Abstract
Hypertension is a leading risk factor for cardiovascular and chronic kidney disease. A new rodent model (transgenic male Cyp1a1–Ren2 rats) provides reversible induction of hypertension through the addition of indole-3-carbinol (I3C) to the diet, without the need for surgical intervention, thus giving researchers control over both the onset of hypertension and its magnitude (I3C dose-dependency). We here report the breeding performance and productivity of Cyp1a1–Ren2 rats. Despite being transgenic, these animals proved to be efficient breeders. In addition to confirming inducible and reversible dose-dependent hypertension (by using I3C doses of 0.125%, 0.167%, and 0.25% [w/w] in the diet for 14 d, followed by normal chow for 4 d), we demonstrated that hypertension can be sustained chronically (14 wk) by continuous dosing with I3C (0.167% [w/w]) in the diet. In chronically dosed male rats, systolic blood pressure continued to rise, from 173 ± 11 mm Hg after 1 mo to 196 ± 19 mm Hg after 3 mo, with no adverse phenotypic features observed. In conclusion, Cyp1a1–Ren2 rats are a useful animal model to investigate hypertension-induced end-organ damage and potential new therapeutic targets to manage hypertension.
Abbreviations: I3C, indole-3-carbinol; SBP, systolic blood pressure
Hypertension has repeatedly been shown to be a leading risk factor contributing to global death rates.1,3,6,20,24,32 As much as 44% of the world population older than 20 y is hypertensive19 (that is, blood pressure of greater than 140/90 mm Hg). The sequelae of chronically elevated blood pressure manifest as cerebrovascular disease, cardiac disease, and chronic kidney diseases, imposing an ever-increasing fiscal load on the delivery of health care.1,2,35,36 The American Heart Association reports that 69% of cases presenting with a myocardial infarction and 74% of cases with reported chronic heart failure have a history of hypertension.1 In the United States alone, the prevalence of hypertension were anticipated to increase by more than 7% and the incidence of cardiovascular disease by 40.8% by the year 2030,12 leading to a projected annual cost estimate for the treatment of hypertension alone of more than $US340 billion.1 However, recent estimates suggest that the prevalence of hypertension will rise more rapidly, with the hypertensive population now estimated to increase by more than 8% by the year 2030 (bringing the rate to almost 50% of the adult population).1
To investigate key regulatory components that influence blood pressure, several transgenic hypertensive rat models have been developed. One such model are transgenic Cyp1a1–Ren2 rats, in which hypertension can be induced reversibly by diet, without the need for surgical intervention.19 In this line, the expression of mouse Ren2 cDNA is under the control of an inducible cytochrome p450-1a1 promoter, which is integrated into the Y chromosome of Fischer 344 rats19,26 and is therefore active only in males. Dietary administration of indole-3-carbinol (I3C) activates the promoter gene (Cyp1a1) and thus increases the production of Ren2.17,19 I3C is a naturally occurring, nontoxic, xenobiotic found in cruciferous vegetables (such as broccoli) that acts as a benign inducer with a short half-life. The production of Ren2, primarily in the liver, on induction of the Cyp1a1 promoter through I3C,18,31 leads to increased circulating renin levels, activation of the renin–angiotensin–aldosterone system and a consequent increase in blood pressure. After the withdrawal of I3C from the diet, the production of mouse renin falls, and blood pressure returns to previous, normotensive levels.19This model, therefore, allows inducible and reversible hypertension through modulation of the renin–angiotensin–aldosterone system. Importantly, the extent of hypertension is I3C dose-dependent,26,29 allowing tight control of blood pressure. Thus, both the timing of the onset of hypertension and its magnitude can be controlled in this unique animal model, in which the effect of various antihypertensive treatments can be assessed.
Although the transgenic Cyp1a1–Ren2 rat model has been used widely to study hypertension and associated organ damage during the 2 decades since its creation,10,14,19,26,28,30 no information has been published regarding the line's breeding characteristics or reproductive performance (such as breeding efficiency and regularity, fertility, and litter size). Similarly, most studies using this model focus on acute hypertension, with few data in terms of chronic hypertension.13,15,30,31 We therefore aimed to outline the breeding performance and productivity of transgenic Cyp1a1–Ren2 rats, demonstrate the inducible and reversible dose-dependent hypertension in this model, and describe the effect of chronic dosing with I3C on inducible hypertension.
Materials and Methods
Animals.
The initial transgenic Cyp1a1-Ren2 rat internal breeding stock was gifted by Professor JJ Mullins (Centre for Cardiovascular Science, University of Edinburgh, United Kingdom). The transgenic Cyp1a1–Ren2 rat colony was held at the University of Otago Animal Resource Unit, and animals were housed under controlled conditions of temperature (approximately 21 °C) and light (12:12-h light:dark cycle), with food (Meat-free Rat and Mouse Diet, Irradiated, Specialty Feeds, Glen Forest, Western Australia, Australia) and tap water provided without restriction. All animals were housed on corn-cob bedding in standard open-top caging, with ‘paper wool’ for nesting and a tunnel provided for enrichment.
