Abstract
Aim:
To estimate the mean lifetime survival benefit, an essential component of health economic evaluations in oncology, of adding tumor treating fields (TTFields) to maintenance temozolomide (TMZ) for newly diagnosed glioblastoma patients.
Methods:
We integrated EF-14 trial data with glioblastoma epidemiology data. The model provided for an evidence-based approach to estimate lifetime survival for the material number of EF-14 trial patients still alive at 5 years.
Results & conclusion:
Patients treated with TTFields and TMZ had an incremental mean lifetime survival of 1.8 years (TTFields/TMZ: 4.2 vs TMZ alone: 2.4). Patients alive at year 2 after starting TTFields had a 20.7% probability of surviving to year 10. The results presented here provide the required incremental survival benefit necessary for a future assessment of the incremental cost–effectiveness of TTFields.
Keywords: : conditional survival, glioblastoma, life years gained, long-term survival, survival model, tumor treating fields
Practice points.
Tumor treating fields (TTFields) for glioblastoma resulted in 5-year survival of 12.8% in the EF-14 trial.
Epidemiological data suggest glioblastoma survival prognosis improves with time.
We combined trial and epidemiological data to model lifetime glioblastoma survival.
Modelling indicates a substantial increase in lifetime survival for GBM patients treated with TTFields.
Glioblastoma (GBM) is the most common and aggressive primary brain malignancy. The estimated incidence of GBM is 12,390 new cases each year in the USA [1]. The age at diagnosis is in the mid-60s in epidemiology reports and in the mid-50s in clinical trial populations [2–4]. The disease progresses rapidly without advanced treatment; however, clinical and epidemiological literature has consistently indicated that a small subset of patients survives to 5, 10 and 15 years [5–7].
Tumor treating fields (TTFields) are low-intensity alternating electric fields delivered at intermediate frequencies intended to disrupt cancer cell division and inhibit tumor growth. TTFields have been studied since the year 2000 in preclinical models and in-clinical trials for GBM and other solid tumor cancers [8,9]. The therapy is delivered to GBM patients by transducer arrays placed on the scalp. TTFields rely on a novel physics-based mechanism of action that is unlike previous applications of electricity or ionizing radiation in medicine [10].
The US FDA approved TTFields as a GBM treatment initially in 2011 for recurrent GBM and later in 2015 for newly diagnosed GBM [8], based on the interim results of the randomized, controlled Phase III EF-14 trial. The final analysis of the EF-14 trial demonstrated that adding TTFields to maintenance temozolomide (TMZ) chemotherapy within the existing standard of care significantly prolonged median overall survival compared with the standard of care alone (20.9 vs 16.0 months; HR: 0.63; p < 0.00006) [11]. The combination of TMZ and TTFields has resulted in the first report from a large clinical trial of 5-year survival in GBM greater than 10% [11]. The survival benefit was achieved without an increase in systemic toxicity or a decrease in quality of life [12]. The clinical use of TTFields is increasing and the therapy is now available in the USA, Germany, Austria, Switzerland, Israel and Japan [13]. The National Comprehensive Cancer Network has added TTFields as a standard-of-care treatment for GBM with a category 1 recommendation based on the EF-14 trial results [14].
An understanding of the predicted prognosis for GBM patients after the clinical trial period is important to facilitate informed clinical, personal and policy decision-making. Specifically, healthcare payers and policymakers often benefit from evaluating the lifetime cost of a therapy against the lifetime clinical benefit. Clinical trials only report data for a specific time period, which is typically a maximum of 5 years in oncology. Healthcare payers require tools to model the expected future costs and survival times for those patients alive at the last reported date of a trial.
The challenge of modeling long-term GBM survival is that the disease is characterized by a period of high mortality after onset, followed by survival probabilities that increase with time from diagnosis [6,15]. Statistical survival extrapolations that are commonly used in outcomes research are based on regression analysis. These parametric distribution models rely on regression analysis of patient level clinical trial data and therefore do not allow for an assumption of a nonconstant hazard function with time from diagnosis [16,17].
Regression-based estimation methods are biased by the initial period of high mortality in GBM and will fail to account for the known presence of long-term survivors in GBM after clinical trial reported outcomes. Notably, there is evidence of TTFields-treated GBM patients surviving to 5 and 10 years after treatment, including after an initial progression of the disease [18–21]. These reported outcomes are consistent with epidemiological reports of long-term GBM survivors [6,22,23].
