103/jcrt.JCRT_237_17. [DOI] [PubMed] [Google Scholar]
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, Mcardle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 2.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. 2014;40(6):753–762. doi: 10.1590/S1677-5538.IBJU.2014.06.06. [DOI] [PubMed] [Google Scholar]
- 4.Ge LN, Wang F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: a systematic review and dose-response meta-analysis of observational studies. Cancer Manag Res. 2018;10:815–825. doi: 10.2147/CMAR.S161876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito H, Kono Y, Murakami Y, et al. Postoperative serum albumin is a potential prognostic factor for older patients with gastric cancer. Yonago Acta Med. 2018;61(1):72–78. doi: 10.33160/yam.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li SQ, Jiang YH, Lin J, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. 2018;7(4):1221–1231. doi: 10.1002/cam4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercier J, Voutsadakis IA. Comparison of hematologic and other prognostic markers in metastatic colorectal cancer. J Gastrointest Cancer. 2018 doi: 10.1007/s12029-018-0108-1. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 9.Zimring JC. Do immune complexes play a role in hemolytic sequelae of intravenous immune globulin? Transfusion. 2015;55(Suppl 2):S86–S89. doi: 10.1111/trf.13158. [DOI] [PubMed] [Google Scholar]
- 10.Petite SE, Bollinger JE, Eghtesad B. Antithymocyte globulin induction therapy in liver transplant: old drug, new uses. Ann Pharmacother. 2016;50(7):592–598. doi: 10.1177/1060028016647974. [DOI] [PubMed] [Google Scholar]
- 11.Meyer EJ, Nenke MA, Rankin W, Lewis JG, Torpy DJ. Corticosteroid-binding globulin: a review of basic and clinical advances. Horm Metab Res. 2016;48(6):359–371. doi: 10.1055/s-0042-108071. [DOI] [PubMed] [Google Scholar]
- 12.Li XH, Gu WS, Wang XP, et al. Low preoperative albumin-to-globulin ratio predict poor survival and negatively correlated with fibrinogen in resectable esophageal squamous cell carcinoma. J Cancer. 2017;8(10):1833–1842. doi: 10.7150/jca.19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Wang W, Meng X, et al. Albumin/globulin ratio is negatively correlated with PD-1 and CD25 mRNA levels in breast cancer patients. Onco Targets Ther. 2018;11:2131–2139. doi: 10.2147/OTT.S159481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Meng X, Liang L, et al. High preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcome. Am J Cancer Res. 2015;5(9):2856–2864. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Zhou Y, Xu Y, Zhu HY, Shi YQ. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour Biol. 2016;37(3):3905–3911. doi: 10.1007/s13277-015-3778-3. [DOI] [PubMed] [Google Scholar]
- 16.Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis. 2013;28(12):1629–1636. doi: 10.1007/s00384-013-1748-z. [DOI] [PubMed] [Google Scholar]
- 17.Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am J Surg. 2013;206(5):764–770. doi: 10.1016/j.amjsurg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Guo HW, Yuan TZ, Chen JX, Zheng Y. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: A meta-analysis. PLoS One. 2018;13(1):e0189839. doi: 10.1371/journal.pone.0189839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. 2018;476:81–91. doi: 10.1016/j.cca.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 20.He J, Pan H, Liang W, et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer. 2017;8(19):4002–4010. doi: 10.7150/jca.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen WZ, Yu ST, Xie R, et al. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget. 2017;8(29):48240–48247. doi: 10.18632/oncotarget.18443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. 2016;9:5317–5328. doi: 10.2147/OTT.S109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koparal MY, Polat F, Çetin S, Bulut EC, Sözen TS. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int Braz J Urol. 2018;44 doi: 10.1590/S1677-5538.IBJU.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Yu W, Zhou LQ, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PLoS One. 2015;10(12):e0144961. doi: 10.1371/journal.pone.0144961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):484.e1–48484. doi: 10.1016/j.urolonc.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Huang H, Li S, et al. The prognostic value of preoperative serum albumin-globulin ratio for high-grade bladder urothelial carcinoma treated with radical cystectomy: A propensity score-matched analysis. J Cancer Res Ther. 2017;13(5):837–843. doi: 10.4103/jcrt.JCRT_237_17. [DOI] [PubMed] [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Dong Q, Huang J. Overexpression of lncRNA PVT1 predicts advanced clinicopathological features and serves as an unfavorable risk factor for survival of patients with gastrointestinal cancers. Cell Physiol Biochem. 2017;43(3):1077–1089. doi: 10.1159/000481719. [DOI] [PubMed] [Google Scholar]