The colony was monitored according to FELASA guidelines7 through quarterly serologic testing for specific pathogens (Table 1). The testing was performed by Cerberus Labs (Cerberus Sciences, Melbourne, Victoria, Australia). Rats were negative for all agents except Helicobacter spp. and Pneumocystis carinii.
Table 1.
Results of annual health testing of the Cyp1a1–Ren2 rat colony
| Organism | Status | |
| Bacteria and fungi | ||
| Cilia-associated respiratory bacillus | Negative | |
| Citrobacter rodentium | Negative | |
| Clostridium piliforme | Negative | |
| Corynebacterium kutscheri | Negative | |
| Helicobacter bilis | Negative | |
| Helicobacter ganmani | Negative | |
| Helicobacter hepaticus | Negative | |
| Helicobacter mastomyrinus | Negative | |
| Helicobacter rodentium | Negative | |
| Helicobacter spp. | Positive | |
| Helicobacter typhlonius | Negative | |
| Mycoplasma pulmonis | Negative | |
| Pasteurella pneumotropica | Negative | |
| Pasteurellaceae group | Negative | |
| Pneumocystis carinii | Positive | |
| Salmonella spp. | Negative | |
| Streptobacillus moniliformis | Negative | |
| Streptococcus group A (β hemolytic) | Negative | |
| Streptococcus group B (β hemolytic) | Negative | |
| Streptococcus group C (β hemolytic) | Negative | |
| Streptococcus group G (β hemolytic) | Negative | |
| Streptococcus pneumoniae (α hemolytic) | Negative | |
| Ectoparasites | ||
| Myobia musculi | Negative | |
| Myocoptes musculinus | Negative | |
| Radfordia affinis | Negative | |
| Endoparasites | ||
| Aspiculuris tetraptera (pinworm) | Negative | |
| Giardia muris | Negative | |
| Spironucleus muris | Negative | |
| Syphacia muris (pinworm) | Negative | |
| Nonpathogenic protozoa | ||
| Entamoeba muris | Negative | |
| Tritrichomonas muris | Negative | |
| Viruses | ||
| Adenovirus type 1 | Negative | |
| Adenovirus type 2 | Negative | |
| Kilham rat virus | Negative | |
| Pneumonia virus of mice | Negative | |
| Rat coronavirus | Negative | |
| Rat minute virus | Negative | |
| Rat parvovirus | Negative | |
| Rat theilovirus | Negative | |
| Reovirus type 3 | Negative | |
| Sendai virus | Negative | |
| Toolan H1 virus | Negative | |
Colony breeding pairs were monitored regularly to assess and characterize breeding performance, including recording the number of litters, litter size, sex ratio, survival, overall health, and behavioral traits. Continuous, inbred (brother–sister) pairs were used for breeding. Randomly selected litters were weighed daily to obtain a standard weight curve for the colony.
All Cyp1a1-Ren2 rats used for experiments were obtained from internal breeding stock and were housed in pairs or in groups of 4 rats per cage. All experiments were approved by the Animal Ethics Committee of the University of Otago (approval no. AEC 51/13), in accordance with the guidelines of the New Zealand Animal Welfare Act.25
Experimental protocol.
I3C dose–response curve.
Standard pelleted rat chow (Specialty Feeds) was ground and blended to a fine powder. I3C (Chem-Impex International, Wood Dale, IL) was added to the powdered diet to produce final concentration (w/w) of 0.125%, 0.167%, and 0.25%. Cyp1a1–Ren2 rats housed in pairs or triplicates were randomly assigned to a dose group at 8 wk of age and allotted 50 g of powdered chow daily per rat. Similarly, 8-wk-old female Cyp1a1–Ren2 rats were assigned to receive either standard chow or 0.167% (w/w) I3C diet. Food was weighed and topped up every morning.
To demonstrate the dose-dependent effect of I3C on blood pressure, Cyp1a1–Ren2 rats experienced a 14-d dosage regimen using different percentages of I3C: the Cyp1a1 gene inducer. Male and female Cyp1a1–Ren2 rats (age, 8 wk; n = 29) were randomly assigned to 1 of 3 groups and received I3C (0.125%, 0.167%, or 0.25% [w/w]) in the diet. Rats were weighed and food was replenished daily, with systolic blood pressure (SBP) measured every 3 to 4 d. At the end of the 14-d I3C dosing period, 6 female rats were euthanized by using halothane. At day 14 or 18 after SBP recordings, male rats from each group were euthanized by using halothane, and tissue (heart and kidneys) was harvested for further analyses.
Plasma from cardiocentesis of normotensive male Cyp1a1–Ren2 rats (maintained on standard chow) and hypertensive male Cyp1a1–Ren2 rats (maintained on diet containing 0.167% [w/w] I3C) was analyzed for renin by using a commercial inhouse ELISA assay (EndoLabs, Christchurch Hospital, Christchurch, New Zealand) on day 14.
Chronic elevation of blood pressure.
