Abstract
Xenotransplantation can provide a solution to the current shortage of human organs for patients with terminal renal failure. The increasing availability of genetically engineered pigs, effective immunosuppressive therapy, and antiinflammatory therapy help to protect pig tissues from the primate immune response and can correct molecular incompatibilities. Life-supporting pig kidney xenografts have survived in NHP for more than 6 mo in the absence of markers of consumptive coagulopathy. However, few reports have focused on the physiologic aspects of life-supporting pig kidney xenografts. We have reviewed the literature regarding pig kidney xenotransplantation in NHP. The available data indicate (1) normal serum creatinine, (2) normal serum electrolytes, except for a trend toward increased calcium levels and a transient rise in phosphate followed by a fall to slightly subnormal values, (3) minimal or modest proteinuria without hypoalbuminemia (suggesting that previous reports of proteinuria likely were due to a low-grade immune response rather than physiologic incompatibilities), (4) possible discrepancies between pig erythropoietin and the primate erythropoietin receptor, and (5) significant early increase in kidney graft size, which might result from persistent effects of pig growth hormone. Further study is required regarding identification and investigation of physiologic incompatibilities. However, current evidence suggests that, in the absence of an immune response, a transplanted pig kidney likely would satisfactorily support a human patient.
Abbreviations: GTKO, α1,3-galactosyltransferase gene knockout; mAb, monoclonal antibody
The increasing number of patients in need of organ transplants has stimulated research into the use of pig organs for transplantation,12,13 but numerous immunologic and physiologic barriers must be overcome. These barriers have developed due to the evolutionary divergence of the species over more than 80 million years.28-30 The immunologic aspects of xenotransplantation have been studied in considerable detail, but except for discrepancies in coagulation,15,20,56 physiologic compatibility between these species has received little attention.31,36,63 However, physiologic incompatibilities may have important implications for the success of pig-to-human kidney xenotransplantation.
The major immune barriers to xenotransplantation have largely been overcome by genetically engineering organ-source pigs: (1) by deleting antigens against which humans have natural (preformed) antibodies, the most important of which is galactose-α1,3-galactose (Gal), and (2) by inserting human transgenes that help to protect the graft against human complement-mediated injury or human coagulation dysfunction, both of which contribute to graft failure (Figure 1). In addition, the pig antigens N-glycolylneuraminic acid (Neu5Gc) and Sda (β4GalNT2) are known to play a role in xenograft rejection (Figure 1). In addition, novel immunosuppressive agents, such as those that block T cell costimulation (that is, signal 2), are more successful in suppressing the adaptive immune response to xenografts than are the conventional pharmacologic agents that are used in current clinical allotransplantation and that suppress the recognition of donor antigens (that is, signal 1).
Figure 1.
Key advances in the genetic engineering of pigs
The transplantation of kidneys from genetically engineered pigs combined with effective immunosuppressive therapy has significantly increased the survival duration of life-supporting kidney grafts in NHP.32,33,38-40,46 For example, survival times of 160 and 310 d were achieved in 2 rhesus macaques after the transplantation of kidneys from α1,3-galactosyltransferase gene-knockout (GTKO) pigs (which lacked the major target for primate antipig antibodies, Gal) that also expressed the human complement-regulatory protein CD55 (which provides additional protection from antibody-dependent complement-mediated rejection; GTKO–CD55 pigs).32,33 Immunosuppressive therapy was based on the blockade of a T-cell costimulation molecule, CD154, by using a monoclonal antibody. The same group also reported the 499-d survival of another GTKO–CD55 pig kidney graft in a rhesus monkey.40 A rhesus macaque experienced extended survival (435 d) after the transplantation of a kidney from a GTKO–β4GalNT2-KO pig (that is, lacking both Gal and Sda).46 Our own group achieved prolonged survival (136, 237, and 260 d) of kidney grafts from pigs with multiple genetic modifications (‘6-gene’ pigs, although with different combinations of genetic modification) in 3 baboons that received antiCD40 monoclonal antibody as a costimulation blockade molecule.38,39 These prolonged survival times have allowed for observations regarding the function of pig kidneys in the ‘foreign’ NHP environment.
The aim of the present brief review is to compare the known similarities and differences in physiology between pigs and primates (including humans) and to discuss the feasibility of producing pigs whose kidneys are physiologically compatible with humans. Even if the pathobiologic problems of pig kidney xenotransplantation were overcome, clinical applications of this technology would remain unsuccessful unless the pig transplants are effective in performing the functions of a human kidney.
Are there physiologic incompatibilities between pig and primate?
Pig kidneys are similar in structure, physiology (for example, glomerular filtration rate and total kidney blood flow), and relative size to human kidneys,6,12,41 but physiologic incompatibilities may arise.36,64 The kidneys are located in the highest part of the pig's body, which might influence renal blood flow; however, the renal blood flow of pigs is reported to be similar to that of humans.57
Mean WBC and RBC counts are significantly (P < 0.01) higher in healthy pigs than in baboons and humans (Table 1).22 Whereas BUN, serum creatinine, sodium, and chloride values are comparable among all 3 species, pigs exhibit higher (P < 0.01) potassium, calcium, and phosphorus values than baboons and humans (Table 1).22 Levels of total protein and albumin appear to be significantly (P < 0.01) lower in pigs than in baboons and humans (Table 1).22
Table 1.
Normal hematology and clinical chemistry values in pigs, baboons, and humans22
| Pigs (mean ± 1 SD) | Baboons (mean ± 1 SD) | Humans (range) | ||
| Hematology | ||||
| WBC (no./mm3) | 18.6 ± 6.4a | 10.0 ± 1.7 | 3.8–10.6 | |
| RBC (×106/mm3) | 6.9 ± 1.1a | 5.1 ± 0.1 | 3.73–4.89 | |
| Hgb (g/dL) | 10 ± 1.4 | 12.7 ± 0.3 | 11.6–14.6 | |
| Hct (%) | 37.9 ± 8.7b | 39.9 ± 1.3 | 34.1–43.3 | |
| Platelets (×103/mm3) | 507 ± 156 | 419 ± 61 | 156–369 | |
| Clinical chemistry | ||||
| BUN (mg/dL) | 12.4 ± 5.1 | 14.3 ± 4.0 | 8.0–26.0 | |
| Creatinine (mg/dL) | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.5–1.4 | |
| Sodium (mmol/L) | 143 ± 4.0 | 146 ± 2.0 | 136–146 | |
| Potassium (mmol/L) | 5.3 ± 1.0a | 3.8 ± 0.5 | 3.5–5.0 | |
| Chloride (mmol/L) | 105 ± 1.9 | 108 ± 4.0 | 95–110 | |
| Calcium (mg/dL) | 11.0 ± 0.8a | 9.5 ± 0.5 | 8.4–10.2 | |
| Phosphorus (mg/dL) | 8.6 ± 3.2a | 4.3 ± 1.8 | 2.5–4.5 | |
| Total protein (g/dL) | 5.2 ± 1.1a | 6.8 ± 0.3 | 6.3–7.7 | |
| Albumin (g/dL) | 2.6 ± 0.6a | 4.1 ± 0.3 | 3.4–5.0 |
Significant (P < 0.01) difference between pigs compared with baboons and humans
Significant (P < 0.01) difference between pigs compared with baboons
Few data are available from experimental studies in pig-to-NHP models of kidney transplantation, but the following comments can be made in regard to specific aspects of renal function.