- 29.Liang RF, Li JH, Li M, Yang Y, Liu YH. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin Chim Acta. 2017;474:155–158. doi: 10.1016/j.cca.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Xia Q, Liu J, Wu C, et al. Prognostic significance of (18)FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging. 2015;15:19. doi: 10.1186/s40644-015-0055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of albumin/globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res. 2018;38(4):2329–2334. doi: 10.21873/anticanres.12478. [DOI] [PubMed] [Google Scholar]
- 32.Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment albumin-to-globulin ratio in patients with non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(3):e655–e661. doi: 10.1016/j.clgc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 33.He X, Guo S, Chen D, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer. 2017;8(2):258–265. doi: 10.7150/jca.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang Z, Wang J, Wang X, et al. Preoperative serum apolipoprotein A-I levels predict long-term survival in non-muscle-invasive bladder cancer patients. Cancer Manag Res. 2018;10:1177–1190. doi: 10.2147/CMAR.S165213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolan RD, Mcsorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi: 10.1038/s41416-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Yang L, Xia L, Chen Y. High C-reactive protein/albumin ratio predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2018;10:371–381. doi: 10.2147/CMAR.S155604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J, Chen S, Chen Y, Li S, Xu D. Combination of CRP and NLR: a better predictor of postoperative survival in patients with gastric cancer. Cancer Manag Res. 2018;10:315–321. doi: 10.2147/CMAR.S156071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galun D, Bogdanovic A, Djokic Kovac J, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing country. Cancer Manag Res. 2018;10:977–988. doi: 10.2147/CMAR.S161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YJ, Yang X, Zhang WB, et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag Res. 2017;9:443–451. doi: 10.2147/CMAR.S146827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Su S, Guo Y. The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(48):84506–84514. doi: 10.18632/oncotarget.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;56(11):749–755. doi: 10.4111/kju.2015.56.11.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22(11-12):1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 43.Kang SC, Kim HI, Kim MG. Low serum albumin level, male sex, and total gastrectomy are risk factors of severe postoperative complications in elderly gastric cancer patients. J Gastric Cancer. 2016;16(1):43–50. doi: 10.5230/jgc.2016.16.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai CC, You JF, Yeh CY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. 2011;26(4):473–481. doi: 10.1007/s00384-010-1113-4. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysi-ologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 46.Gatta A, Verardo A, Bolognesi M, Hypoalbuminemia BM. Hypoalbu-minemia. Intern Emerg Med. 2012;7(S3):193–199. doi: 10.1007/s11739-012-0802-0. [DOI] [PubMed] [Google Scholar]
- 47.Göransson J, Jonsson S, Lasson A. Pre-operative plasma levels of C-reactive protein, albumin and various plasma protease inhibitors for the pre-operative assessment of operability and recurrence in cancer surgery. Eur J Surg Oncol. 1996;22(6):607–617. doi: 10.1016/s0748-7983(96)92398-7. [DOI] [PubMed] [Google Scholar]
- 48.Barbosa-Silva MC. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248–254. doi: 10.1097/MCO.0b013e3282fba5d7. [DOI] [PubMed] [Google Scholar]
