Abstract
Unexpected mortality occurred in a group of 12 NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) and 12 NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ (NRG) immunodeficient mice. At 10 d after routine bone marrow–liver–thymus humanization surgery, 9 mice were found dead without observation of initiating clinical signs; 1 d later (day 11), 3 additional mice showed signs of morbidity, including severe hunching, lateral recumbency, slow movement, shallow respiration, and decreased response to external stimulus. All remaining mice rapidly decompensated and were found dead or were euthanized within 4 d after the first death. Histopathology revealed severe ascending pyelonephritis with numerous yeast. Cultures in some mice were positive for Enterococcus faecalis or Staphylococcus xylosus, 2 bacteria considered commensals in rodents. In addition, Candida albicans was cultured from some animals. Further investigation revealed that a restraining device used for tail vein injections was the likely fomite harboring Candida organisms. These findings indicate that ascending pyelonephritis, with Candida as the etiologic agent, can cause significant mortality in NSG and NRG immunodeficient mice.
Abbreviations: BLT, bone marrow–liver–thymus; HSC, hematopoietic stem cells; NOD, nonobese diabetic; NSG, NODCg-Prkdcscid Il2rgtm1Wjl/SzJ; NRG, NODCg-Rag1tm1Mom Il2rgtm1Wjl/ SzJ; PAS, periodic acid–Schiff
Successful transplantation of human hematopoietic tissues, including fetal liver, thymus, and hematopoietic stem cells (HSC), into SCID mice has allowed for reconstitution of the human immune system in a murine model, thereby providing novel insights into the evaluation of human-specific diseases and human responses to therapeutics. In recent years, these chimeric mice have become increasingly popular in translational biomedical research and have been used to study immune responses after HIV infection, engraftment of primary human tumors, and human cell therapy.3 In this study, we completed bone marrow–liver–thymus (BLT) humanization, which provides an HLA-matched human thymus and bone marrow transplant. Mice that receive this type of surgery typically begin to have human cells in the periphery at approximately 8 wk after surgery and are fully reconstituted by 12 to 16 wk.20 To assure appropriate and consistent responses for translational research, it is imperative to create and adhere to strict sanitization and husbandry protocols for these immunodeficient mice, because they have several genetic mutations that increase their susceptibility to various bacterial, viral and fungal infections. In this report, we describe an acute onset of morbidity and mortality in a group of immunodeficient mice engrafted with human HSC and tissue. The human immune system mouse models we used here included NSG and NRG mutant strains, which mice carry 2 mutations on the NOD/ShiLtJ genetic background. Both mouse models carry a complete null allele of the IL2 receptor common γ chain (IL2rgnull), which abrogates murine signaling for the IL2, IL4, IL7, and IL15 cytokine receptors, among others.28 One difference between these strains is that NSG mice have a SCID mutation, whereas NRG mice have a targeted knockout mutation in recombination activating gene 1 (Rag1).28
Both humanized immune mouse models were derived from nonobese diabetic (NOD) mice, an important model of autoimmune type 1 diabetes. The NOD strain was developed during selection for a cataract-prone strain, when repeated brother– sister mating resulted in a subline that spontaneously developed diabetes.16,18 NOD mice typically develop diabetes at approximately 12 to 14 wk of age, and they are also prone to other autoimmune-related syndromes, including sialoadenitis, thyroiditis, peripheral neuropathy, and prostatitis.1 In addition, NOD mice have immune defects, including impaired NK cell function and IL15 signaling.22,28,32 Importantly, NOD mice possess an allele for SIRPα that is essential to successful engraftment of human cells and tissues.33 The sum of the genetic modifications to the immune systems of these mice renders them severely immunocompromised and highly susceptible to infection by not only pathogenic but also commensal organisms.
History and clinical presentation.