Serum creatinine.
In reports of life-supporting pig kidney xenotransplantation in NHP extending back more than a decade, the serum creatinine level essentially remained stable and largely within the normal human range (Figure 2 A)16,32,38,39,70 but in early studies was higher than the normal range.64 In view of the improved protection from immune-mediated injury provided by current genetically engineered pig kidneys, these data indicate that a persistently high creatinine is more likely to have been the result of immune-related graft dysfunction rather than of physiologic incompatibility, which is no longer seen.
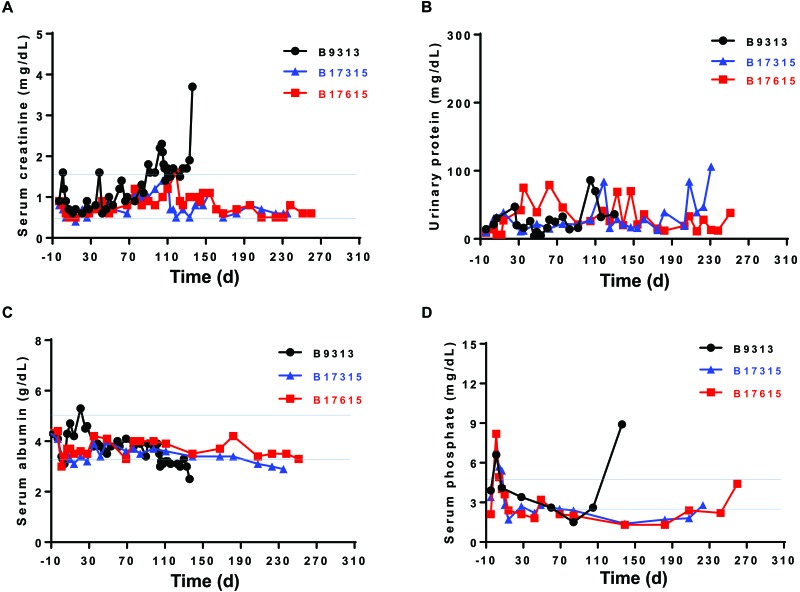
Figure 2.
(A) Serum creatinine, (B) urinary protein, (C) serum albumin, and (D) serum phosphate levels in 3 baboons (nos. 9313, 17315, and 17615) whose genetically engineered pig kidney grafts functioned for 136, 237, and 260 d, respectively, before infectious complications necessitated termination of the experiments. Blue lines indicate the normal range of serum creatinine in humans. Reprinted with permission from reference 39.
However, we have observed that baboons with pig kidney grafts intermittently develop features of hypovolemia and dehydration (that is, transient increases in serum creatinine) in the absence of features of immune rejection. The infusion of normal saline reduces serum creatinine levels to the normal human range. We have now documented this phenomenon in 4 consecutive baboon recipients at various time points after pig kidney transplantation. The baboons remained fully alert and active, maintained a steady body weight, and did not drink or urinate more or less than usual, yet, when examined, had a low central venous pressure and dehydrated skin and tissues. The intravenous infusion of several hundred milliliters (approximately 100 mL/kg) of normal saline was required to raise the venous pressure of these animals to a normal level. Alternatively, we have administered saline subcutaneously on a daily basis to maintain our NHP recipients in a well-hydrated state. A possible cause of this intermittent phenomenon is discussed in the section Plasma renin.
Serum proteins.
Proteinuria, even in small amounts, is considered pathologic (for example, in glomerulopathies and allograft rejection), because the human glomerular membrane normally is impermeable to proteins.47 In contrast, in adult pigs, environmental and physical stresses can readily induce increased proteinuria.24,35 Furthermore, proteinuria occurs in healthy neonatal pigs, where it is associated with immature proximal renal tubules.2,5 Proteinuria is, therefore, a feature of renal dysfunction, but it may be related to causes other than graft rejection.
In the early studies of pig-to-NHP kidney xenotransplantation, moderate to severe proteinuria and hypoalbuminemia were documented, necessitating frequent intravenous administration of human albumin to maintain the serum albumin concentration within the normal range.3,7,17,64,70 In another study, proteinuria and hypoalbuminemia occurred in NHP recipients at levels consistent with nephrotic syndrome, despite normal renal histology.70
Our own more recent experience38,39 and that of other colleagues32 is of minimal or modest proteinuria (Figure 2 B), with no accompanying hypoalbuminemia (Figure 2 C)—probably because the recipient's immune response was well controlled due to genetic modification of the donor pig and because of pharmacologic intervention. This modest proteinuria was associated with prolonged graft survival in both studies.32,38,39 In another study of pig-to-NHP kidney xenotransplantation, despite normal renal function, recipients developed proteinuria with morphologic changes (podocyte effacement).66 However, treatment with the antiCD20 (B cell) monoclonal antibody rituximab successfully delayed the development of proteinuria, possibly due to the prevention of pig podocyte disruption.66 Although changes in serum creatinine and protein or albumin can be associated with changes in the recipient's body weight, none of our 3 baboons lost weight (data not shown), suggesting that loss of protein mass was not a feature in the lack of proteinuria.
These observations suggest that the proteinuria reported in early studies was due to a low-grade immune response (for example, activation or injury of the vascular endothelium by complement or antipig antibodies) rather than from physiologic incompatibilities between pig and primate.
Serum electrolytes.
Studies of pig-to-NHP kidney xenotransplantation have indicated that most serum electrolytes (for example, sodium, chloride, potassium, calcium) remain within normal limits in recipients with well-functioning pig kidney grafts, although serum calcium can rise.11,32,36,38,39,64 Serum phosphate can rise transiently immediately after transplantation, but thereafter falls and remains in the low-to-normal range;32,36,38,39,64 this effect may be due to the slightly greater glomerular filtration rate in pigs . However, kidney allotransplantation in NHP has likewise been associated with a reduction in serum phosphate (Figure 2 D).58 The cause of decreased phosphate levels during the period of stable pig xenograft function is uncertain.