- 49.Brenner DA, Buck M, Feitelberg SP, Chojkier M. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest. 1990;85(1):248–255. doi: 10.1172/JCI114419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mcmillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, Mcardle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 51.Laursen I, Briand P, Lykkesfeldt AE. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. Anticancer Res. 1990;10(2A):343–351. [PubMed] [Google Scholar]
- 52.Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001;93(12):490–493. [PMC free article] [PubMed] [Google Scholar]
- 53.Gómez P, Beltrán ME, Rábago M. Immunoelectrophoretic demonstration of albumin in breast cancer. Arch Invest Med. 1983;14(3):241–245. [PubMed] [Google Scholar]
- 54.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med. 1980;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 55.Chandra RK. Nutrition and immunology: from the clinic to cellular biology and back again. Proc Nutr Soc. 1999;58(3):681–683. doi: 10.1017/s0029665199000890. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Wang F, Li S, et al. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep. 2018;38(4):BSR20180214. doi: 10.1042/BSR20180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Shao Y, Wang K, et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:6701–6710. doi: 10.2147/OTT.S108469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obata J, Kikuchi E, Tanaka N, et al. C-reactive protein: a bio-marker of survival in patients with localized upper tract urothelial carcinoma treated with radical nephroureterectomy. Urol Oncol. 2013;31(8):1725–1730. doi: 10.1016/j.urolonc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka N, Kikuchi E, Shirotake S, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol. 2014;65(1):227–234. doi: 10.1016/j.eururo.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 60.Saito K, Kawakami S, Ohtsuka Y, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int. 2007;100(2):269–273. doi: 10.1111/j.1464-410X.2007.06934.x. [DOI] [PubMed] [Google Scholar]
- 61.Mehrabani D, Shamsdin SA, Dehghan A, Safarpour A. Clinical signifi-cance of serum vascular endothelial growth factor and complement 3a levels in patients with colorectal cancer in southern Iran. Asian Pac J Cancer Prev. 2014;15(22):9713–9717. doi: 10.7314/apjcp.2014.15.22.9713. [DOI] [PubMed] [Google Scholar]
- 62.Cohen MH, Makuch R, Johnston-Early A, et al. Laboratory parameters as an alternative to performance status in prognostic stratifi-cation of patients with small cell lung cancer. Cancer Treat Rep. 1981;65(3-4):187–195. [PubMed] [Google Scholar]
- 63.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 64.Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Relevant data for AGR and clinicopathologic features
| Tumor grade
| ||||
|---|---|---|---|---|
| High grade | Low grade | |||
|
| ||||
| Study | Low AGR | High AGR | Low AGR | High AGR |
| Fukushima et al 20181 | 31 | 22 | 15 | 37 |
| He et al 20172 | 166 | 195 | 205 | 329 |
| Koparal et al 20183 | 32 | 29 | 37 | 64 |
| Liu et al 20164 | 35 | 40 | 119 | 102 |
| Niwa et al 20185 | 53 | 96 | 59 | 156 |
| Zhang et al 20156 | 5 | 37 | 73 | 72 |
|
| ||||
| Lymphovascular invasion | ||||
|
| ||||
| Low AGR | High AGR | |||
|
| ||||
| Study | yes (+) | no (−) | yes (+) | no (−) |
| Fukushima et al 20181 | 26 | 20 | 15 | 44 |
| Liu et al 20177 | 40 | 70 | 16 | 63 |
| Zhang et al 20156 | 13 | 65 | 15 | 94 |
|
| ||||
| pT status | ||||
|
| ||||
| pT3/4 | pTa-2 | |||
|
| ||||
| Study | Low AGR | High AGR | Low AGR | High AGR |
| Fukushima et al 20181 | 25 | 9 | 21 | 50 |
| He et al 20172 | 83 | 35 | 288 | 489 |
| Koparal et al 20183 | 18 | 10 | 51 | 83 |
| Liu et al 20164 | 69 | 33 | 85 | 109 |
| Liu et al 20177 | 42 | 27 | 68 | 52 |
| Zhang et al 20156 | 21 | 19 | 57 | 90 |
|
| ||||
| pN status | ||||
|
| ||||
| Low AGR | High AGR | |||
|
| ||||
| Study | Positive (+) | Negative (−) | Positive (+) | Negative (−) |
| Fukushima et al 20181 | 1 | 45 | 1 | 58 |
| He et al 20172 | 47 | 324 | 19 | 505 |
| Liu et al 20164 | 49 | 105 | 14 | 128 |
| Liu et al 20177 | 24 | 86 | 9 | 70 |
| Zhang et al 20156 | 5 | 73 | 3 | 106 |
Abbreviation: AGR, albumin-to-globulin ratio.