Abstract
Cyanide is a readily available and potentially lethal substance. Oral exposure can result in larger doses, compared with other routes. Currently, there are no antidotes specific for use in the treatment of oral cyanide poisoning, and studies cannot be done in humans. We report on a new large animal model of oral cyanide toxicity to evaluate potential antidotes. Six female swine (Sus scrofa; weight, 45 to 55 kg) were anesthetized, intubated, and instrumented. Animals received a KCN bolus of either 5 or 8 mg/kg delivered via orogastric tube. Time to apnea was recorded; parameters monitored included heart rate, respiratory rate, blood pressure, pulse oximetry, end-tidal CO2, arterial blood gasses, and lactate concentrations. The Welch t test was used to calculate confidence intervals, mean, and standard deviation, and a Kaplan–Meier survival curve was used to compare survival between the 2 groups. At baseline, all animals in both groups were similar. Animals in the 5-mg/kg group had a more rapid time to apnea (5.1 ± 2.1 min), longer time to death (48.5 ± 38.1 min), and a greater rate of survival than the 8-mg/kg group (apnea, 10.6 ± 10.7 min; death, 26.1 ± 5.8 min). All animals displayed signs of toxicity (acidemia, hyperlactatemia, hypotension, apnea). We here report a large animal (swine) model of oral cyanide poisoning with dose-dependent effects in regard to time to death and survival rate. This model likely will be valuable for the development of medical countermeasures for oral cyanide poisoning.
Cyanide is lethal to humans.1-5,27 A primary mechanism of toxicity is the inhibition of complex IV (cytochrome a3) in the electron transport chain, thus compromising oxidative phosphorylation and leading to severe end-organ damage.1-5,7-8 The NIH Strategic Plan and Research Agenda for Medical Countermeasures specifies that ingested cyanide is a leading threat—unique from inhaled and intravenous cyanide—because of the potentially higher cyanide dose, accessibility, delayed kinetics of absorption, and vulnerability of food and water supplies.5,6,9,12,15,16,23,28,29 When ingested, cyanide salts are exposed to the acidic environment of the stomach and form hydrogen cyanide, which gets absorbed and can cause systemic toxicity. Events over the past 2 decades have revealed the interest of various terrorist network groups in using cyanide in attacks.1,5,6,9,11-13,17-20,23,27-29 According to the Centers for Disease Control and Prevention, the potential LD50 for oral KCN exposure is 1.8 to 7.3 mg/kg, but this figure is not well defined and is extrapolated from case report studies. Furthermore, the LD50 for oral cyanide in swine is not well established, and dosing regimens must be based on previous studies performed mostly on small to moderate-sized animals. The LD50 of oral cyanide in small to moderate-sized animal models range from 3 to 8 mg/kg, and these data were used to determine dosing regimens for swine toxicity studies.2-4,7,8,14-16,21,22,24
Currently, no countermeasures are established for oral cyanide poisoning. Regulations of the US Food and Drug Association provide guidance on the new drug approval process when antidotes for chemical threats cannot be tested directly on human subjects for safety reasons.10 For scenarios where human studies are not feasible, the FDA (through application of the Animal Rule) can approve new therapeutics that have been studied in well-characterized animal models—preferably 2 species, one of which is a large mammal.10 Swine (Sus scrofa) are a large mammalian species that has been used in several toxicologic studies.2-4 In these studies, pigs provided a reliable and reproducible model system.
Potential therapeutics and countermeasures for oral cyanide exposures have been studied in small animal models, including mice.20-24 Small animals have several limitations, including difficulty with scaling dosages and challenges with hemodynamic, clinical, and laboratory monitoring.20-24 In addition, there are key differences between the physiologic and metabolic characteristics of some species and humans.2-4,7,8,10,11,14,16,21,22,24-26 As a result, the toxicokinetic and pharmacodynamic principles of oral chemicals and antidotes observed in those species may not apply to humans. Studies of oral cyanide toxicity and treatment involving a large animal model that has similar physiology, size, and anatomy to humans would be useful in developing new antidotes.