Serum uric acid.
In humans, uric acid is an end-product of purine metabolism, but in lower mammals, including pigs, it is further oxidized by urate oxidase.51 Hyperuricemia is unlikely to be problematic in pig kidney xenotransplantation, because pig kidneys can eliminate uric acid both by filtration and secretion.62 This added function of pig kidneys should prove an advantage over human kidneys, which cannot oxidize uric acid.
Plasma renin.
Renin (produced by the kidney),27 cleaves angiotensinogen (produced by the liver) to angiotensin I, which is further cleaved to angiotensin II, which regulates body fluid volume and potassium balance. Pig renin cannot cleave human angiotensinogen.59,68 The relatively normal fluid balance and maintenance of body weight in NHP with well-functioning life-supporting pig kidney grafts32,38,39,64 may indicate an alternative regulatory mechanism to maintain fluid balance despite decreased renin activity (for example, antidiuretic hormone).
Alternatively, the state of intermittent hypovolemia or dehydration that we have documented and that results in an increase in serum creatinine (see earlier) may be associated with abnormalities of renin function. Baboons with pig kidney grafts apparently are unaware that they are becoming dehydrated, because their fluid intake does not match their clinical need (even though their urinary output isn't excessive). Patients with pig kidney grafts, therefore, may need to drink large volumes of fluid, even though they do not feel thirsty.
Erythropoietin.
The amino-acid identity between pig and human erythropoietin (produced by the kidney) is approximately 82%.18,69 Life-supporting pig-to-NHP renal xenotransplantation was associated with the gradual development of normocytic, normochromic anemia in the absence of treatment with recombinant human erythropoietin.64 This effect possibly resulted from molecular incompatibility between pig erythropoietin and the primate erythropoietin receptor.64 Alternatively, drug-associated myelosuppression combined with frequent blood draws for laboratory tests might have induced the observed anemia.
Whether pig erythropoietin functions adequately in humans or NHP is unclear. In our own studies, we relatively frequently draw considerable volumes of blood from pharmacologically myelosuppressed recipient NHP; we therefore routinely administer recombinant human erythropoietin to recipient baboons and have maintained a stable hematocrit. However, even if pig erythropoietin does not adequately function in humans, the administration of recombinant human erythropoietin should resolve any deficiency, as it does in NHP. Furthermore, an alternative solution is to genetically engineer pigs to produce human erythropoietin, which thus would interact with the human erythropoietin receptors of recipients.
Kidney size and growth.