The objective of this study was to develop a model to estimate GBM survival that integrates clinical trial data with real-world reported outcomes for GBM populations. The model benefited from the availability of 5-year survival data of the EF-14 trial and multiple epidemiological studies of long-term survival outcomes in GBM.
Materials & methods
Integrated survival model approach
A Bayesian area under the curve survival model framework was constructed to estimate the overall life expectancy of newly diagnosed GBM patients. The incremental mean survival benefit was calculated as the difference between the two survival curves (AUCincremental = AUCTTF+TMZ – AUCTMZ). The model was programed to represent a lifetime horizon, modeling patients from the start of TTFields with maintenance TMZ versus maintenance TMZ alone. Patients were assumed to start treatment at the age of 56 years, consistent with the EF-14 trial population. Survival was estimated over the next 40 years.
The model estimated both mean life years and conditional survival probabilities for long-term survivors. Conditional survival is defined as the probability of a patient surviving for y additional years given that they had already survived to x years from starting treatment or diagnosis [24].
The integrated survival model was designed to replicate the EF-14 trial design and population. The EF-14 trial enrolled 695 patients with GBM who had undergone maximal safe surgery, including biopsy only when surgery was not possible and completed 60 Gy of radiation with concurrent TMZ without tumor progression. Patients were randomized 2:1 to receive either TTFields with maintenance TMZ or maintenance TMZ alone.
The EF-14 Kaplan–Meier (K–M) survival data by year is reported for each arm in Table 1 and is based on the published final analysis of the trial data reported in 2017 [11]. The reported 5-year survival was 12.8% for patients treated with TTFields and maintenance TMZ versus 4.5% for patients treated with maintenance TMZ alone (p = 0.004). The hazard ratio between the two arms was 0.63 (95% confidence interval [CI] 0.53–0.76; p = 0.00006). The K–M survival curves demonstrated that the benefit of adding TTFields was maintained throughout the entire 5-year trial period [11]. A subgroup or responder-based survival model was beyond the scope of this analysis and was not considered meaningful as the benefit of TTFields was not restricted to a specific group of patients [11].
Table 1. . EF-14 survival rates.
| Survival | TTFields with maintenance TMZ (%) | Maintenance TMZ alone (%) |
|---|---|---|
| Year 1 survival | 73.2 | 65.3 |
| Year 2 survival | 43.1 | 30.7 |
| Year 3 survival | 25.9 | 16.3 |
| Year 4 survival | 19.6 | 7.9 |
| Year 5 survival | 12.8 | 4.5 |
TMZ: Maintenance temozolomide; TTFields: Tumor treating fields.
Data taken from [11].
The integrated survival model then synthesized the EF-14 K–M survival data from treatment initiation until year 5 with epidemiological survival rates in GBM from year 5 to year 15. Patients alive at year 15 are assumed to return to the baseline mortality rate of the age-adjusted US population [25].
The survival results were calculated with and without a 3% discount rate applied to future health outcomes. The use of a discount rate is common in health outcome and health economic studies, representing the theoretical higher value of near-term versus long-term survival and the 3% rate was selected based on current guidelines for US studies [26]. One-way and probabilistic sensitivity analyses were performed to assess uncertainty. Bayesian 95% credible ranges (CR) were estimated for each model outcome.
Selection of epidemiology data
The epidemiological data was selected based on a literature search. The MEDLINE® database of the US National Library of Medicine was accessed via the PubMed® website. A Boolean word search was conducted using the keyword combination ‘glioblastoma’ and ‘long-term survival’ or ‘conditional survival’. Of the 473 publications screened, 22 publications were reviewed in full text and five publications were selected for a detailed review.
All five publications indicated that the probability of surviving GBM increased as patients survived longer from diagnosis and that the first 2 years after diagnosis were the period of the highest mortality hazard rates [6,15]. Two publications based on single institution reports were then excluded in favor of larger epidemiological populations [15,27].
The review of the three epidemiological studies identified the introduction of TMZ in 2005 to be a potential confounding factor [6,28,29]. TMZ became the principal chemotherapy used to treat GBM in 2005 after demonstrating a significant survival benefit both in median survival and 5-year survival [4].