Previously, we studied acute intravenous cyanide toxicity in a swine model.3,4 Swine is a reasonable option to test countermeasures, given the similar size, cardiovascular system, and gastrointestinal system between swine and humans.24-26 The use of a large animal model will help further understanding of oral cyanide poisoning through increased knowledge of the physiologic and biochemical effects that occur but cannot be measured in smaller mammals. Studies in large animals can help to determine the necessary effective dose in humans and for use in Good Laboratory Practice and Phase I studies. Here we report a new large animal (swine) model of oral cyanide toxicity, which we developed to study oral cyanide poisoning and evaluate potential countermeasures.
Materials and Methods
Adolescent female Yorkshire swine (Sus scrofa; weight, 45 to 55 kg) were used for this study. Anesthesia was induced by using ketamine (10 to 20 mg/kg IM; MWI, Boise, ID) and isoflurane (MWI) by nosecone. Animals were intubated with a cuffed 8.0-mm endotracheal tube (Teleflex, Morrisville, NC), an orogastric tube (B Braun, Boise, ID) was placed, and peripheral venous access obtained. Sedation was maintained (Fabius GS, Drager, Houston, TX) by using 1%,3% isoflurane and 0.4 FiO2. Tidal volume was set at 8 mL/kg and a respiratory rate of 16 to 20 breaths per minute, and the minute volume was adjusted to maintain an end-tidal CO2 of 38 to 42 mm Hg. A 7.5-mL/kg bolus of 0.9% saline (B Braun) was given prior to central line placement. The external jugular and femoral artery were visualized (M9 ultrasound system, Mindray, Mahwah, NJ), and central venous and arterial access was obtained. Pulse oximetry, body temperature, invasive blood pressure, and ECG were monitored (Infinity Delta Monitor, Drager) throughout the experiment. Once vascular access was obtained, the mechanical ventilator was turned off, and isoflurane and FiO2 were weaned to 0.8% to 1% and 0.21, respectively.
Once the pig was stabilized and breathing spontaneously without mechanical ventilation, as indicated by a minute volume greater than 5.0 L and PaCO2 less than 45, gastric contents were sampled to assess pH. KCN (Sigma Aldrich, St Louis, MO) was diluted in saline and delivered as a one-time bolus dose through the orogastric tube. After administration of KCN, ventilation status was monitored by using end-tidal CO2, arterial blood gasses, and time to apnea, defined as fewer than 6 breaths per minute, was recorded. Untreated control animals were instrumented and monitored identically to the KCN exposure groups. The pigs were observed continuously for 90 min or until death, defined as a mean arterial pressure of less than 30 mm Hg for 10 min. Physiologic variables including heart rate, respiratory rate, minute volume, end-tidal CO2, and mean arterial pressure were monitored continuously and recorded every 5 min. Laboratory studies including chemistry, arterial blood gasses, and lactate concentration were obtained every 10 min. At the end of the study, pigs were euthanized by using sodium pentobarbital (100 mg/kg IV).
All experiments were approved by the University of Colorado's IACUC and complied with the regulations and guidelines of the Animal Welfare Act and AALAC. Prism 7.0 software (GraphPad, La Jolla, CA) was used for statistical analysis. The Welch t test was used to calculate confidence intervals, mean, and standard deviation. A Kaplan–Meier survival curve was used to compare survival rate between concentrations of ingested cyanide.