Female NSG (n = 12) and NRG (n = 12) immunodeficient mice were purchased from Jackson Laboratories (Bar Harbor, ME) to compare humanization between these strains by using the same donor cells and tissue. NSG and NRG mice were housed according to strain in IVC cage racks (Sealsafe Plus, Tecniplast USA, West Chester, PA) and were provided autoclaved chow (no. 5K52, LabDiet, St Louis, MO) and acidified water without restriction. All cages were autoclaved before being cleaned; underwent cage washing; filled with fresh bedding (Beta Chip, Northeastern Products, Warrensburg, NY), a cotton square, and a tunnel (Modak Mouse Tube, Braintree Scientific, Braintree, MA) as enrichment; and then autoclaved. All procedures were approved by the Animal Care and Use Committee of White Oak Federal Research Center and were performed in an AAALAC-accredited facility. Mice (age, 8 to 10 wk) underwent a routine BLT humanization surgery, as previously described.35 Briefly, CD34+ cells were isolated and purified from autologous fetal liver and cultured overnight in sterile stem-cell medium containing growth factors and human serum albumin but no fetal bovine serum. Bone marrow ablation was achieved by using a single dose of pharmaceutical-grade treosulfan prepared aseptically immediately prior to injection at 2 gm/kg IP 24 h prior to HSC injection. Stem cell injection was performed immediately after completion of surgery. Surgery was performed in class IIA biologic safety cabinets under sterile conditions. Anesthesia was induced with avertin (200 to 240 mg/kg IP), which was prepared fresh by combining 2,2,2-tribromoethanol, tert-amyl alcohol, and pharmaceutical-grade water for injection, filter-sterilized by using a 0.22-μm filter, and protected from light during surgery.
Each mouse was placed on its right side, fur was moistened with 70% ethanol, and then the mouse's side was shaved by using a sterile surgical blade. The site was sterilized through 3 alternating applications of povidone–iodine and 70% ethanol; the skin dried completely between applications. Sterile surgical instruments were used to create a left lateral incision into the body wall sufficient to elevate and expose the left kidney. The renal capsule was nicked, and 2 segments of human fetal thymus (approximately 0.5 mm × 0.5 mm) adjacent to a similarly sized segment of fetal liver were implanted and secured beneath the renal capsule. The kidney was replaced into the abdominal cavity, and the body wall and skin were apposed by using a simple interrupted suture pattern of 5-0 and 4-0 polydioxanone suture, respectively. All surgical and suture instruments were replaced between strains, such that no item during surgery or post-operative recovery was used for both strains. After surgery and recovery from anesthesia, all mice were placed successively in a restraining device (Rotating Tail Injector, Braintree Scientific), and 2.5 × 105 CD34+ HSC were infused into each mouse through tail vein injection. Mice were monitored twice daily after surgery and recovered uneventfully. Protocol procedures to prevent infection were strictly implemented and included decontamination of all items with 70% ethanol when they entered the room and autoclaving of all appropriate items, including cages, surgical instruments, transport containers, and miscellaneous medical supplies, such as gauze. In addition, routine use of items supplied as sterile, such as needles, syringes, surgical gloves, and other items was a standard operating procedure.
At 10 d after surgery, 9 mice were found dead during the routine morning cage checks, without any prior clinical indication of morbidity at the previous afternoon's observations. On the morning of day 11, 4 additional mice were found dead, with another 3 showing signs of morbidity including severe hunching, lateral recumbency, slow movements, shallow respiration, and decreased response to external stimuli. In addition, 1 mouse demonstrated neurologic signs, including head tilt and circling to the right. The 3 mice that demonstrated signs of morbidity on the morning of day 11 were immediately submitted live to the pathology service at NIH for full diagnostic necropsy. Within 14 d after surgery, all 24 mice were either found dead or were euthanized by using an overdose of avertin or carbon dioxide. Samples for histology and bacteriology were collected from mice that were either recently dead (less than 2 h) or moribund and euthanized by study investigators.
Microbiology.