Epidemiological reports that included pre- and post-2005 populations were subject to data censoring requirements that may have biased the reporting of the conditional survival rate from 5 years to 10 years after diagnosis. Specifically, the benefit of TMZ was available for analysis at the 5-year survival point but only patients from the pre-TMZ era were available for analysis at the 10-year survival mark.
The epidemiological data published by Porter et al. was selected for inclusion in the survival model based on its homogeneous population of patients who were treated prior to the introduction of TMZ. Porter et al. reported primary malignant and nonmalignant brain tumor cases diagnosed from 1985–2005 from the National Cancer Institute Surveillance, Epidemiology and End Results Program registries, including 5991 GBM patients. This study provided survival probabilities through 15 years after diagnosis with GBM. The probability of surviving GBM to 10 years and 15 years given survival to 5 years and 10 years was 70.4% (95% CI: 55.6–81.2%) and 84.0% (95% CI: 38.9–96.8), respectively [6].
The model utilized weekly cycles to calculate survival and converted the long-term conditional survival probabilities to weekly mortality probabilities. The 70.4% probability of surviving at year 10 given survival to year 5 was converted to a weekly survival probability of 0.9987 and inversely a weekly mortality probability of 0.0013. To test the sensitivity of the survival results to the accuracy of the epidemiological data utilized in this study, we varied the reported long term survival rates by ±20% for the period following year 5.
Additional parametric modeling
Parametric distribution models, including exponential, Weibull, log-logistic, and log-normal functions, of the EF-14 trial K–M survival data were developed for validity testing against the available reported real-world outcomes for long-term survival. This approach to test regression-based parametric models was previously reported by Holland et al. [30]. The parametric models were also developed to allow for use in probabilistic sensitivity analysis. The best parametric fit was assessed using a combination of Akaike's information criterion and face validity inspection for the 5-year trial data period.
Results
Mean lifetime survival estimated by the integrated survival model
Survival benefits were estimated over a lifetime horizon and represent the mean survival accrued for a population of newly diagnosed GBM patients treated with and without adding TTFields to maintenance TMZ. The estimated mean lifetime survival was 4.2 years (95% CR: 3.8–4.6) when TTFields was added to maintenance TMZ and 2.4 years (95% CR: 2.3–2.6) for patients treated with maintenance TMZ alone, accounting for 1.8 incremental life years gained (LYG; 95% CR: 1.5–2.1). The resulting estimate of LYGs was 1.2 years after applying a 3% discount rate (95% CR: 1.1–1.4).
To test the sensitivity of the results to the epidemiology data utilized in this study, one-way sensitivity analysis varied the long-term survival rates reported by Porter et al. by 20%. Decreasing the epidemiology survival rates by 20% estimated a mean survival benefit of 1.4 years (undiscounted). Increasing the epidemiology survival rates by 20% resulted in an estimated survival benefit of 2.2 years (undiscounted).
Conditional survival estimated by the integrated survival model
The conditional probability for patients alive 2 years after starting treatment to survive to years 3, 4, 5, 10 and 15 are presented in Table 2. Patients treated with TTFields and maintenance TMZ who were alive at year 2 after starting treatment had a 29.4% probability of surviving to year 5 (95% CR: 24.4–31.2%) and a 20.7% probability of surviving to year 10 (95% CR: 14.0–24.6%). For patients treated with maintenance TMZ alone, the probability of surviving from year 2 to year 5 was 14.7% (95% CR: 18.5–23.7%) and the probability of surviving from year 2 to year 10 was 10.3% (95% CR: 11.2–18.3%).
Table 2. . The conditional survival rates estimated by the integrated survival model.
| Survival to year given 2-year survival | TTFields with maintenance TMZ | Maintenance TMZ alone |
|---|---|---|
| Year 2 | 100% | 100% |
| Year 3 | 59.6% | 53.1% |
| Year 4 | 45.3% | 25.7% |
| Year 5 | 29.4% | 14.7% |
| Year 10 | 20.7% | 10.3% |
| Year 15 | 17.4% | 8.7% |
The conditional survival rates estimated by the integrated survival model at future time points given a patient has survived to 2 years.
TMZ: Maintenance temozolomide; TTFields: Tumor treating fields.
Outcomes of regression-based parametric modeling and validity testing
The best fit for the TTFields with maintenance TMZ arm was the log-normal distribution and the best fit for the maintenance TMZ alone arm was the log-logistic distribution (Table 3). Despite being the best fit, the parametric curve for the maintenance TMZ alone arm visibly underestimated the EF-14 K–M survival results when plotted.