Results
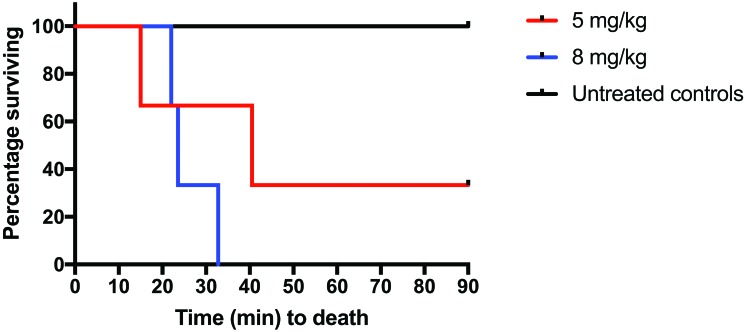
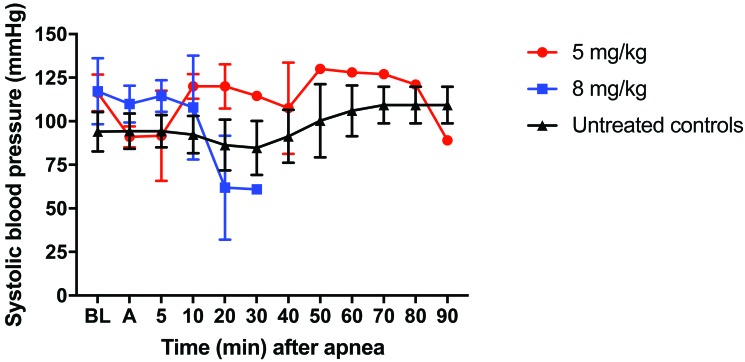
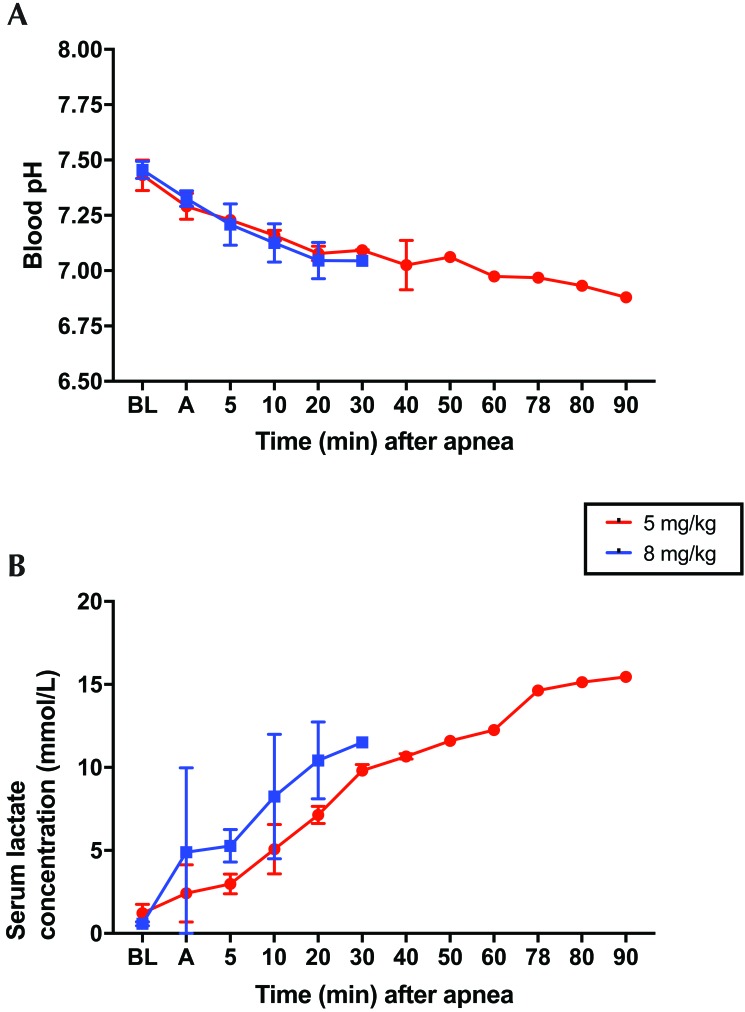
At baseline, all physiologic parameters, including weight, pH, lactate, CO2, and blood pressure, were similar in all groups (Table 1). Survival in the 5-mg/kg group (n = 3) was lower (33.3%) compared with the control group (100%; n = 3; P = 0.116). Survival at 90 min in the 8-mg/kg group (0%; n = 3) was significantly (P = 0.025) lower than the control group (100%; Figure 1). After oral KCN administration, all pigs began to develop hypotension before becoming apneic (Figure 2). Hypotension was more profound and persistent in the group treated with 8 mg/kg KCN. One animal in the 5-mg/kg group did not return to spontaneous ventilation, as indicated by a minute volume of less than 1 L and CO2 of 96.3 mm Hg but maintained a mean arterial pressure of greater than 30 mm Hg for the 90-min duration of the study. Animals in the 5 mg/kg group had a more rapid time to apnea but a longer time to death and a greater rate of survival, compared with the 8-mg/kg group (Table 2). In addition to hypotension, all KCN-exposed animals developed acidemia, as indicated by a decrease in serum pH (Figure 3 A) and an increase in serum lactate concentration (Figure 3 B). At the time of death, animals in both groups had similar physiologic parameters (Table 2). Animals treated with 8 mg/kg KCN PO died faster than animals treated with 5 mg/kg.