Early studies reported an increase in kidney graft size (Figure 3).63 We observed a similar increase in our recent studies, but in every case a partial stricture of the ureter had developed; this stricture was not always related to the ureterovesical anastomosis and therefore may have been due to the immune response, thus potentially complicating interpretation of the increase in graft size.38,39 In addition, relief of the partial stricture achieved only a minimal reduction in pig kidney size.38,39 Other investigators have not mentioned an increase in kidney graft size,16,32,61,71 nor has rapid growth been reported after heterotopic pig heart xenotransplantation in baboons.37,48,49
Figure 3.
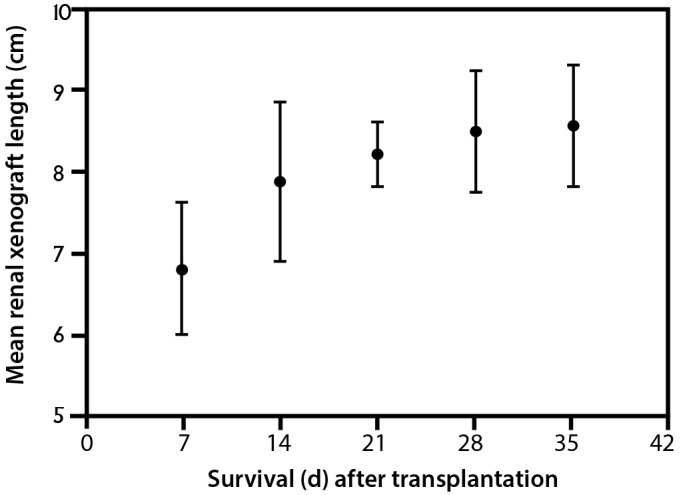
Growth of pig kidney grafts in cynomolgus monkey recipients (n = 6). Based on the data from reference 63.
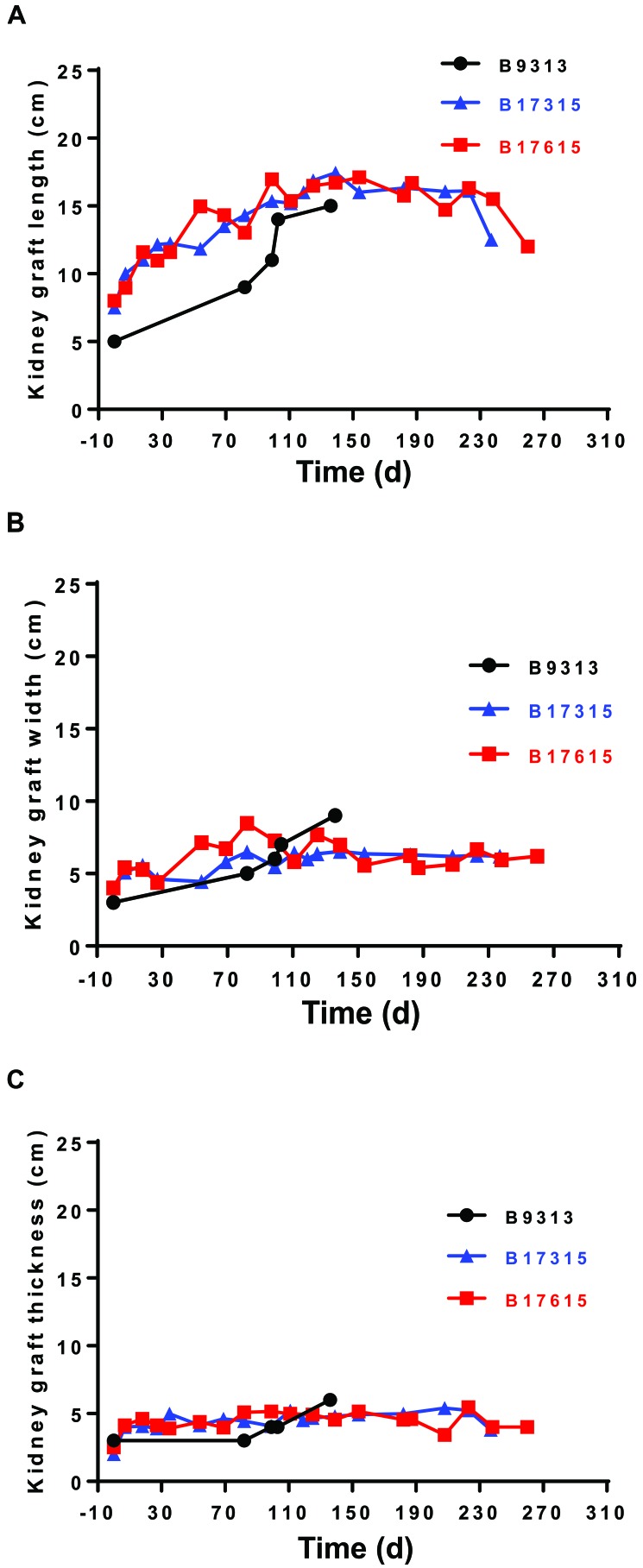
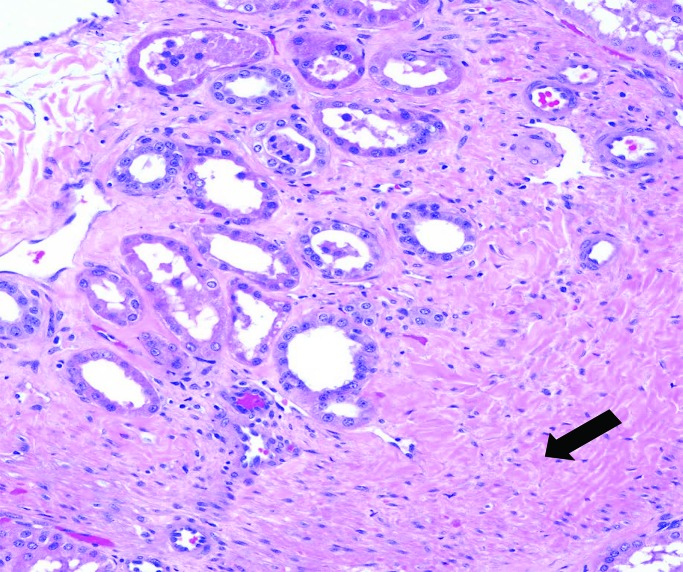
In our recent report, pig kidney grafts increased markedly in length (at least 2-fold; less so in other dimensions) during the first 1 to 2 mo, after which growth slowed and then plateaued (Figure 4 A through C).38,39 This time-scale correlates relatively well with the original observation,63 although kidney graft growth in that study plateaued slightly sooner than in ours. The changes in our animals38,39 were not a result of an acute cellular or antibody-mediated immune response, when interstitial hemorrhage, thrombosis, and cellular infiltrates might increase graft weight and size, because histology of the grafts showed no features of rejection (Figure 5) but revealed considerable interstitial expansion and fibrosis.
Figure 4.
Increases in the (A) length, (B) width, and (C) thickness of the pig kidneys in 3 baboons (nos. 9313, 17315, and 17615) with genetically-engineered pig kidney grafts that functioned for 136, 237, and 260 d, respectively; all of the experiments were terminated due to infectious complications.
Figure 5.
Microscopic appearance of a genetically engineered pig kidney graft in a baboon at necropsy (at 260 d after transplantation), showing expansion of the interstitial tissue, with some fibrosis (arrow), but no features of rejection. Reprinted with permission from reference 39.
The cause of the initial rapid growth in size (which in our experience seemed disproportionate to the effect of a partial stricture of the ureter) remains uncertain. This effect might be associated with compensatory enlargement when one immature kidney (taken from a 6- to 8-wk-old pig) takes the place of 2 relatively mature kidneys excised from an 8-kg baboon. Relatively rapid growth in kidney allografts occurs when kidneys from infants or children are transplanted into adult recipients.26,52
However, there is a recognized discrepancy in the rate of organ growth between pig and NHP. Pigs (and pig organs) grow at a much faster rate than primates,63 with Yorkshire swine reaching a body weight of more than 50 kg by 6 mo of age (Figure 6),8,72 whereas baboons, rhesus monkeys, and cynomolgus monkeys (all Old World NHP) are unlikely to weigh more than 4 to 6 kg at that age.1,9,43
Figure 6.
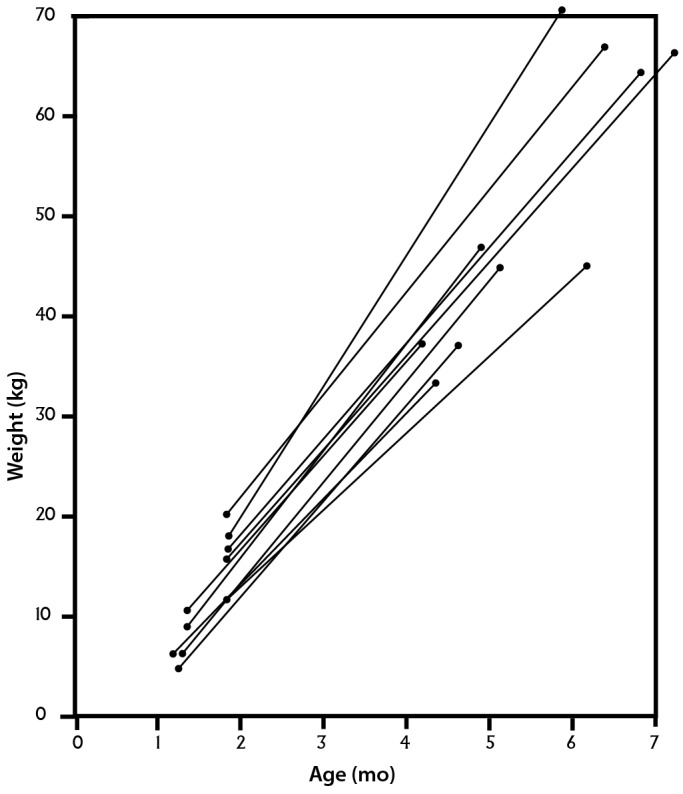
Increase in weight of healthy Yorkshire pigs during the first 6 mo of life. Reprinted with permission from reference 72.