Male transgenic Cyp1a1–Ren2 rats were maintained on either pelleted standard chow (Specialty Feeds) or pelleted standard chow with addition of 0.167% (w/w) I3C (SF13-086, Specialty Feeds) for 14 wk. All animals were housed under controlled conditions in groups of 4 and had free access to water and food. Rats were weighed and food was replenished weekly, with SBP measured every 4 wk. At the end of the 14-wk period, rats were euthanized by using halothane.
Physiologic measurements.
All rats were acclimated through daily handling and were weighed (to the nearest gram) at 8 wk of age. Weights were calculated as the gain in weight from this start point to account for variability in initial weight. SBP was measured in conscious habituated rats by using tail-cuff plethysmography (NIBP controller, AD Instruments, New Zealand) and recorded (PowerLab 4SP, AD Instruments). Animals were given 30 min to acclimate prior to the blood pressure recording procedure, and a heat lamp was used to gently warm the tail prior to SBP readings. Data were captured and analyzed by using Chart (version 7, AD Instruments). A minimum of 10 clear recordings were obtained from each rat during each session.
Statistical significance (that is, P < 0.05) was assessed by using 2-way ANOVA with Bonferroni posthoc analysis (KaleidaGraph, Synergy Software, Reading, PA).
Results
Transgenic Cyp1a1–Ren2 rat colony.
Compared with outbred stocks, inbred strains typically show lower productivity—not only in frequency of productive matings but also smaller litters and fewer litters during a dam's lifetime. However, despite being an inbred strain, many of the reproductive features observed in the Cyp1a1–Ren2 rat colony were typical of those in outbred strains. Sexual maturity occurred similarly to other common rat strains, and first litters typically were born within 1 mo after initial pairing of breeders. Decreased breeding performance in the Cyp1a1–Ren2 rat colony was due to a slower reproductive cycle (average, 28 to 35 d) compared with the typical duration (20 to 24 d). However, Cyp1a1–Ren2 rats displayed a normal gestational period (20 to 24 d).
Fertility in the colony varied widely during the first few generations, with some pairs never producing a litter. However, careful colony maintenance (for example, culling of nonproductive pairs) eliminated this infertility within a few generations. In addition, Cyp1a1–Ren2 rats in this colony frequently produced the first litter within expected timeframes (that is, within 30 d of pairing) but then had a long period with no visible conception (around 50 to 70 d before visible pregnancy), followed by regular and efficient production after the birth of the second (that is, delayed) litter. This pattern was not restricted to specific breeding lines within the colony or associated with any noted excessive stress or unusual behavior. This characteristic appears to be strain-specific and not one that has been reported previously for Cyp1a1–Ren2 rats. Despite this reproductive behavior, the generation time of these rats was normal, with gestation of approximately 3 wk followed by 3 wk to weaning. Surprisingly, this colony of inbred transgenic Cyp1a1–Ren2 rats produced litter sizes comparable to those of outbred, nontransgenic strains, with average litters of 12 ± 4 pups.
To date, the colony of Cyp1a1–Ren2 rats at the University of Otago has produced only 2 cases of birthing defects. In both cases, pups were born with microphthalmia (microeye), and their lineages were removed from the breeding colony. No strain-specific behaviors have been distinguished within the Cyp1a1–Ren2 rat colony, nor are any specific behaviors documented for this transgenic strain. No data available suggest that Cyp1a1–Ren2 rats experience any adverse effects due to the genetic manipulation itself.
Standard growth curve of Cyp1a1–Ren2 rats.
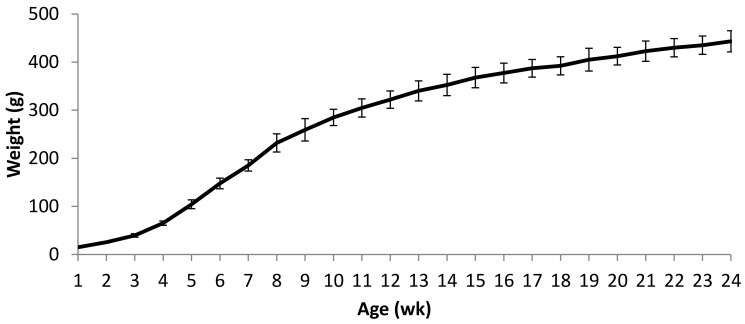
Prior to weaning, pups showed a steady and rapid daily weight gain. All pups were weaned at 21 d of age, and a small reduction in the rate of weight gain was noted during the 2 to 3 d after weaning but quickly reverted to a steady gain. Weight records were continued in male Cy1a1Ren2 rats maintained on normal pelleted chow until 24 wk of age (Figure 1). Rats maintained steady and strong growth until 8 wk of age (mean ± 1 SD, 232 ± 19 g), which then continued at a slower rate until 24 wk of age (443 ± 22 g).
Figure 1.
Standard weight curve for normotensive male Cyp1a1–Ren2 rats maintained on normal chow until 24 wk of age. Data are shown as mean ± 1 SD (n = 43).
I3C dose curves.
Weight.