Table 1.
Characteristics at baseline
| 5 mg/kg KCN | 8 mg/kg KCN | 95% CI | |
| Weight (kg) | 50.1 ± 7.0 | 51.5 ± 6.4 | −13.9 to 16.6 |
| KCN dose (mg/kg PO) | 5.22 ± 0.44 | 8.14 ± 0.08 | −0.54 to 4.3 |
| pH | 7.43 ± 0.07 | 7.46 ± 0.04 | −0.12 to 0.17 |
| CO2 (mm Hg) | 42 ± 7.9 | 43.7 ± 2.6 | −15.9 to 19.3 |
| Lactate (mmol/L) | 1.22 ± 0.53 | 0.58 ± 0.12 | −1.9 to 0.6 |
| Systolic blood pressure (mm Hg) | 116 ± 11 | 116 ± 19 | −40 to 40 |
Data are given as mean ± 1 SD.
Figure 1.
The increased time to death in the 5-mg/kg group compared with the 8-mg/kg group demonstrates a dose-dependent response to KCN toxicity. Compared with untreated controls, the 8-mg/kg animals had increased (P = 0.025) mortality.
Figure 2.
Animals challenged with 5 or 8 mg/kg KCN developed hypotension prior to apnea. One of the 3 pigs that received 5 mg/kg KCN PO survived until the end of the study despite remaining apneic; all animals in the 8-mg/kg group continued to become hypotensive until death. A, anesthesia; BL, baseline.
Table 2.
Characteristics of pigs at death after KCN challenge
| 5 mg/kg | 8 mg/kg | 95% CI | |
| Time to apnea (min) | 5.12 ± 2.13 | 10.64 ± 10.7 | −19.77 to 30.81 |
| Time to death (min) | 48.52 ± 38.13 | 26.11 ± 5.81 | −114.3 to 69.5 |
| pH | 6.99 ± 0.14 | 7.02 ± 0.05 | −0.26 to 0.32 |
| CO2 (mm Hg) | 75.3 ± 18.2 | 56.9 ± 3.6 | −61.5 to 24.7 |
| Lactate (mmol/L) | 10.92 ± 4.36 | 11.17 ± 2.07 | −8.89 to 9.39 |
| Systolic blood pressure (mm Hg) | 64.0 ± 35.4 | 49.7 ± 9.5 | −278.7 to 250 |
Data are presented as mean ± 1 SD.
Figure 3.
All pigs developed acidosis after KCN challenge. (A) Blood pH decreased over time until death occurred (mean: 49 min [5 mg/kg pigs] or 26 min [8 mg/kg pigs]) or until the end of the study. (B) Blood lactate concentrations increased over time until death occurred (mean: 49 min [5 mg/kg pigs] or 26 min [8 mg/kg pigs]) or until the end of the study. A, anesthesia; BL, baseline.
Discussion
Several government agencies highlight the threat of oral cyanide and the need for an antidote approved for cyanide poisoning through this route of exposure.19,27-29 A well-characterized model for oral cyanide poisoning is needed to develop effective countermeasures. Here we report a reproducible swine model of oral cyanide poisoning that is dose-dependent in regard to time to death and survival and demonstrates clinical effects that are similar to the toxicity of oral cyanide in humans.3,4 Human exposure to cyanide results in significant cellular dysfunction, presenting as sedation, apnea, hypotension, and metabolic acidosis with significant hyperlactatemia.1-4 In our current study, swine exposed orally to KCN had similar physiologic effects and demonstrated a similar time course as had been reported for human exposures.1-4 All of our KCN-treated pigs died, except for one animal in the 5-mg/kg group. Although this remaining pig maintained a mean arterial pressure of greater than 30 mm Hg for the duration of the study, it likely would have died at a later point than 90 min (the end of our observation period), given that it never returned to spontaneous ventilation.