Three mice were submitted live for diagnostic necropsy, and blood samples and spleen swabs were cultured. The only organism identified was Candida albicans, which was present in the spleen swabs from 2 animals; the remaining mouse had negative blood and spleen cultures. At that time, the growth of yeast was suspected to be due to contamination of the cultures. Subsequently, swabs from the skin, spleen, heart, and abdominal cavity of 5 mice that were either recently found dead or euthanized due to moribundity were submitted for culture and bacteriology. Yeast species were obtained from within the heart and abdomen of 2 different mice and identified as Candida albicans. Two bacteria, Staphylococcus xylosus and Enterococcus faecalis, were cultured from the skin, abdominal cavity, spleen, or heart of these 5 mice. S. xylosus and E. faecalis are presumed to be nonpathogenic bacterial organisms and are commonly identified in immunocompetent laboratory mouse strains.11 Although yeast species were cultured from 4 animals, further investigation of the organism that led to the demise of all 24 mice was not initiated until histopathology confirmed severe, ascending pyelonephritis and tubulointerstitial nephritis, with the presence of numerous yeast, hyphae, and pseudohyphae that stained positive for periodic acid–Schiff (PAS) in all of the 14 mice evaluated.
To determine whether the yeast species cultured through swabs at necropsy was the same organism found in tissues, we sought to identify the species and genera by using PCR analysis. We attempted to isolate yeast DNA from formalin-fixed, paraffin-embedded blocks by using 2 different kits (Gentra Puregene Tissue Kit, Qiagen, Germantown, MD, and RecoverAll Total Nucleic Acid Isolation Kit for FFPE, Ambion, Thermo Fisher Scientific, Waltham, MA). We assessed the sizes of all fragments isolated (High Sensitivity DNA Kit, Agilent Technologies, Santa Clara, CA), but the DNA was too fragmented to confirm the species identity from tissue.
Pathology.
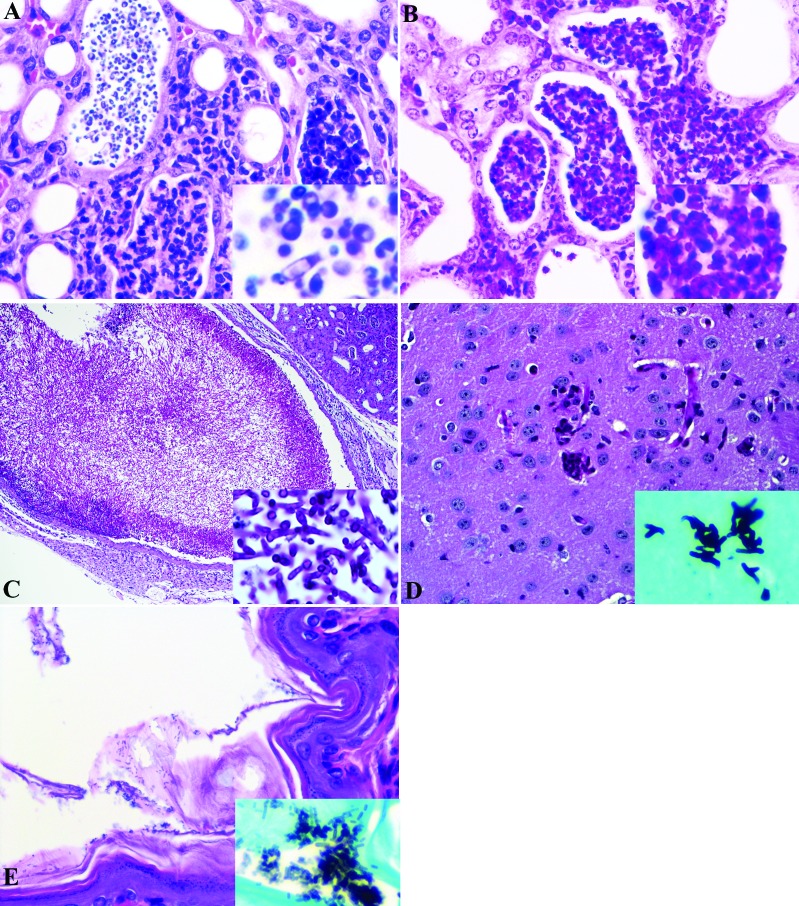
In total, 14 mice (11 found recently dead or euthanized by investigators and 3 that were submitted live for diagnostic pathology) were necropsied and submitted for histopathology. Grossly, the 3 mice submitted for diagnostic necropsy were thin and lean and exhibited mild dehydration. Bilaterally, the kidneys were pale. All other tissues were unremarkable. Mice that were found dead or euthanized by investigators exhibited various degrees of autolysis. Important histopathologic findings included bilateral, ascending suppurative pyelonephritis with numerous PAS-positive hyphae, pseudohyphae, and yeast (Figure 1). Renal tubular epithelial atrophy, tubulointerstitial suppurative nephritis with intralesional yeast organisms (Figure 1), proteinaceous casts and fibrosis were also appreciated. ‘Fungal balls’ composed of yeast organisms admixed with sloughed epithelial cells and degenerative neutrophils, as described in human Candida infections,15,36 appeared in the proximal ureter of 1 mouse (Figure 1). Regions of suppurative inflammation within the kidneys were PAS-positive and gram-negative (Figure 1), indicating yeast as the primary cause of ascending pyelonephritis.