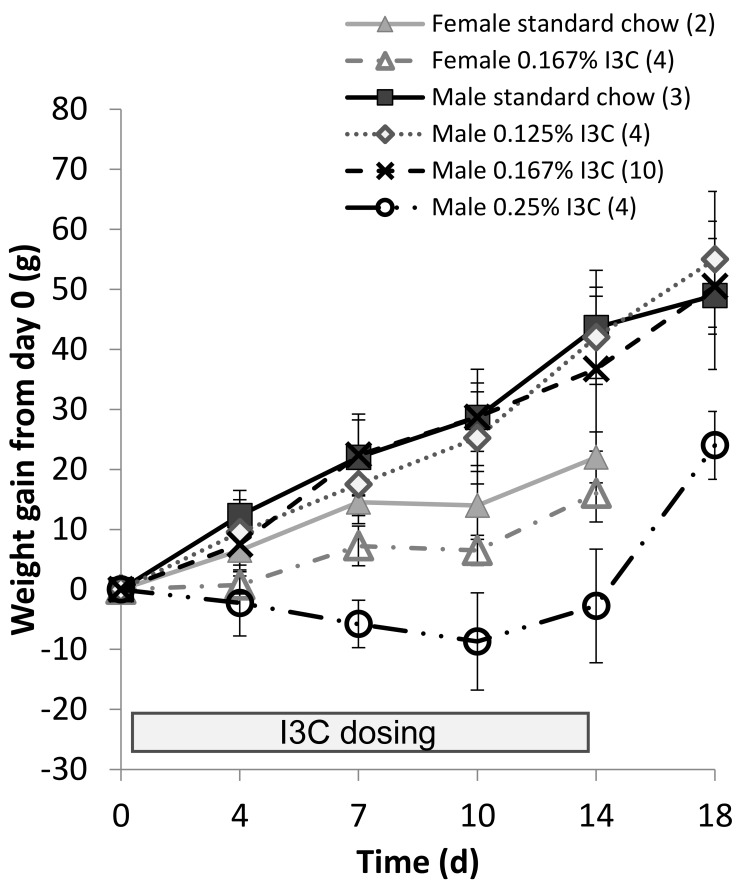
Female Cyp1a1–Ren2 rats, which lack the transgene, showed a steady weight gain on standard powdered chow from day 0. Female Cyp1a1–Ren2 rats placed onto a diet containing 0.167% (w/w) I3C showed an initial delay in weight gain when compared with females on standard chow, and this delay continued until day 14 (16 ± 5 g compared with 22 ± 4 g, respectively; Figure 2). Male Cyp1a1–Ren2 rats maintained on chow containing either 0.125% or 0.167% (w/w) I3C showed a weight gain from day 0, similar to that of male rats fed standard chow (Figure 3). In contrast, male Cyp1a1–Ren2 rats fed the highest dose (0.25% w/w I3C), failed to show a gain in weight over the 14-d dosing period (-2.8 ± 9.5g, Figure 2). Following removal of the I3C from the diet on day 14 and returning to normal chow, rats increased weight.
Figure 2.
Weight gain from day 0 of transgenic Cyp1a1–Ren2 rats maintained on different doses of dietary indole-3-carbinol (I3C). At day 14, dietary I3C was removed and normal chow administered. The number of animals in each experimental group is given in parentheses. Data are shown as mean ± 1 SD.
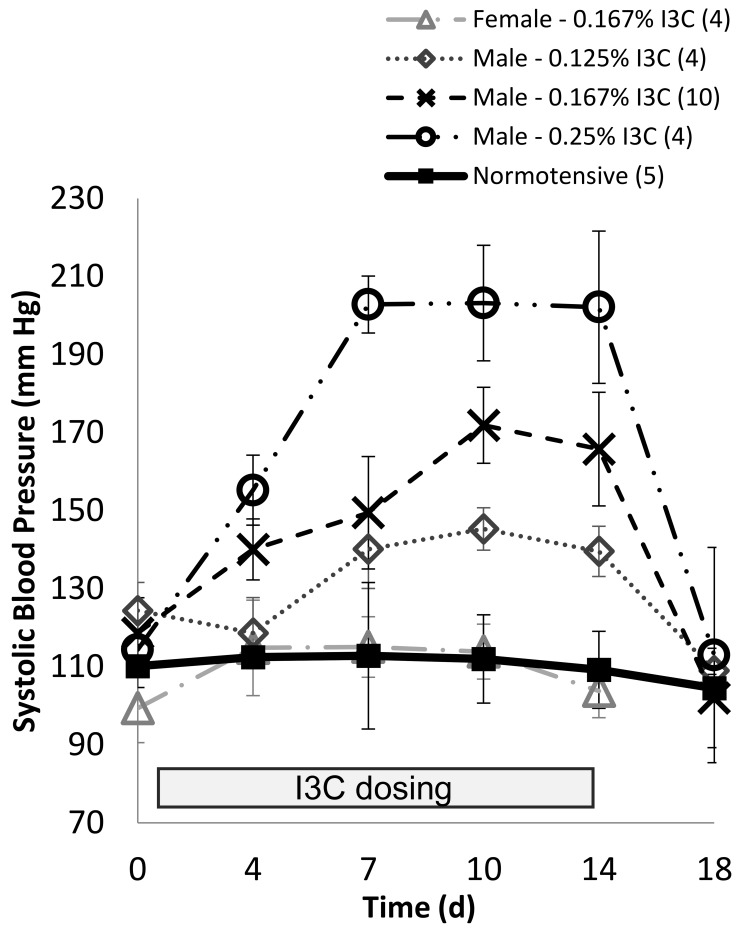
Figure 3.