As with human toxicity, higher doses of oral KCN proved to be more toxic in pigs. The group of swine challenged with 8 mg/kg of oral KCN had a 100% mortality rate, a more rapid decrease in blood pressure, and higher rise in serum lactate concentration. The dose-dependency of oral cyanide has clinical implications, given that large gastrointestinal reservoirs of cyanide can occur after ingestion, which in turn may require increased doses of antidote compared with conventional dosing. In humans, exposures to oral KCN result in signs of toxicity within minutes, which was similar to the onset of toxicity in our pigs. In our study, the 5-mg/kg group became apneic sooner than the other group, but other markers (lactate, time to death) reflect that 8 mg/kg of KCN is more toxic, in a dose-dependent manner. Because death occurred at different time points throughout the experiment, intergroup differences in blood pH and lactate were not statistically significant. In addition, the shorter time to apnea observed for the 5-mg/kg group may be secondary to a small sample size.
Previous reports describe using small animal models to study oral cyanide. In 2016, a mouse model of oral poisoning was used to study cyanide toxicokinetics.22 In that study, the authors determined the LD50 of oral cyanide in these mice, noted that the natural history of oral cyanide toxicity is similar in mice and humans, and concluded that a reproducible small animal model can be used to test potential countermeasures for oral cyanide toxicity. A limitation of the cited study22 is the use of a small animal model, where monitoring of cardiovascular parameters can prove to be challenging. In addition, given the large size difference between mice and humans, scaling the dose of any potential therapeutics might be difficult. Furthermore, mice may be more resistant to the effects of oral cyanide exposure than humans. This effect may be secondary to the potential differences in gastric pH of the 2 species. Human gastric pH generally ranges from 1.5 to 3, whereas in mice, depending on whether they are fed (or not), gastric pH may be as high as 4. HCN is formed more favorably in an acidic milieu after the ingestion of cyanide salts such as KCN, and these differences in pH may explain potential differences in the toxic response to oral cyanide exposure across species.20-22
In studies using animals to model human toxicity, the use of moderate-sized species such as rabbits, offers various advantages compared with smaller animals, such as mice. For example, rabbits are more amenable to cardiovascular monitoring in real-time than are mice. In addition, rabbits have a similar gastric pH compared with humans, which has implications regarding the bioavailability of toxic compounds and potential oral countermeasures in vivo.22 A 2017 study reported data from rabbits and potential oral cyanide countermeasures. In particular, the authors used continuous-wave near-infrared tissue spectroscopy system to monitor for toxicity in real-time and found that a combination of oral glycine and sodium thiosulfate may be useful for treating high-dose acute cyanide ingestion.7 The authors highlighted the difference between the metabolic rates of rabbits and humans as a limitation of using rabbits for these types of studies. Of the 2 species, rabbits have a higher metabolic rate, and this difference has implications regarding the toxicokinetics of oral poisonings, time window for antidote administration, and response to potential oral antidotes.
To compliment earlier studies, a large animal model for cyanide toxicity is needed. Rabbits and swine complement each other well in terms of drug development and assessment of physiologic and biochemical variables.1-4,7 The FDA Animal Rule likely will require 2 animal models (including at least one large animal species) for testing the efficacy of oral cyanide countermeasures.10 The selection of swine as a model of cyanide toxicity is appropriate for several reasons. First, the size of swine is similar to humans, and the allometric conversion of drug doses is similar to the human equivalent dose.25 With the potential for increased amount of antidote needed in oral cyanide exposures, the need for significant scaling of drug dosing can be better extrapolated to humans by using of a large animal model. In addition, swine and humans are gastrointestinally and cardiovascularly similar.2,14,24,25 These similarities support the use of swine to model human toxicity of oral poisons like cyanide, because swine and humans have demonstrated similar systemic absorption of such chemicals.2-4,14,24-25 Future studies should evaluate promising countermeasures in our swine model—similar to what has been done for intravenous KCN models—in experiments that reflect clinically relevant scenarios.3,4,7,8
Our study has limitations. This study was a pilot study, and the sample size was small. In addition, drug kinetics were not studied; however, all pigs demonstrated severe toxicity as evidenced by apnea, acidemia, and hyperlactatemia, similar to the clinical signs of KCN poisoning in previous experiments.3,4 Furthermore, the pigs were observed for only a short period of time, and potential delayed sequela from KCN poisoning were not characterized. However, we plan to develop specific countermeasures for acute oral KCN poisoning soon after ingestion for prehospital and mass-casualty settings. In addition, we anesthetized the pigs for dosage, which was required by our IACUC and might influence data from the model. We did not test a broad range of KCN doses, because the development of a new large animal models is expensive, which thus limits the number of experiments that can be performed. We did not assess a countermeasure or antidote, given that our objective was to develop and present the current model; we have studies ongoing in this model to evaluate several potential treatments and to further understand the mechanisms of oral KCN toxicity. Furthermore, we report an animal model for human toxicity.