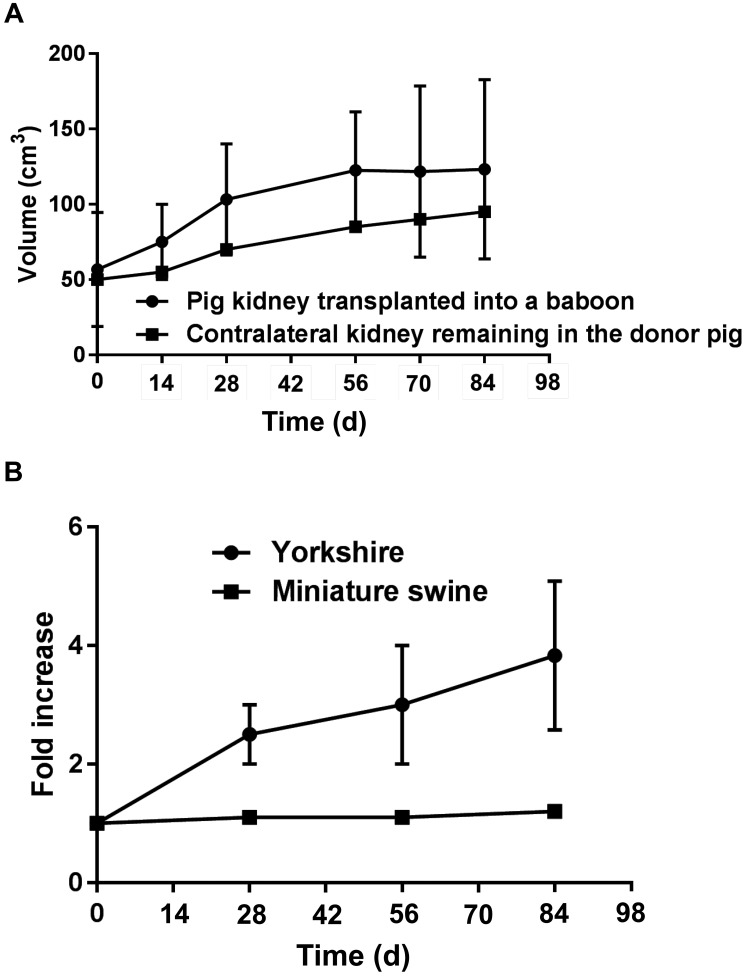
Recently, growth of a transplanted miniature swine kidney after GTKO pig-to-baboon kidney xenotransplantation (n = 6) was similar to that of the contralateral kidney that was retained in the miniature swine donor, which was maintained to measure autologous kidney growth (Figure 7 A).65 Furthermore, in a pig allotransplantation model (n = 5), a Yorkshire pig kidney graft exhibited rapid growth after transplantation into a miniature swine, reaching 3.7 times its initial volume over 3 mo. This growth rate compared with an increase in volume of only 1.2 times over the same period when a miniature swine kidney was transplanted into another miniature swine (Figure 7 B).65
Figure 7.
(A) The growth of a miniature swine pig kidney graft transplanted into a baboon was similar to that of the contralateral kidney, which was retained in the donor. The graft in the baboon increased in volume by 2.1-fold, whereas the kidney that remained in the donor increased 1.6-fold, suggesting maintenance of a growth stimulus after transplantation. Longitudinal data obtained from 3 of 6 baboons with pig kidney grafts formed the basis of this study. Based on data from reference 65. (B) Growth of a Yorkshire pig kidney graft compared with that of a miniature swine kidney graft during the first 3 mo after transplantation into miniature swine recipients. The volume of the graft from the Yorkshire pig increased 3.7-fold, whereas that from the miniature swine increased only 1.2-fold. Based on data from reference 65.
One group60,65 has suggested that the rapid growth of pig kidney xenografts in small baboons is largely associated with intrinsic (that is, graft-associated) factors rather than with factors in the recipient environment. From our own observations, we agree with this conclusion, even though partial ureteric stricture may have played a minor role. The other authors suggested that rapid growth may result in deterioration of renal function due to a form of ‘abdominal compartment syndrome.’ That is, the rapid growth of the kidney in the confined space of the abdomen may have led to insufficient intragraft blood flow, leading to cortical ischemia and graft dysfunction. This effect might be associated with a rise in serum creatinine without evidence of rejection. However, although we have seen a rapid growth of the pig kidney, we have not observed any persistent decrease in its function.
Although the intermittent dehydration (discussed earlier) might represent transient impairment of function, it is rapidly and entirely reversible. By increasing the circulating blood volume through the administration of intravenous or subcutaneous fluid, the intragraft blood flow can be improved, at least temporarily, resulting in a reduction in serum creatinine. We think it unlikely that abdominal compartment syndrome would be so easily reversible. Together, these studies suggest that the pig kidney graft continues to grow at its normal rate (as it would in the native donor pig) for several weeks after transplantation into an NHP. Thereafter, its growth correlates with the growth rate of the recipient NHP. The mechanism behind this sequence of events remains uncertain. Although rapid growth of a pig kidney in the abdomen of the recipient may not be problematic, rapid growth of a pig heart within the confines of the recipient's chest would likely restrict function.
In view of the observations regarding the initial rapid growth of the transplanted kidneys, it could be argued that all grafts for clinical xenotransplantation should be from miniature swine (of which there are several different breeds that reach adult sizes ranging from 30 to 200 kg), rather than from domestic pigs, such as Large White Landrace. However, several points require consideration. First, miniature swine grow more slowly than domestic pigs; therefore, if an organ is required for a large human recipient, miniature swine would need to be housed for a much longer period, thus adding to logistics and cost. Second, some miniature swine do not grow to the size required to provide an organ sufficient for a large human adult. Third, domestic swine are currently available that carry 6 genetic manipulations, and it might be easier to inhibit the growth of these pigs by further genetic manipulation (discussed later) than continue to genetically engineer miniature swine, in which this field is less advanced. Nevertheless, with further experimental experience, a case might be made that miniature swine may become advantageous in this regard. In a recent study,34 inactivation of the growth hormone receptor gene in pigs led to markedly decreased levels of insulin-like growth factor 1 and thus to decreased body and organ growth. This group is inactivating the growth hormone receptor gene in GTKO/hCD46/hThrombomodulin pigs to reduce the growth of the pig heart after orthotopic cardiac xenotransplantation in baboons.34
Differences between pig and primate growth hormone may be a critical factor for organ growth after transplantation. One group reported that pig growth hormone does not activate human growth hormone receptors due to amino acid differences in growth hormone structure between the 2 species.4 In contrast, human growth hormone has a biologic effect in pigs.55 Perhaps residual pig growth hormone is present in the graft after transplantation and leads to the initial increase in size (as it would if the kidney had remained in the rapidly growing juvenile pig). This rapid growth may cease when the residual pig growth hormone is depleted. The subsequent more gradual increase in pig kidney graft size might be associated with the effect of human (or NHP) growth hormone. Further investigation of these observations and of this hypothesis is required.