Systolic blood pressure (SBP) in transgenic 8-wk old Cyp1a1–Ren2 rats maintained on different dietary doses of indole-3-carbinol (I3C) for 14 d. On day 14, rats were returned to standard chow. Data are shown as mean ± 1 SD. The number of animals in each experimental group is shown in parentheses.
Blood pressure.
Female and male Cyp1a1–Ren2 rats maintained on the standard diet were shown to have a SBP of 111 ± 7 mm Hg. Similarly, female rats maintained on an I3C dose of 0.167% were recorded to have a mean SBP of 109 ± 7mm Hg (Figure 3). After 14 d on a dietary dose of 0.125% I3C, male Cyp1a1–Ren2 rats had an increase in SBP to 140 ± 6 mm Hg, which was significantly (P > 0.01) higher than in normotensive males. After 14 d on a diet containing 0.167% I3C, male rats had a SBP of 165 ± 15 mm Hg, which was significantly (P > 0.01) higher than both normotensive male rats and male rats on 0.125% I3C. Animals maintained on the highest dose (0.25% I3C) had a SBP of 202 ± 20 mm Hg (Figure 3), again significantly (P > 0.001) higher than all other groups. By 4 d after the return to standard rat chow, SBP in all groups had returned to the normotensive range (107 ± 5 mm Hg; Figure 3).
Plasma renin in hypertensive animals (maintained on 0.167% [w/w] I3C diet) was significantly (P < 0.01) elevated after 14 d compared with normotensive animals (22 ± 4 and 11 ± 3 nmol/L, respectively).
Chronic elevation of blood pressure in Cyp1a1–Ren2 rats.
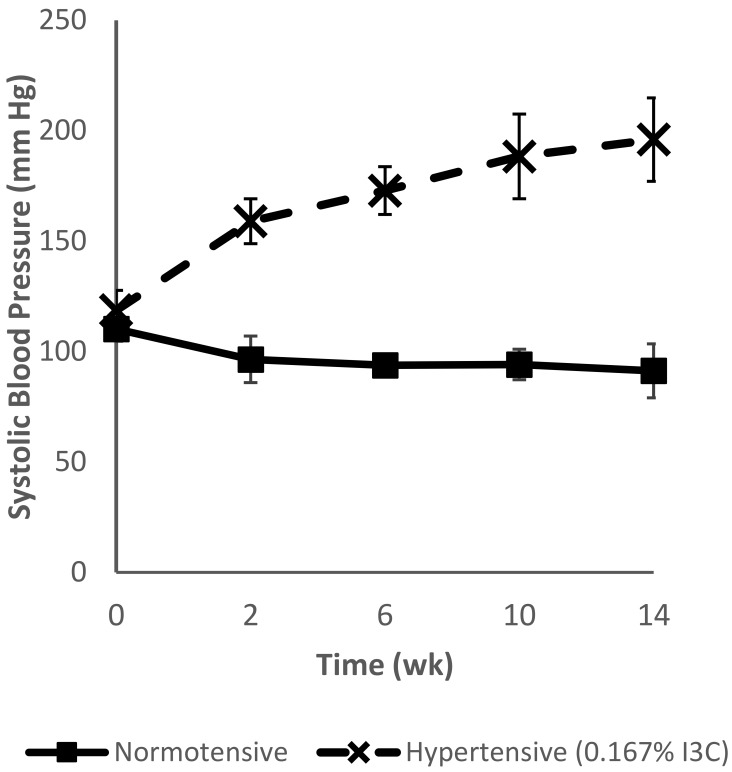
Feeding I3C to male transgenic Cyp1a1–Ren2 rats led to a steady increase in SBP over time (Figure 4). In contrast, male Cyp1a1–Ren2 rats maintained on normal chow showed no increase in SBP over the 14 wk. After the initial 2 wk of dietary dosing with 0.167% (w/w) I3C, SBP rose to 159 ± 10 mm Hg (giving similar results to the I3C dose curve, Figure 3). Furthermore, SBP rose to 173 ± 11 mm Hg after an additional 4 wk and to 196 ± 19 mm Hg after 3 mo (Figure 4).
Figure 4.
Chronic elevation (3 mo) of systolic blood pressure in male Cyp1a1–Ren2 transgenic rats maintained on a diet containing 0.167% (w/w) indole-3-carbinol (I3C; hypertensive, dashed line) or standard rat chow (normotensive, solid line). Hypertensive animals were given an initial 14-d ramping period on a diet containing 0.167% I3C to establish hypertension, whereas normotensive animals remained on normal chow. Data are shown as mean ± 1 SD (n = 8).