Although swine are a great choice in terms of their physiologic and size similarities to humans, these species nonetheless display key differences. For example, the CYP1 and CYP2 families of enzymes differ markedly between the 2 species. CYP1 activity is sex-related in both species. However, CYP1 activity is higher in female pigs compared with male. Whereas clear sex-associated differences in CYP2 activity occur in swine, these differences are not as pronounced in humans. In addition, CYP2 enzyme variations in humans are primarily due to polymorphisms, whereas most of the variations in individual CYP2 in swine are secondary to sex hormones.2,11 These examples are only some of the limitations in using an animal model to study human toxicity.
Here we report a large animal (swine) model of oral cyanide poisoning with dose-dependent toxic effects in regard to time to death and survival. This model would be valuable for federal agencies and industry in need of developing medical countermeasures to treat oral cyanide poisoning.28,29
Acknowledgments
We thank the Department of Emergency Medicine (University of Colorado) for providing funding for this study.
References
- 1.Baskin SI, Brewer TG. 1997. Cyanide poisoning, p 272–286. Chapter 10. In: Sidell FR, Takafuji ET, Franz DR, Medical aspects of chemical and biological warfare. Washington (DC): TMM Publications. [Google Scholar]
- 2.Bassols A, Costa C, Eckersall PD, Osada J, Sabrià J, Tibau J. 2014. The pig as an animal model for human pathologies: a proteomics perspective. Proteomics Clin Appl 8:715–731. 10.1002/prca.201300099. [DOI] [PubMed] [Google Scholar]
- 3.Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA. 2012. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med 59:532–539. 10.1016/j.annemergmed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Bebarta VS, Tanen DA, Boudreau S, Castaneda M, Zarzabal LA, Vargas T, Boss GR. 2014. Intravenous cobinamide versus hydroxocobalamin for acute treatment of severe cyanide poisoning in a swine (Sus scrofa) model. Ann Emerg Med 64:612–619. 10.1016/j.annemergmed.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beering P. 2002. Threats on tap: understanding the terrorist threat to water. Journal of Waste Resources Planning and Management 128:163–167. 10.1061/(ASCE)0733-9496(2002)128:3(163). [DOI] [Google Scholar]
- 6.Berlinger J, [Internet] 2016. Man sprayed poison on open food at grocery stores, FBI 2016. [Cited 18 January 2018]. Available at: http://www.cnn.com/2016/05/05/health/michigan-food-contamination-poison/.
- 7.Brenner M, Azer SM, Oh KH, Han CH, Lee J, Mahon SB, Du X, Mukai D, Burney T, Saidian M, Chan A, Straker DI, Bebarta VS, Boss GR. 2017. Oral glycine and sodium thiosulfate for lethal cyanide ingestion. J Clin Toxicol 7:584–602. 10.4172/2161-0495.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, Mukai D, Patterson S, Mohammad O, Sharma VS, Boss GR. 2010. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med 55:352–363. 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Director of National Intelligence. [Internet]. 2015. Terror franchise: the unstoppable assassin. Techs vital role for its success. [Cited 18 January 2018]. Available at: http://www.dni.gov/files/documents/ubl/english/Terror Franchise.pdf.
- 10.Food and Drug Administration (FDA). [Internet]. 2015. Product development under the Animal Rule. Industry guidance. [Cited 08 August 2018]. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm399217.pdf.