Figure 1.
(A) Suppurative tubulointerstitial nephritis with intratubular yeast organisms, mild interstitial fibrosis, and variable tubular ectasia and atrophy. Kidney, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ humanized mouse. Hematoxylin and eosin stain. Inset: Higher magnification of yeast, with occasional pseudohyphae. (B) Gram stain of section in panel A, showing suppurative tubulointerstital nephritis without the presence of bacteria. Kidney, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ humanized mouse. Inset: Higher magnification, illustrating the absence of bacterial organisms. (C) A sheet of yeast organisms admixed with sloughed epithelial cells; degenerative neutrophils occlude the proximal ureteral lumen. Fungal ball, kidney, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ humanized mouse. PAS stain. Inset: Higher magnification, showing yeast, septate hyphae, and occasional pseudohyphae. Etiology consistent with Candida spp. (D) Multifocally expanding the neuropil are few, often coalescing, nodules predominately composed of degenerate and viable neutrophils. Brain, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ humanized mouse. Hematoxylin and eosin stain. Inset: Higher magnification, showing yeast and septate hyphae within inflamed regions of neuropil. Grocott–Gomori methenamine silver stain. (E) Regionally extensive, intralesional yeast organisms. Stratum corneum, forestomach, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ humanized mouse. Hematoxylin and eosin stain. Inset: Higher magnification of yeast organisms positive for Grocott–Gomori methenamine silver stain.
Of the 3 mice that were submitted for diagnostic necropsy, 2 had mild, multifocal suppurative meningoencephalitis (Figure 1); the third exhibited encephalitis only. There was no evidence of otitis that could have contributed to the clinically observed neurologic signs within the decalcified heads of these 3 mice. One brain, excised from the skull, was submitted for histopathology from the group of 11 mice necropsied by investigators and revealed similar histopathologic findings. Yeast-like structures, albeit rare, were admixed within the suppurative inflammation in the brains of the euthanized mice that were considered positive for Grocott–Gomori methenamine silver (GMS) stain (Figure 1) and negative by Gram staining (not shown). In addition, GMS-positive yeast or fungal organisms were observed in the stomach along regions of focally extensive epithelial degeneration or loss (Figure 1). However, structurally, these organisms were smaller than yeast within the kidney and brain. Additional findings supportive of systemic disease and impending sepsis in 1 of the 3 submitted mice included pulmonary vein necrosis and focally extensive pleural to interstitial necrosis. Inflammation was present mainly in kidneys (14 of 14 animals) and less often in brain (4 of 14) and stomach (5 of 14). The organisms associated with these regions of inflammation were GMS-positive but gram-negative in the kidneys and brain; in contrast, the regions in stomach were GMS-negative but gram-positive (Table 1).
Table 1.