Discussion
A persistent difficulty in the investigation of hypertension is the availability of appropriate models that reproduce the clinical situation. In humans, elevation of blood pressure occurs slowly and insidiously, whereas most animal models of hypertension involve an intervention that produces a rapid elevation in blood pressure. The development of Cyp1a1–Ren2 transgenic rats in which blood pressure can be manipulated by a dietary additive has made possible the investigation of hypertension under controlled settings.19 In contrast to other genetic and transgenic hypertensive rat models (including Spontaneously Hypertensive Rats,4,9,21 Dahl salt-sensitive rats,11,21 and transgenic m(Ren2)27 rats),5,23 hypertension in Cyp1a1–Ren2 transgenic rats can be induced at any chosen age or time, allowing tailoring of experimental design and exploration of the effect of hypertension over time and on animals of different ages. In addition, this model permits dose-dependent titration of hypertension, which enables the investigation of a broad range of elevated blood pressures, from prehypertension to malignant hypertension, simply by adjusting the level of I3C in the diet. In contrast to other hypertensive rat models, Cyp1a1–Ren2 rats have a relatively slow ramping of blood pressure, which is more closely related to the development of hypertension in the clinical setting. This incremental development of hypertension consequently provides a more realistic model in which to study the effects of increased pressure on vascular structure and function.
The experiments reported here verified the absence of any effect of I3C (inducer) on blood pressure in female rats lacking the Ren2 gene. When male Cyp1a1–Ren2 rats received I3C in the diet for 2 wk, SBP was elevated in a dose-dependent fashion, with higher doses of I3C leading to higher levels of SBP (Figure 3). These results are similar to previous findings,29 although, in contrast, our animals fed a diet containing I3C at 0.167% (w/w) showed no growth retardation. When I3C-treated male Cyp1a1–Ren2 rats returned to normal chow, their SBP returned to normotensive values within 48 h, consistent with other published studies.13,18,30
A previous study29 showed that the addition of 0.125% (w/w) I3C in the diet, male Cyp1a1–Ren2 rats achieved a stable mean arterial pressure of approximately 170 ± 5 mm Hg after 6 wk on the diet. Similarly, a later study30 showed a continued rise in mean arterial blood pressure up to 28 d after the commencement of I3C, although this progression was far slower than that seen in the first 2 wk. In contrast an earlier study,29 we noted no plateau in blood pressure during the 14-wk time frame, and SBP continued to rise from 173 ± 11 mm Hg after 1 mo to 196 ± 19 mm Hg after 3 mo on the I3C diet (Figure 4). Interestingly, the diet-manipulated animals (maintained on 0.167% [w/w] I3C) showed no adverse phenotypic features related to their elevated blood pressure, such as lethargy, hunched posture, piloerection, and lower body weight gain, as have previously been reported, albeit associated with an I3C dose of 0.3% (w/w).18,27,29
Correct establishment and management of a rodent colony are essential, and knowledge of commonalities and deficiencies of a strain can be critical to maintaining a good colony and therefore also good research stock. Generally, laboratory rats become sexually mature between 5 and 10 wk of age;22,33 consequently, breeding animals are often paired at approximately 10 wk of age to obtain the maximal reproductive performance.33,34 Typically laboratory rats breed for 5 to 8 mo, producing 5 to 6 litters,16 but this number can be reduced or extended due to strain-specific characteristics or mutant phenotypes that affect fertility. In addition, interstrain differences can influence litter size, although 10 to 14 pups is common and expected.22
The performance (or productivity) of a colony is traditionally defined according to the fecundity of the breeder females (although reproductive failures may be due to the female, the male, or both). Because mating usually occurs within 24 h of placing sexually mature rats together,34,37 with pregnancy visible and fetuses palpable often by day 14 and litters typically born by day 23 (ranging from 21 to 25 d),34,37 a general rule is that if no visible signs of pregnancy are noted within 60 d from pairing or the birth of the previous litter, the breeding pair is considered nonproductive and is removed from the colony.22,33,34 Assessment of breeding performance also considers hybrid vigor, litter size, strain-specific genetic mutations or transgenes (such as reduced fertility, effects of mammary gland function, or lethal embryonic effects) as well as behaviors (such as high aggression or poor mothering instincts). This practice helps to ensure that peak breeding efficiency is sustained, expected reproductive characteristics of the strain are maintained, and breeders are assessed sufficiently to select appropriate replacement stock. This continuous evaluation will identify reproductive problems before the colony is in reproductive crisis.22,33,34
Cyp1a1–Ren2 rats proved to be efficient and good breeders, comparable to common outbred laboratory rat strains. However, we noticed several characteristics while breeding this unique transgenic strain. First, despite being an inbred transgenic rat model, Cyp1a1–Ren2 rats produced large litters (that is, 10 to 16 pups). These litter sizes were much larger than reported for Fischer 344 rats (the background strain for Cyp1a1–Ren2 rats) from both Envigo (worldwide) and Janvire Laboratories (France, Europe), which both report an average litter of fewer than 8 pups. Although this characteristic is not undesirable, it is unusual and therefore should be noted. Second (and more important to the management of the colony) was a prolonged reproductive cycle (that is, 28 to 35 d) compared with other laboratory rat strains. Furthermore, we observed repeatedly that, after the birth of the first litter, dams seemed to ‘skip’ either the second or third litter, resulting in periods of 60 to 70 d with no visible pregnancy. However, after this litter-free spell, rats produced efficiently from then on. Despite all efforts to remove this lag period, it appears to be a strain characteristic of the transgenic Cyp1a1–Ren2 rat. Further investigation is required to document these findings; regardless, breeding pairs of Cyp1a1–Ren2 rats should be afforded more than 60 d before being removed from the colony.