- 11.Helke KL, Nelson KN, Sargeant AM, Jacob B, McKeag S, Haruna J, Vemireddi V, Greeley M, Brocksmith D, Navratil N, Stricker-Krongrad A, Hollinger C. 2016. Pigs in toxicology: breed differences in metabolism and background findings. Toxicol Pathol 44:575–590. 10.1177/0192623316639389. [DOI] [PubMed] [Google Scholar]
- 12.Keim ME. 2006. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med 21 Suppl 2:s56–s60. 10.1017/S1049023X00015910. [DOI] [PubMed] [Google Scholar]
- 13.Khan AS, Swerdlow DL, Juranek DD. 2001. Precautions against biological and chemical terrorism directed at food and water supplies. Public Health Rep 116:3–14. 10.1016/S0033-3549(04)50017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi E, Hishikawa S, Teratani T, Lefor AT. 2012. The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplant Res 1:1–9. 10.1186/2047-1440-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. 2008. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci 101:101–111. 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Mahon SB, Mukai D, Burney T, Katebian BS, Chan A, Bebarta VS, Yoon D, Boss GR, Brenner M. 2016. The vitamin B12 analog cobinamide is an effective antidote for oral cyanide poisoning. J Med Toxicol 12:370–379. 10.1007/s13181-016-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy A, [Internet] 2016Chemistry graduate who threatened, kill supermarket shoppers with cyanide in a £2million blackmail plot is jailed for 7 years. [Cited 18 January 2018]. Available at: http://www.dailymail.co.uk/news/article-3771823/Chemistry-graduate-threatened-kill-supermarket-shoppers-cyanide-2million-blackmail-plot-jailed-seven-years.html.
- 18.Moore J, [Internet] 2017ISIS supporters call for poisoning of food in grocery stores across U.S. and Europe. [Cited 18 January 2018]. Available at: http://www.newsweek.com/isis-supporters-call-poisoning-grocery-stores-us-and-europe-660750.
- 19.National Consortium for the Study of Terrorism and Responses to Terrorism (START). [Internet]. 2017Global terrorism database: University of Maryland. [Cited 18 January 2018]. Available at: http://www.start.umd.edu/gtd/.
- 20.Newhouse K, Chiu N. 2010Toxicological review of hydrogen cyanide and cyanide salts. Washington (DC): US Environmental Protection Agency. [Google Scholar]
- 21.Reid FM, Jett DA, Platoff GE, Jr, Yeung DT, Babin M. 2016Animal models for testing antidotes against an oral cyanide challenge. Fort Belvoir (VA): Defense Technical Information Center. [Google Scholar]
- 22.Sabourin PJ, Kobs CL, Gibbs ST, Hong P, Matthews CM, Patton KM, Sabourin CL, Wakayama EJ. 2016. Characterization of a mouse model of oral potassium cyanide intoxication. Int J Toxicol 35:584–603. 10.1177/1091581816646973. [DOI] [PubMed] [Google Scholar]
- 23.Soeriaatmadja W, [Internet] 2016. Indonesia on alert for cyanide attacks. [Cited 18 April 2018.] Available at: https://www.straitstimes.com/asia/se-asia/indonesia-on-alert-for-cyanide-attacks.
- 24.Swindle MM. 2012. The development of swine models in drug discovery and development. Future Med Chem 4:1771–1772. 10.4155/fmc.12.113. [DOI] [PubMed] [Google Scholar]
- 25.Swindle MM, Markin A, Herron AJ, Clubb FJ, Jr, Frazier KS. 2012. Swine as models in biomedical research and toxicology testing. Vet Pathol 49:344–356. 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JP, Marrs TC. 2012. Hydroxocobalamin in cyanide poisoning. Clin Toxicol (Phila) 50:875–885. 10.3109/15563650.2012.742197. [DOI] [PubMed] [Google Scholar]
- 27.University of Maryland and the Department of Homeland Security. [Internet]. 2013. Background report—ricin and cyanide use by AQAP. [Cited 18 January 2018]. Available at: http://www.start.umd.edu/sites/default/files/files/publications/br/STARTBackgroundReport_RicinMailings_April2013.pdf.
- 28.US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. 2007. NIH strategic plan and research agenda for medical countermeasures against chemical threats. Bethesda (MD): National Institutes of Health. [Google Scholar]
- 29.US Department of Health and Human Services. [Internet].2016. PHEMCE. 2016 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan. [Cited 19 May 2018]. Available at: https://www.phe.gov/Preparedness/mcm/phemce/Documents/2016-phemce-sip.pdf