Although both growth hormone (produced by the anterior pituitary gland) and insulin-like growth factor 1 are growth stimulants for many organs, including kidney,10,21,67 a key question is why these hormones might be retained after transplantation in the kidney but not the heart, which, in the abdominal heterotopic position, apparently does not continue to grow rapidly.37,48-50 The fact that the kidney grafts are life-supporting, whereas heterotopic (that is, abdominally placed) heart grafts do not support a vascular circulatory system (and therefore are not fully functional), may be relevant.19 Recently, survival after orthotopic heart transplantation (in which the heart supports the entire circulation) has been extended to 90 d, during which the heart graft grew abnormally rapidly.19,34
In summary, pigs might be a solution to the problem of organ donor shortage. Recent progress in the survival of kidneys transplanted from genetically engineered pigs into NHP that were supported with effective immunosuppressive regimens and antiinflammatory therapy indicates that the immunologic barriers are being overcome and that clinical trials of pig kidney xenotransplantation are likely to be considered soon.14 Although few to date, most experimental data from pig-to-NHP models of kidney xenotransplantation imply that the pig kidney will function satisfactorily within a human recipient, provided that the patient's hydration and volemic states are monitored carefully. However, further evaluation of the ability of pig erythropoietin to maintain adequate erythrocyte numbers in primate recipients is required. Questions also remain regarding the cause of the rapid growth of pig kidneys early after transplantation and whether this phenomenon will be problematic when pig kidneys are transplanted into humans.
Acknowledgments
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
References
- 1.Andrade MC, Ribeiro CT, Silva VF, Molinaro EM, Gonçalves MA, Marques MA, Cabello PH, Leite JP. 2004. Biologic data of Macaca mulatta, Macaca fascicularis, and Saimiri sciureus used for research at the Fiocruz Primate Center. Mem Inst Oswaldo Cruz 99:581–589 10.1590/S0074-02762004000600009. [DOI] [PubMed] [Google Scholar]
- 2.Baintner K, Kerenyi T, Cseplo A. 1989. Tubular reabsorption of protein by porcine kidneys during neonatal alimentary proteinuria. Acta Vet Hung 37:69–74. [PubMed] [Google Scholar]
- 3.Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, Boldrin M, Seveso M, Dall'Olmo L, Frison L, De Benedictis G, Bernardini D, Thiene G, Cozzi E, Ancona E. 2004. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant 4:475–481 10.1111/j.1600-6143.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 4.Behncken SN, Rowlinson SW, Rowland JE, Conway-Campbell BL, Monks TA, Waters MJ. 1997. Aspartate 171 is the major primate-specific determinant of human growth hormone. Engineering porcine growth hormone to activate the human receptor. J Biol Chem 272:27077–27083 10.1074/jbc.272.43.27077. [DOI] [PubMed] [Google Scholar]
- 5.Bergelin IS, Karlsson BW. 1975. Functional structure of the glomerular filtration barrier and the proximal tubuli in the developing foetal and neonatal pig kidney. Anat Embryol (Berl) 148:223–234 10.1007/BF00319845. [DOI] [PubMed] [Google Scholar]
- 6.Breimer ME, Svalander CT, Haraldsson B, Björck S. 1996. Physiological and histological characterisation of a pig kidney in vitro perfusion model for xenotransplantation studies. Scand J Urol Nephrol 30:213–221 10.3109/00365599609181302. [DOI] [PubMed] [Google Scholar]
- 7.Bühler L, Yamada K, Alwayn I, Kitamura H, Basker M, Barth RN, Appel J, Awwad M, Thall A, White-Scharf ME, Sachs DH, Cooper DK. 2001. Miniature swine and hDAF pig kidney transplantation in baboons treated with a nonmyeloablative regimen and CD154 blockade. Transplant Proc 33:716 10.1016/S0041-1345(00)02220-X. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Kaiser MS, Dekkers JC. 2012. Bayesian analysis of the effect of selection for residual feed intake on growth and feed intake curves in Yorkshire swine. J Anim Sci 90:127–141 10.2527/jas.2011-4293. [DOI] [PubMed] [Google Scholar]
- 9.Choi K, Chang J, Lee MJ, Wang S, In K, Galano-Tan WC, Jun S, Cho K, Hwang YH, Kim SJ, Park W. 2016. Reference values of hematology, biochemistry, and blood type in cynomolgus monkeys from Cambodian origin. Lab Anim Res 32:46–55 10.5625/lar.2016.32.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cingel-Ristić V, Flyvbjerg A, Drop SL. 2004. The physiological and pathophysiological roles of the GH–IGF axis in the kidney: lessons from experimental rodent models. Growth Horm IGF Res 14:418–430 10.1016/j.ghir.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AJ, Larson TS, Dean P, Logan J, Diamond L, McGregor CG, Stegall MD. 2001. Renal physiology in pig-to-baboon xenografts. Transplant Proc 33:727–728 10.1016/S0041-1345(00)02226-0. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DKC, Ye Y, Rolf LL, Jr, Zuhdi N. 1991. The pig as potential organ donor for man, p 481–500. In: Cooper DKC, Kemp R, Reemtsma K, White DJG, Xenotransplantation, 1st ed. Heidelberg (Germany):Springer; 10.1007/978-3-642-97323-9_30 [DOI] [Google Scholar]
- 13.Cooper DKC, Gollackner B, Sachs DH. 2002. Will the pig solve the transplantation backlog? Annu Rev Med 53:133–147 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DKC, Wijkstrom M, Hariharan S, Chan JL, Singh A, Horvath K, Mohiuddin M, Cimeno A, Barth RN, LaMattina JC, Pierson RN., 3rd 2017. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation 101:1551–1558 10.1097/TP.0000000000001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan PJ, Robson SC, d'Apice AJ. 2011. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant 16:214–221 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cozzi E, White DJ. 1995. The generation of transgenic pigs as potential organ donors for humans. Nat Med 1:964–966 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 17.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, Bradley JR, Thiru S, Davies HF, Wallwork J, White DJG, Goddard M, Friend PJ. 2003. Maintenance triple immunosuppression with cyclosporine A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation 10:300–310 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 18.David RB, Blom AK, Sjaastad OV, Harbitz I. 2001. The porcine erythropoietin gene: cDNA sequence, genomic sequence, and expression analysis in piglets. Domest Anim Endocrinol 20:137–147 10.1016/S0739-7240(01)00089-3. [DOI] [PubMed] [Google Scholar]
- 19.Denner J, Reichart B, Längin M. 2018. Does size matter? Xenotransplantation 25:e12383; Epub ahead of print 10.1111/xen.12383. [DOI] [PubMed] [Google Scholar]