Table 3. . Akaike information criterion scores.
| Distribution | TTFields with maintenance TMZ | Maintenance TMZ alone |
|---|---|---|
| Exponential | 5335.59 | 5392.48 |
| Weibull | 5286.62 | 5319.66 |
| Log-Normal | 5227.00 | 5332.34 |
| Log-Logistic | 5227.56 | 5306.78 |
AIC scores for parametric fits to EF-14 trial Kaplan–Meier overall survival data. Best fits (lowest scores) are bolded.
AIC: Akaike information criterion; TMZ: Maintenance temozolomide; TTFields: Tumor treating fields.
The parametric models also estimated conditional survival from year 5 to year 10 of 21.9% and 24.0% for treatment with TTFields and maintenance TMZ versus maintenance TMZ alone, respectively (Table 4). These results substantially underestimated survival compared with real world outcomes reported in large epidemiological studies [6,15,27–30].
Table 4. . Comparison of parametric-estimated conditional survival rates to real-world reported outcomes in glioblastoma.
| Conditional probability of survival for each 5-year interval | Parametric model: TTFields with maintenance TMZ (%) | Parametric model, maintenance TMZ alone (%) | Survival rates prior to TMZ (Porter et al.) (%) |
|---|---|---|---|
| Year 5 to year 10 | 21.9 | 24.0 | 70.4 |
| Year 10 to year 15 | 32.4 | 42.7 | 84.0 |
| Year 15 to year 20 | 40.5 | 54.8 | N/A |
TMZ: Maintenance temozolomide; TTFields: Tumor treating fields.
Discussion
GBM is a highly aggressive tumor that requires intensive treatment to maximize survival. The disease affects a relatively young population, indicating that the disease often strikes during the peak productive years for adults. The age of the patients also indicates that successful intervention has the potential to produce substantial survival benefits for those who survive the early stages of the disease when measured over the remaining lifetime of the patients.
The integrated survival model allows for the synthesis of 5-year survival data from a large randomized controlled trial and real-world outcomes for GBM patients alive 5 to 15 years after diagnosis. This integrated modeling approach relied on actual reported outcomes to estimate future survival and did not rely on statistical extrapolations and assumptions.
Regression-based parametric models produced survival estimates that were inconsistent with both the EF-14 trial data and epidemiological data. The parametric models estimated survival rates after year 5 that were substantially below the real-world outcomes reported by Porter et al. Additionally, the parametric models estimated a higher hazard of death after year 5 for patients treated with TTFields and maintenance TMZ than for patients treated with maintenance TMZ alone; a finding that was inconsistent with the EF-14 K–M survival data, which reported lower mortality rates for TTFields treated patients during the entire trial period [11]. The reason for this discrepancy is the constant hazard function for death overtime that is inherent to regression-based statistical parametric models was not observed in the EF-14 trial or previous analysis of GBM survival data [11,15].
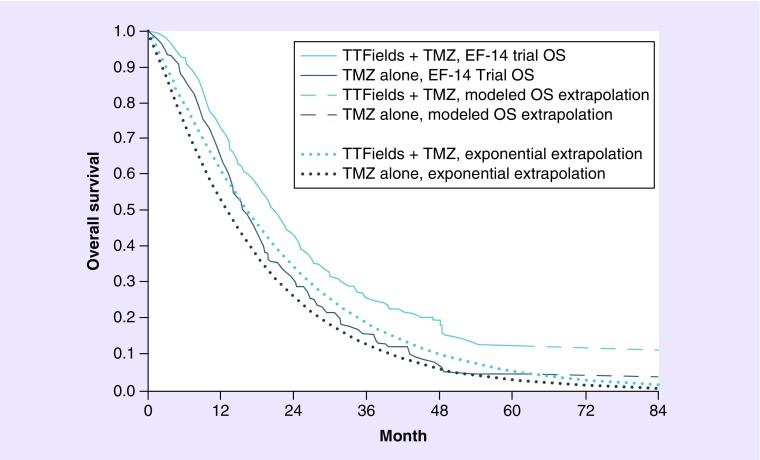
The limitations of statistical extrapolation of GBM survival can be observed in the only prior attempt to model lifetime survival based on the EF-14 trial data, which estimated GBM survival using exponential extrapolation of median EF-14 survival rates [31]. We plotted the exponential extrapolation method against the reported EF-14 K–M survival curves in Figure 1. The exponential extrapolation had the worst fit by Akaike's information criterion testing (Table 3) and was a poor visual fit to the EF-14 K–M survival curves in Figure 1. Specifically, estimated 5-year survival for patients treated with TTFields and maintenance TMZ was only 5.5%, substantially below the actual reported K–M result of 12.8%.