Summary of histopathologic lesions (no. of animals affected [no. examined]) after experimental humanization surgery in NSG and NRG immunodeficient mice
| Histopathologic Diagnosis | Mice submitted live | Mice found dead (within 2 h) or euthanized |
| Kidneys | ||
| Ascending pyelonephritis with yeast | 3 (3) | 11 (11) |
| Brain | ||
| Meningoencephalitis | 2 (3) | 0 (11) |
| Encephalitis | 1 (3) | 1 (11) |
| Lungs | ||
| Mild pulmonary vein necrosis with necrosis and hemorrhage | 1 (3) | 0 (11) |
| Mild, focally extensive pleural necrosis with hemorrhage | 1 (3) | 0 (11) |
| Stomach | ||
| Mild, mulifocal suppurative gastritis | 2 (3) | 3 (11) |
| Mild, multifocal epithelial degeneration and loss | 1 (3) | 0 (11) |
| Squamous nodules | 1 (3) | 0 (11) |
| Spleen | ||
| Mildly increased extramedullary hematopoiesis | 3 (3) | 6 (6) |
| Oral cavity | ||
| Minimal gingivitis | 3 (3) | 0 (0) |
| Bone marrow | ||
| Mild atrophy | 2 (3) | 0 (0) |
| Ovary | ||
| Teratoma with cysts | 1 (1) | 0 (0) |
| Ears | ||
| Otitis | 0 (3) | 0 (0) |
No significant findings in heart or liver of either group.
Histopathology confirmed severe, ascending pyelonephritis and tubulointerstitial nephritis, with the presence of myriad of PAS-positive yeast, hyphae, and pseudohyphae in all 14 examined mice. Given the complete lack of bacterial organisms within regions where yeast was present within suppurative inflammation, it is unlikely that the microbiologically identified organisms E. faecalis (a common gut bacteria) and S. xylosus (a nonpathogenic dermal bacterial species) contributed to the acute deaths.
Follow-up investigation.
After pathologic identification of fungal species in the proximal ureter and kidneys of all mice 14 examined, we sought to identify the source of the infectious agent. Two groups of 12 mice underwent surgery, and all items used were either prepared aseptically and sterilized, purchased sterile, or autoclaved to ensure sterility. Prior to this round of surgery, we had successfully completed BLT humanization surgery more than a dozen times with no acute infection observed. We evaluated all items used for these surgeries, and the only ones to which all mice were exposed were the pharmaceutical products, including tribromoethanol, carprofen, enrofloxacin, and saline; HSC; and the rodent restrainer. Each of the pharmaceutical compounds was prepared aseptically from pharmaceutical-grade reagents, except for tribromoethanol which was sterile-filtered prior to administration. HSC were isolated aseptically from fetal liver and cultured overnight. Had a microbial organism been present, it likely would have been discovered through culture contamination, because the medium was free of antibiotics. In addition, all of these products were administered by injection, and had one of them been responsible for the acute infection in all mice, the animals likely would have become ill much more rapidly than was observed.
We considered the rodent restrainer, because the illness appeared to be an ascending infection, causing pyelonephritis in all mice. This device was disinfected with 70% ethanol between surgeries, but it was not disinfected between individual mice during injection of HSC after surgery. We were unable to culture the device concurrently to confirm it as the source, because several weeks elapsed between the loss of mice and documentation of histopathologic findings. Prior to the investigation and histopathologic confirmation of ascending pyelonephritis with intralesional yeast, the infection was thought to be bacterial in etiology, and the entire procedure room, including all equipment, was thoroughly decontaminated. Immediately after the identification of yeast in tissues, we cultured microbiologic swabs of the biosafety cabinets, ventilation duct, and other equipment in the animal room, but no organisms were grown.