- 20.Dorling A, Lechler RI. 2001. Disordered thromboregulation after xenografting. Curr Opin Organ Transplant 6:36–41 10.1097/00075200-200103000-00007. [DOI] [Google Scholar]
- 21.Ece A, Çetinkaya S, Ekşioğlu S, Şenel S, Özkasap S, Giniş T, Sen V, Şahin C. 2014. Kidney growth and renal functions under the growth hormone replacement therapy in children. Ren Fail 36:508–513 10.3109/0886022X.2013.875834. [DOI] [PubMed] [Google Scholar]
- 22.Ekser B, Bianchi J, Ball S, Iwase H, Walters A, Ezzelarab M, Veroux M, Gridelli B, Wagner R, Ayares D, Cooper DK. 2015. Comparison of hematologic, biochemical, and coagulation parameters in α1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and 4 primate species. Xenotransplantation 19:342–354. Available at https://www.ncbi.nlm.nih.gov/pubmed/23145497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J. 2015. Evaluation of human and nonhuman primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 22:194–202 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehlhaber K, Krüger G, Schubert V, Rolletschek H. 1989. [[Urinary protein level determination as a rapid method for the detection of contamination of slaughtered swine.]] Arch Exp Veterinarmed 43:855–862 [[Article in German]]. [PubMed] [Google Scholar]
- 25.Fodor WL, Williams BL, Matis LA, Madri JA, Rollins SA, Knight JW, Velander W, Squinto SP. 1994. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci USA 91:11153–11157 10.1073/pnas.91.23.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foss A, Line PD, Brabrand K, Midtvedt K, Hartmann A. 2007. A prospective study on size and function of paediatric kidneys (<10 years) transplanted to adults. Nephrol Dial Transplant 22:1738–1742 10.1093/ndt/gfm080. [DOI] [PubMed] [Google Scholar]
- 27.Hackenthal E, Paul M, Ganten D, Taugner R. 1990. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70:1067–1116 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 28.Hammer C. 1989. Evolutionary considerations in xenotransplantation. p 115–125. Proceedings of the International Congress, Xenograft 25, Arden House, Harriman, New York, 11–13 November 1988. In: Hardy MA, Xenograft 25. Amsterdam (Netherlands): Elsevier. [Google Scholar]
- 29.Hammer C. 1991. Evolutionary, physiological, and immunological considerations in defining a suitable donor for man, p 429–438.In: Cooper DKC, Kemp E, Reemtsma K, White DJG, Xenotransplantation, 1st ed. Heidelberg (Germany): Springer; 10.1007/978-3-642-97323-9_27 [DOI] [Google Scholar]
- 30.Hammer C. 1997. Evolutionary obstacles to xenotransplantation, p 716–735. In: Cooper DKC, Kemp E, Platt JL, White DJG, Xenotransplantation: the transplantation of organs and tissues between species, 2nd ed. Heidelberg (Germany): Springer. [Google Scholar]
- 31.Hammer C. 1998. Physiological obstacles after xenotransplantation. Ann N Y Acad Sci 862:19–27 10.1111/j.1749-6632.1998.tb09113.x. [DOI] [PubMed] [Google Scholar]
- 32.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, Ford ML, Lutz AJ, Tector M, Newell KA, Tector AJ, Adams AB. 2015. Pretransplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-toprimate kidney transplant model. Xenotransplantation 22:221–230 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higginbotham L, Kim S, Mathews D, Stephenson A, Breeden C, Larsen C, Ford M, Newell K, Tector A, Adams A, [Internet] 2016. Late renal xenograft failure is antibody-mediated: description of the longest-reported survival in pig-to-primate renal xenotransplantation. [Cited 25 July 2018]. Available at: https://atcmeetingabstracts.com/abstract/late-renal-xenograft-failure-is-antibody-mediated-description-of-the-longest-reported-survival-in-pig-to-primate-renal-xenotransplantation/.
- 34.Hinrichs A, Klymiuk N, Reichart B, Kessler B, Kurome M, Bähr A, Dahlhoff M, Wolf E. 2017. Inactivation of the GHR gene—a strategy to overcome excess growth of orthotopic pig-to-baboon cardiac xenografts? Xenotransplantation 24: 39–40. Abstract (#O6.2). [Google Scholar]
- 35.Hohage H, Kleyer U, Brückner D, August C, Zidek W, Spieker C. 1997. Influence of proteinuria on long-term transplant survival in kidney transplant recipients. Nephron 75:160–165 10.1159/000189525. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. 2006. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation 13:488–499 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 37.Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, Klein E, Wagner R, Long C, Thacker J, Li J, Zhou H, Jiang M, Nagaraju S, Zhou H, Veroux M, Bajona P, Wijkstrom M, Wang Y, Phelps C, Klymiuk N, Wolf E, Ayares D, Cooper DK. 2015. Pig-to- baboon heterotopic heart transplantation—exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of 3 costimulation blockade-based regimens. Xenotransplantation 22:211–220 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, Ezzelarab M, Long C, Klein E, Wagner R, Phelps C, Ayares D, Shapiro R, Humar A, Cooper DK. 2015. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 22:302–309 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, Liu H, Long C, Wang Y, Cassano A, Klein E, Phelps C, Ayares D, Humar A, Wijkstrom M, Cooper DKC. 2017. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 24:1–31 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Higginbotham LB, Mathews DV, Breeden C, Dong Y, Stephenson A, Larsen CP, Ford M, Tector J, Adams AB. 2017. CD4 depletion is necessary and sufficient for long-term pig-to-nonhuman primate renal xenotransplant survival. Abstract presented at the 2017 American Transplant Congress, Chicago, Illinois, 29 April–3 May 2017. Am J Transplant 17 Suppl 3. Abstract 479. [Google Scholar]
- 41.Kirkman RL. 1989. Of swine and men: organ physiology in different species, p 125–131. In: Hardy MA, Xenograft 25. Amsterdam (Netherlands): Elsevier. [Google Scholar]
- 42.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. 2002. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089–1092. 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 43.Leigh S. 2009. Growth and development of baboons, p 57–88. Chapter 4. In: VandeBerg JL, Williams-Blangero S, Tardif SD, The baboon in biomedical research. Heidelberg (Germany): Springer; DOI: 10.1007/978-0-387-75991-3_4. [DOI] [Google Scholar]
- 44.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, Regensburg M, Duffield M, French AJ, Williams L, Baker L, Brandon MR, Xing PX, Kahn D, McKenzie IF. 2004. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in nonimmunosuppressed baboons. Xenotransplantation 11:171–183 10.1046/j.1399-3089.2003.00103_11_2.x. [DOI] [PubMed] [Google Scholar]