The development of transgenic Cyp1a1–Ren2 rats19 has allowed increased control of the study of hypertension. Because the breeding characteristics of these transgenic animals have not been published previously, here we report that these rats breed surprisingly well despite being an inbred and transgenic strain. In addition, we showed that Cyp1a1–Ren2 rats maintained on decreased titrated doses of dietary I3C can tolerate prolonged periods of sustained hypertension but without the ill effects reported by others.15,29 Given that hypertension repeatedly has been shown to be a major risk factor contributing to global death rates,1,8,20,24 animal models such as Cyp1a1–Ren2 rats may hold key components to therapeutic developments targeting hypertension, and further research to clarify the dose-dependent response, especially long-term, in this model is needed.
Acknowledgments
Part of this work was presented at meetings of the Australian and New Zealand Society of Nephrology and the American Society of Nephrology. Funding was provided by the Department of Medicine (University of Otago, New Zealand), a Laurenson Award from the Otago Medical Research Foundation, and the Maurice and Phyllis Paykel Trust. We thank Professor JJ Mullins (Centre for Cardiovascular Science, University of Edinburgh, United Kingdom) for providing the transgenic Cyp1a1–Ren2 rat internal breeding stock.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee 2017. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135:e146–e603. 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. 2013. Renal microvascular dysfunction, hypertension, and CKD progression. Curr Opin Nephrol Hypertens 22:1–9. 10.1097/MNH.0b013e32835b36c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey RM. 2015. The intrarenal renin–angiotensin system in hypertension. Adv Chronic Kidney Dis 22:204–210. 10.1053/j.ackd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Doggrell SA, Brown L. 1998. Rat models of hypertension, cardiac hypertrophy, and failure. Cardiovasc Res 39:89–105. 10.1016/S0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 5.Engler S, Paul M, Pinto YM. 1998. The TGR(mRen2)27 transgenic rat model of hypertension. Regul Pept 77:3–8. 10.1016/S0167-0115(98)00120-7. [DOI] [PubMed] [Google Scholar]
- 6.Falaschetti E, Mindell J, Knott C, Poulter N. 2014. Hypertension management in England: a serial cross-sectional study from 1994 to 2011. Lancet 383:1912–1919. 10.1016/S0140-6736(14)60688-7. [DOI] [PubMed] [Google Scholar]
- 7.FELASA Working Group on Animal Health. Kraft V, Deeny AA, Blanchet HM, Boot R, Hansen AK, Hem A, van Herck H, Kunstyr I, Milite G, Needham JR, Nicklas W, Perrot A, Rehbinder C, Richard Y, De Vroey G. 1994. Recommendations for the health monitoring of mouse, rat, hamster, guinea pig, and rabbit breeding colonies. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management November 1992. Lab Anim 28:1–12.doi: 10.1258/002367794781065933 [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee 2013. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129:e28–e292. 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes AC, Falcão-Pires I, Pires AL, Brás-Silva C, Leite-Moreira AF. 2012. Rodent models of heart failure: an updated review. Heart Fail Rev 18:219–249. 10.1007/s10741-012-9305-3. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez AA, Prieto MC. 2015. Renin and the (pro)renin receptor in the renal collecting duct: role in the pathogenesis of hypertension. Clin Exp Pharmacol Physiol 42:14–21. 10.1111/1440-1681.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasenfuss G. 1998. Animal models of human cardiovascular disease, heart failure, and hypertrophy. Cardiovasc Res 39:60–76. 10.1016/S0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research 2011. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123:933–944. 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 13.Heijnen BF, Pelkmans LP, Danser AJ, Garrelds IM, Mullins JJ, De Mey JG, Struijker-Boudier HA, Janssen BJ. 2013. Cardiac remodeling during and after renin-angiotensin system stimulation in Cyp1a1–Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 15:69–81. [DOI] [PubMed] [Google Scholar]
- 14.Heijnen BFJ, Nelissen J, van Essen H, Fazzi GE, Cohen Tervaert JW, Peutz-Kootstra CJ, Mullins JJ, Schalkwijk CG, Janssen BJ, Struijker-Boudier HA. 2013. Irreversible renal damage after transient renin–angiotensin system stimulation: involvement of an AT1 receptor-mediated immune response. PLoS One 8:1–17. 10.1371/journal.pone.0057815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijnen BFJ, Peutz-Kootstra CJ, Mullins JJ, Janssen BJA, Struijker-Boudier HAJ. 2011. Transient renin–angiotensin system stimulation in an early stage of life causes sustained hypertension in rats. J Hypertens 29:2369–2380. 10.1097/HJH.0b013e32834cfcf4. [DOI] [PubMed] [Google Scholar]