Discussion
Here we report an outbreak of ascending pyelonephritis with numerous yeast in a group of 12 NSG and 12 NRG immunodeficient mice that underwent BLT humanization surgery 10 d earlier. The purpose of the surgery was to compare BLT humanization between these strains of immunocompromised mice by using the same donor and surgical staff to limit potential variability. To this end, we used different surgical packs, recovery cages, and routine surgical supplies for each mouse strain. The only exposures common to all mice were the HSC, pharmaceutical products, and rodent restrainer. All of the pharmaceuticals used were prepared aseptically by using pharmaceutical-grade reagents or were filter-sterilized (pore size, 0.22 μm) prior to use. The HSC were cultured overnight in antibiotic-free medium. All pharmaceuticals and HSC were injected by using sterile needles and syringes for each mouse, making it unlikely that Candida was transmitted through these products. The remaining common exposure was the rodent restrainer. Although this device might seem an unlikely fomite, Candida species readily form biofilms on a variety of medical devices, including catheters, pacemakers, dental devices, and contact lenses.21 In addition, mature biofilms can form within 24 h on materials common in medical devices.21 A factor that might enhance the spread of infection from a biofilm is that many mice spontaneously urinate during restraint, thus potentially facilitating the transmission of pathogens from a biofilm to a mouse.
Biofilms have 4 stages, including the formation of pseudohyphae and hyphae,21 which have increased capability to invade immunocompromised hosts. Importantly, biofilms, unlike suspension cultures, are highly resistant to elimination with a variety of disinfectants, including povidone–iodine, sodium hypochlorite, 70% alcohol, and Ecodiol (Prodene Klint, Alby sur Cheran, France); in the study, only chlorhexidine had efficacy against Candida biofilms.34 In the current case, the sanitizer used to disinfect the equipment was 70% ethanol. Although this disinfectant is effective against numerous bacteria and viruses, 70% ethanol has limited to no effect on yeast, even in suspension.19 After this incident, MB10 or Vimoba (Quip Laboratories, Wilmington, DE)—which contain chlorine dioxide as the principal active ingredient and one that is effective on most yeast, bacterial, and many viral organisms—was used as a biocide for future disinfection. This measure, coupled with storage of the restrainer within a closed bin, has prevented any outbreak of opportunistic Candida infection in our humanized mouse colony to date.
Although it seemed unlikely that all mice exposed to a commonly used restraining device could experience ascending pyelonephritis, it is important to consider how experimental mouse models of Candida infection respond to infection. In a study comparing the susceptibility of different mouse strains to Candida after intravenous injection of 5.5 to 6.5 Log10 blastospores, death occurred in 2 to 5 d, with CBA/H, BALB/c, and C57BL/6 having greatest to least susceptibility, respectively.2 In addition, healthy male BALB/c mice injected intravenously with either 5 × 105 or 1 × 105 blastospores experienced 100% mortality in 7 or 14 d, respectively, with the cause of mortality identified as sepsis.29 Healthy immunocompromised SCID and NSG mice inoculated intravenously with 1 × 104 Candida cells died as early as 10 d after injection, with approximately 60% of the NSG mice experiencing mortality.23 These results suggest that the time course for sepsis and death in the mice we describe here would have been more rapid than 10 d, given the treatments and procedures that the animals received. With regard to the time required to ascend the reproductive tract, female BALB/c mice in which pseudoestrus had been induced were vaginally inoculated with 5 × 105 bioluminescent Candida blastospores and were observed using imaging techniques for 30 d. Dissemination of Candida to organs distant from the vaginal lumen was observed as early as 2 d after infection,5 supporting the conclusion that exposure to the urinary tract could lead to ascending pyelonephritis according to the observed time course.
Furthermore, in each of the previous studies, blastospores served as the inoculum, but hyphal forms are known to be integrally associated with pathogenicity.31,37 An investigation into the intravenous pathogenicity of hyphae-producing Candida compared with mutant strains that produced no hyphae revealed significant differences in morbidity, mortality, and pathology. In addition, mice that received the hyphae-producing strain compared with the mutant ones died in 1 d compared with 13 d after inoculation with the same number of organisms.24 Healthy mice, even in immunocompromised strains, primarily require the presence of neutrophils to combat Candida infection.10,17,23 In the current case report, the mice were from highly immunocompromised strains that had been given a chemotherapeutic agent 20 h prior to surgery to ablate existing leukocytes, and they received a perisurgical broad-spectrum antibiotic; both of these events likely markedly increased their susceptibility to Candida infection.