- 45.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, Yin F, Blankenship RL, Paris LL, Tector AJ. 2013. Double-knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 20:27–35 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 46.Martens G, Estrada J, Sidner RA, Butler JR, Reyes L, Ladowski J, Kim S, Tector M, Adams A, Tector AJ. 2017. Porcine GGTA1/β4GalNT2 gene knockout reduces antibody binding and achieves 1-y life-supporting renal xenograft in pig-to-rhesus model. Xenotransplantation 24:12–15. [Google Scholar]
- 47.Miltényi M. 1979. Urinary protein excretion in healthy children. Clin Nephrol 12:216–221. [PubMed] [Google Scholar]
- 48.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, Eckhaus MA, Seavey C, Ayares D, Pierson RN, 3rd, Horvath KA. 2012. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant 12:763–771 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, Eckhaus M, Reimann KA, Klymiuk N, Wolf E, Ayares D, Horvath KA. 2013. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 14:488–489 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3rd, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson RN, 3rd, Belli AJ, Wolf E, Klymiuk N, Phelps C, Reimann KA, Ayares D, Horvath KA. 2016. Chimeric 2C10R4 antiCD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 7:1–10 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oda M, Satta Y, Takenaka O, Takahata N. 2002. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 19:640–653 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 52.Pape L, Hoppe J, Becker T, Ehrich JH, Neipp M, Ahlenstiel T, Offner G. 2006. Superior long-term graft function and better growth of grafts in children receiving kidneys from paediatric compared with adult donors. Nephrol Dial Transplant 21:2596–2600 10.1093/ndt/gfl119. [DOI] [PubMed] [Google Scholar]
- 53.Petersen B, Ramackers W, Tiede A, Lucas-Hahn A, Herrmann D, Barg-Kues B, Schuettler W, Friedrich L, Schwinzer R, Winkler M, Niemann H. 2009. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation 16:486–495 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 54.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. 2003. Production of α 1,3-galactosyltransferase-deficient pigs. Science 299:411–414 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pursel VG, Rexroad CE, Jr, Bolt DJ, Miller KF, Wall RJ, Hammer RE, Pinkert CA, Palmiter RD, Brinster RL. 1987. Progress on gene transfer in farm animals. Vet Immunol Immunopathol 17:303–312 10.1016/0165-2427(87)90149-8. [DOI] [PubMed] [Google Scholar]
- 56.Robson SC, Cooper DK, d'Apice AJ. 2000. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 7:166–176 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 57.Sachs DH. 1994. The pig as a potential xenograft donor. Vet Immunol Immunopathol 43:185–191 10.1016/0165-2427(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 58.Schuurman HJ, Menninger K, Audet M, Kunkler A, Maurer C, Vedrine C, Bernhard M, Gaschen L, Brinkmann V, Quesniaux V. 2002. Oral efficacy of the new immunomodulator FTY720 in cynomolgus monkey kidney allotransplantation, given alone or in combination with cyclosporine or RAD. Transplantation 74:951–960 10.1097/00007890-200210150-00009. [DOI] [PubMed] [Google Scholar]
- 59.Sen S, Hirawawa K, Smeby RR, Bumpus FM. 1971. Measurement of plasma renin substrate using homologous and heterologous renin. Am J Physiol 221:1476–1480. [DOI] [PubMed] [Google Scholar]
- 60.Shah JA, Tanabe T, Yamada K. 2017. Role of intrinsic factors in the growth of transplanted organs following transplantation. J Immunobiol 2:122 [doi: 10.4172/2476-1966.1000122.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, Sachs DH, Colvin RB. 2005. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to- baboon kidney xenografts. J Am Soc Nephrol 16:2732–2745 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 62.Simmonds HA, Hatfield PJ, Cameron JS, Cadenhead A. 1976. Uric acid excretion by the pig kidney. Am J Physiol 230:1654–1661. [DOI] [PubMed] [Google Scholar]
- 63.Soin B, Ostlie D, Cozzi E, Smith KG, Bradley JR, Vial C, Masroor S, Lancaster R, White DJ, Friend PJ. 2000. Growth of porcine kidneys in their native and xenograft environment. Xenotransplantation 7:96–100 10.1034/j.1399-3089.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 64.Soin B, Smith KG, Zaidi A, Cozzi E, Bradley JR, Ostlie DJ, Lockhart A, White DJ, Friend PJ. 2001. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int 60:1592–1597 10.1046/j.1523-1755.2001.00973.x. [DOI] [PubMed] [Google Scholar]
- 65.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, Asfour A, Danton M, Boyd L, Dardenne Meyers A, Ekanayake-Alper DK, Sachs DH, Yamada K. 2017. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant 17:1778–1790 10.1111/ajt.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. 2014. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol 25:737–744 10.1681/ASN.2013040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Buul-Offers SC, Van Kleffens M, Koster JG, Lindenbergh-Kortleve DJ, Gresnigt MG, Drop SL, Hoogerbrugge CM, Bloemen RJ, Koedam JA, Van Neck JW. 2000. Human insulin-like growth factor (IGF) binding protein 1 inhibits IGF1-stimulated body growth but stimulates growth of the kidney in snell dwarf mice. Endocrinology 141:1493–1499 10.1210/endo.141.4.7418. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Liang TC. 1994. Substrate specificity of porcine renin: P1′, P1, and P3 residues of renin substrates are crucial for activity. Biochemistry 33:14636–14641 10.1021/bi00252a032. [DOI] [PubMed] [Google Scholar]
- 69.Wen D, Boissel JP, Tracy TE, Gruninger RH, Mulcahy LS, Czelusniak J, Goodman M, Bunn HF. 1993. Erythropoietin structure–function relationships: high degree of sequence homology among mammals. Blood 82:1507–1516. [PubMed] [Google Scholar]
- 70.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. 2005. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1, 3-galactosytransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11:32–34 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 71.Yamada K, Tasaki M, Sekijima M, Wilkinson RA, Villani V, Moran SG, Cormack TA, Hanekamp IM, Hawley RJ, Arn JS, Fishman JA, Shimizu A, Sachs DH. 2014. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig-to-baboon xenotransplantation model. Transplantation 98:411–418. 10.1097/TP.0000000000000232.Transplantation 98:e77.Hawley, RJ added. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye Y, Niekrasz M, Kosanke S, Welsh R, Jordan HE, Fox JC, Edwards WC, Maxwell C, Cooper DK. 1994. The pig as a potential organ donor for man. A study of potentially transferable disease from donor pig to recipient man. Transplantation 57:694–702 10.1097/00007890-199403150-00011.Author: Read proofs carefully. This is your ONLY opportunity to make changes. NO further alterations will be allowed after this point. [DOI] [PubMed] [Google Scholar]