- 16.Herrera VLM, Ruiz-Opazo N. 2005. Genetic studies in rat models: insights into cardiovascular disease. Curr Opin Lipidol 16:179–191. 10.1097/01.mol.0000162323.77666.5e. [DOI] [PubMed] [Google Scholar]
- 17.Howard CG, Mitchell KD. 2012. Renal functional responses to selective intrarenal renin inhibition in Cyp1a1–Ren2 transgenic rats with ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 302:F52–F59. 10.1152/ajprenal.00187.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard CG, Mullins JJ, Mitchell KD. 2010. Transient induction of ANG II-dependent malignant hypertension causes sustained elevation of blood pressure and augmentation of the pressor response to ANG II in Cyp1A1–Ren2 transgenic rats. Am J Med Sci 339:543–548. 10.1097/MAJ.0b013e3181d82a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. 2001. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem 276:36727–36733. 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 20.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. 2005. Global burden of hypertension—analysis of worldwide data. Lancet 365:217–223. 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Nangaku M, Navar LG, Nishiyama A. 2007. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59:251–287. 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 22.Krinkle GJ. 2000The laboratory rat—the handbook of experimental animals. London (United Kingdom): Academic Press. [Google Scholar]
- 23.Lee MA, Bohm M, Paul M, Bader M, Ganten U, Ganten D. 1996. Physiological characterization transgenic of the hypertensive transgenic rat TGR(mRen2)27. Am J Physiol 270:E919–E929. [DOI] [PubMed] [Google Scholar]
- 24.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry for Primary Industries New Zealand. [Internet]. 2018. Animal Welfare Act 1999. [Cited 24 August 2018]. Available at: https://www.mpi.govt.nz/law-and-policy/legal-overviews/animal-welfare/
- 26.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. 2006. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1–Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst 7:74–86. 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell KD, Mullins JJ. 2005. Enhanced tubuloglomerular feedback in Cyp1a1–Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol 289:F1210–F1216. 10.1152/ajprenal.00461.2004. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz RM, Graciano ML, Mullins JJ, Mitchell KD. 2007. Aldosterone receptor antagonism alleviates proteinuria but not malignant hypertension in Cyp1a1–Ren2 transgenic rats. Am J Physiol Renal Physiol 293:F1584–F1591. 10.1152/ajprenal.00124.2007. [DOI] [PubMed] [Google Scholar]
- 29.Peters B, Grisk O, Becher B, Wanka H, Kuttler B, Ludemann J, Lorenz G, Rettig R, Mullins JJ, Peters J. 2008. Dose-dependent titration of prorenin and blood pressure in Cyp1a1–Ren2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J Hypertens 26:102–109. 10.1097/HJH.0b013e3282f0ab66. [DOI] [PubMed] [Google Scholar]
- 30.Peters BS, Dornaika R, Hosten N, Hadlich S, Mullins JJ, Peters J, Rettig R. 2012. Regression of cardiac hypertrophy in Cyp1a1–Ren2 transgenic rats. J Magn Reson Imaging 36:373–378. 10.1002/jmri.23661. [DOI] [PubMed] [Google Scholar]
- 31.Peters BS, Kuttler B, Beineke A, Lorenz G, Thiele A, Nicolai O, Rettig R, Mullins JJ, Peters J. 2010. The renin–angiotensin system as a primary cause of polyarteritis nodosa in rats. J Cell Mol Med 14:1318–1327. 10.1111/j.1582-4934.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulter NR, Prabhakaran D, Caulfield M. 2015. Hypertension. Lancet 386:801–812. 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 33.Sharp PE, LaRegina MC, Suckow MA. 1998The laboratory rat: the laboratory animal pocket reference series. Boca Raton (FL): CRC Press. [Google Scholar]
- 34.Suckow MA, Weisbroth SH, Franklin C. 2005. The laboratory rat, 2nd ed. San Diego (CA): Elsevier Academic Press. [Google Scholar]
- 35.Tsioufis C, Tsiachris D, Kasiakogias A, Dimitriadis K, Petras D, Goumenos D, Siamopoulos K, Stefanadis C. 2013. Preclinical cardiorenal interrelationships in essential hypertension. Cardiorenal Med 3:38–47. 10.1159/000346817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf PA, Abbott RD, Kannel WB. 1991. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22:983–988. 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 37.Wolfensohn S, Lloyd M.1998. Handbook of laboratory animal management and welfare. West Sussex (United Kingdom): John Wiley and Sons. [Google Scholar]