Candida spp. are routinely found in the environment and are not considered pathogenic in healthy animals other than on poorly ventilated areas of skin, including skin folds or flaps and auricular infections.12 In addition, Candida genitourinary infections are not commonly reported in any domestic animal species,4,7,25 but immunocompromised animals have increased susceptibility when exposure occurs.23 In humans, Candida albicans is a common commensal organism of skin and mucosal surfaces8 and does not typically cause infections in healthy persons. Candida infection occurs most commonly in humans with a predisposing condition, such as diabetes, therapy with antibiotics or immunosuppressive agents, very young or old age, and the use of intravenous or indwelling urinary catheters and in patients receiving critical care.14,30,36 Urinary tract infections with Candida are frequently nosocomial and often are associated with biofilms on urinary catheters; the organisms then are able to ascend the urinary tract, causing cystitis and, infrequently, pyelonephritis.13,27 In studies of nosocomial urinary tract infections, Candida was the etiologic agent in 12% to 21% of all infections evaluated.13 Factors that contribute to Candida’s ability to colonize the urinary tract and develop hyphal forms include acid pH and the presence of proteins.8 The critical role of neutrophils in the control of hyphal growth has been demonstrated for human neutrophils and is consistent with observations in mouse models of Candida infection.6
To our knowledge, this case report is the first to describe severe, ascending pyelonephritis with numerous Candida, resulting in systemic disease and acute mortality in an entire surgery cohort of humanized mice. A previous case report described opportunistic ascending urinary bacterial infections in NSG breeding colonies;9 the mice in that report were all healthy breeding animals that were not humanized or otherwise reported to have any additional predisposing factor to support infection. A report on pathology of aged NSG mice found Candida on the skin of mice enrolled in a fur mite study,26 but the mice in that study were housed in a nonbarrier facility and exposed to opportunistic pathogens. The origin of Candida in the present report is unknown, but regardless of origin, the organisms were able to induce significant mortality among these mice. This report underscores the importance of strict animal husbandry, appropriate disinfection and sanitization, and careful management of restraint devices used for severely immunocompromised mouse strains.
Acknowledgments
We thank Steven Soto and Massimo Vazzana for technical support provided in the assessment and management of the animals in the study and Dr Kathleen Gabrielson for consultation regarding study histopathology.
References
- 1.Anderson MS, Bluestone JA. 2005. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23:447–485. 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Ashman RB, Papadimitriou JM. 1987. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol Cell Biol 65:163–171. 10.1038/icb.1987.18. [DOI] [PubMed] [Google Scholar]
- 3.Brehm MA, Shultz LD, Greiner DL. 2010. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 17:120–125. 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Repentigny L. 2004. Animal models in the analysis of Candida host–pathogen interactions. Curr Opin Microbiol 7:324–329. 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. 2006. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb Pathog 40:82–90. 10.1016/j.micpath.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Duggan S, Leonhardt I, Hunniger K, Kurzai O. 2015. Host response to Candida albicans bloodstream infection and sepsis. Virulence 6:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel PLJ, Sobel JD. 1999. Murine models of Candida vaginal infections, p 741–748. In: Zak O, Sande M, Handbook of animal models of infection. London (United Kingdom): Academic Press. [Google Scholar]
- 8.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. 2011. Candida urinary tract infection: pathogenesis. Clin Infect Dis 52 Suppl 6:S437–S451. 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- 9.Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. 2010. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol 48:495–499. 10.1177/0300985810378282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulurija A, Ashman RB, Papadimitriou JM. 1996. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 142:3487–3496. 10.1099/13500872-142-12-3487. [DOI] [PubMed] [Google Scholar]