Figure 1. . Comparison of final EF-14 survival curves (with modeled extrapolation) to the previously reported survival estimates.
OS: Overall survival; TMZ: Temozolomide; TTFields: Tumor treating fields.
The National Institute for Health and Care Excellence in the UK considered a similar survival model structure in its decision to license ipilimumab [32,33]. Recent academic research has also relied on this approach to assess ipilimumab and pembrolizumab [34,35].
The integrated survival model is subject to certain limitations. First, the model relied on trial and epidemiological survival rates as an input. The model therefore combined data from two sources and assumed that patients alive at 5 years in one dataset will have the same future outcomes as patients in the other dataset. The benefit of this approach is that long-term survival is consistent with available data from real-world reported outcomes over decades. The sensitivity analysis demonstrated that even if the modeled survival rate after year 5 was overstated by 20%, the incremental mean lifetime survival benefit of adding TTFields to maintenance TMZ was still substantial at 1.4 years.
Another limitation of the model is that the clinical trial input to the model was a single pivotal trial of TTFields. GBM is a relatively rare disease and multiple pivotal trials are generally not feasible prior to regulatory approval and product launch. The limitation is mitigated by the size and rigor of the EF-14 trial. The EF-14 trial was a multinational randomized controlled trial run in leading cancer institutions specialized in treating CNS tumors and enrolled 695 patients (about 5% of the GBM annual incidence in the USA). This risk is further mitigated by the fact that the survival results for the maintenance TMZ alone arm in the EF-14 trial were consistent with outcomes reported in a prior trial with a comparable design [36].
One more possible limitation of this model is that it does not differentiate between patients with different genetic tumor markers (e.g., MGMT promotor methylation and IDH1 mutation). Patients with methylated MGMT promotors (about 40% of GBM patients) are known to have much longer survival times when receiving TMZ than those with unmethylated promotors [4,7]. In addition, patients with secondary GBM transforming from low-grade astrocytomas to GBM are characterized by mutated IDH1 (about 6% of the GBM population). These patients have significantly longer survival times as well regardless of treatment. Although patients with these different genetic tumor markers were equally distributed between groups in the EF-14 trial, it is unknown whether the incidence of the different genetic markers is the same between the EF-14 trial and the epidemiological data used in this model, since Porter et al. did not report this data.
Conclusion
The integrated survival model provides physicians, patients and payers with the ability to estimate mean lifetime survival in GBM based on the synthesis of the most recent clinical data and epidemiological sources. This approach avoids the limitations of parametric survival models that are based on regression-analysis of patient level trial results. The integrated survival model results indicated that the addition of TTFields to maintenance TMZ resulted in a substantial increase in mean lifetime survival for GBM patients. This estimated increase in mean lifetime survival of 1.8 years is highly significant for a disease with an historical median survival of just over a year.
Footnotes
Availability of data & materials
All data generated or analyzed during this study are included in this published article.