- 11.Gozalo AS, Hoffmann VJ, Brinster LR, Elkins WR, Ding L, Holland SM. 2010. Spontaneous Staphylococcus xylosus infection in mice deficient in NADPH oxidase and comparison with other laboratory mouse strains. J Am Assoc Lab Anim Sci 49:480–486. [PMC free article] [PubMed] [Google Scholar]
- 12.Huffnagle GB, Noverr MC. 2013. The emerging world of the fungal microbiome. Trends Microbiol 21:334–341. 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Finazzi Agro E. 2014. Nosocomial urinary tract infections: a review. Urologia 81:222–227. 10.5301/uro.5000092 [DOI] [PubMed] [Google Scholar]
- 14.Iatta R, Cafarchia C, Cuna T, Montagna O, Laforgia N, Gentile O, Rizzo A, Boekhout T, Otranto D, Montagna MT. 2013. Bloodstream infections by Malassezia and Candida species in critical care patients. Med Mycol 52:264–269. 10.1093/mmy/myt004. [DOI] [PubMed] [Google Scholar]
- 15.Kauffman CA, Fisher JF, Sobel JD, Newman CA. 2011. Candida urinary tract infections—diagnosis. Clin Infect Dis 52 Suppl 6:S452–S456. 10.1093/cid/cir111. [DOI] [PubMed] [Google Scholar]
- 16.Kikutani H, Makino S. 1992. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 51:285–322. 10.1016/S0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 17.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 4:1–10. 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. 1980. Breeding of a nonobese, diabetic strain of mice. Jikken Dobutsu 29:1–13. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST1. Nat Med 12:1316–1322. 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 21.Nobile CJ, Johnson AD. 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. 2003. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 18:41–51. 10.1016/S1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 23.Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JW, Joosten LA, Kurzai O, Netea MG. 2014. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J Immunol 44:2405–2414. 10.1002/eji.201343828. [DOI] [PubMed] [Google Scholar]
- 24.Ryley JF, Ryley NG. 1990. Candida albicans—do mycelia matter? J Med Vet Mycol 28:225–239. 10.1080/02681219080000291. [DOI] [PubMed] [Google Scholar]
- 25.Samaranayake YH, Samaranayake LP. 2001. Experimental oral candidiasis in animal models. Clin Microbiol Rev 14:398–429. 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santagostino SF, Arbona RJR, Nashat MA, White JR, Monette S. 2017. Pathology of aging in NOD SCIDγ female mice. Vet Pathol 54:855–869. 10.1177/0300985817698210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneeberger C, Holleman F, Geerlings SE. 2016. Febrile urinary tract infections: pyelonephritis and urosepsis. Curr Opin Infect Dis 29:80–85. 10.1097/QCO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 28.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise, and challenges. Nat Rev Immunol 12:786–798. 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis 192:336–343. 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 30.Stapleton A. 2002. Urinary tract infections in patients with diabetes. Am J Med 113 Suppl 1A:80–84. 10.1016/S0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 31.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 32.Suwanai H, Wilcox MA, Mathis D, Benoist C. 2010. A defective IL15 allele underlies the deficiency in natural killer cell activity in nonobese diabetic mice. Proc Natl Acad Sci USA 107:9305–9310. 10.1073/pnas.1004492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8:1313–1323. 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 34.Théraud M, Bedouin Y, Guiguen C, Gangneux JP. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J Med Microbiol 53:1013–1018. 10.1099/jmm.0.05474-0. [DOI] [PubMed] [Google Scholar]
- 35.Weaver JL, Boyne M, Pang E, Chimalakonda K, Howard KE. 2015. Nonclinical evaluation of the potential for mast cell activation by an erythropoietin analog. Toxicol Appl Pharmacol 287:246–252. 10.1016/j.taap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein RA, Lundstrom T, Sobel J. 2001. Nosocomial candiduria: a review. Clin Infect Dis 32:1602–1607. 10.1086/320531. [DOI] [PubMed] [Google Scholar]
- 37.Wilson D, Naglik JR, Hube B. 2016. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog 12:1–5. 10.1371/journal.ppat.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]