Financial & competing interests disclosure
The funding for this study was provided by Novocure. G Guzauskas, M Salzberg and B Wang are paid consultants to Novocure. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Authors’ contributions
G Guzauskas and B Wang contributed to conceptualization, methodology, formal analysis, original draft and reviewing and editing. M Salzberg contributed to supervision, validation and reviewing and editing.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro. Oncol. 2014;16(Suppl. 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy: temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro. Oncol. 2016;18(Suppl. 5):v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Davis FG, Mccarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology and end results (SEER) data. Cancer. 1999;85(2):485–491. [PubMed] [Google Scholar]
- 6.Porter KR, Mccarthy BJ, Berbaum ML, Davis FG. Conditional survival of all primary brain tumor patients by age, behavior and histology. Neuroepidemiology. 2011;36(4):230–239. doi: 10.1159/000327752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro. Oncol. 2016;18(10):1338–1349. doi: 10.1093/neuonc/now182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 10.Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl Acad. Sci USA. 2007;104(24):10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taphoorn M, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncology. 2018;4(4):495–504. doi: 10.1001/jamaoncol.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novocure, Inc. 2017. www.novocure.com
- 14.National Comprehensive Cancer Network (NCCN) NCCN Guidelines for central nervous system cancers. 2018. www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- 15.Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Conditional probability of survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2011;29(31):4175–4180. doi: 10.1200/JCO.2010.32.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annemans L, Asukai Y, Barzey V, et al. Outcomes assessment: extrapolation in oncology modeling: novel methods for novel compounds. ISPOR Connections. 2012;18(4) [Google Scholar]
- 17.Benbassat J, Zajicek G, Van Oortmarssen GJ, Ben-Dov I, Eckman MH. Inaccuracies in estimates of life expectancies of patients with bronchial cancer in clinical decision making. Med. Decis. Making. 1993;13(3):237–244. doi: 10.1177/0272989X9301300310. [DOI] [PubMed] [Google Scholar]
- 18.Rulseh AM, Keller J, Klener J, et al. Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World J. Surg. Oncol. 2012;10:220. doi: 10.1186/1477-7819-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villano JL, Williams LE, Watson KS, et al. Delayed response and survival from NovoTTF-100A in recurrent GBM. Medical Oncol. 2013;30(1):338. doi: 10.1007/s12032-012-0338-1. [DOI] [PubMed] [Google Scholar]
- 20.Vymazal J, Wong ET. Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin. Oncol. 2014;41(Suppl. 6):S14–S24. doi: 10.1053/j.seminoncol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Rulseh AM, Šroubek J, Klener J, Vymazal J. 5th Quadrennial Meeting of the World Federation of Neuro-Oncology Societies (WFNOS) Zurich, Switzerland: 2017. Long-term survival in glioblastoma patients after tumor-treating fields (TTFields) therapy. [Google Scholar]
- 22.Gittleman H, Boscia A, Ostrom QT, et al. Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro. Oncol. 2018 doi: 10.1093/neuonc/noy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. a systematic review. J. Clin. Neurosci. 2018;54:7–13. doi: 10.1016/j.jocn.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Henson DE, Ries LA. On the estimation of survival. Semin. Surg. Oncol. 1994;10(1):2–6. doi: 10.1002/ssu.2980100103. [DOI] [PubMed] [Google Scholar]
- 25.Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat. Rep. 2017;66(4):1–64. [PubMed] [Google Scholar]
- 26.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices and reporting of cost–effectiveness analyses: second panel on cost–effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 27.McNamara MG, Lwin Z, Jiang H, et al. Conditional probability of survival and post-progression survival in patients with glioblastoma in the temozolomide treatment era. J. Neurooncol. 2014;117(1):153–160. doi: 10.1007/s11060-014-1368-7. [DOI] [PubMed] [Google Scholar]
- 28.Farah P, Blanda R, Kromer C, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Conditional survival after diagnosis with malignant brain and central nervous system tumor in the United States, 1995–2012. J. Neurooncol. 2016;128(3):419–429. doi: 10.1007/s11060-016-2127-8. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118(22):5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 30.Holland RR, Ellis CA, Geller BM, Plante DA, Secker-Walker RH. Life expectancy estimation with breast cancer: bias of the declining exponential function and an alternative to its use. Med. Decis. Making. 1999;19(4):385–393. doi: 10.1177/0272989X9901900406. [DOI] [PubMed] [Google Scholar]
- 31.Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost–effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro. Oncol. 2016;18(8):1129–1136. doi: 10.1093/neuonc/now102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J, Hatswell AJ, Nathan P, Lebmeier M, Lee D. The predicted impact of ipilimumab usage on survival in previously treated advanced or metastatic melanoma in the UK. PloS ONE. 2015;10(12):e0145524. doi: 10.1371/journal.pone.0145524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute for Clinical Excellence. Ipilimumab for previously treated advanced (unresectable or metastatic) melanoma | Guidance and guidelines | NICE. (TA268) 2012. www.nice.org.uk/guidance/ta268
- 34.Barzey V, Asukai Y, Gueron B, Holmberg C, Kotapati S. Cost–effectiveness of Ipilimumab in previously untreated patients for advanced melanoma in Sweden. Value Health. 2014;17(7):A642–A643. doi: 10.1016/j.jval.2014.08.2322. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Lou Y, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–844. doi: 10.1007/s40273-017-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized Phase III clinical trial. J. Clin. Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]