Abstract
Background: Nursing home (NH) residents with dementia experience high rates of intensive treatment near the end of life. Limited research examines whether treatment is concordant with goals of care (GOC).
Objectives: We analyzed data from the GOC trial to describe family decision makers' preferred GOC and perceptions of goal-concordant care for NH residents with late-stage dementia We compared subsequent treatment orders when families chose a primary goal of comfort versus other goals.
Design: We performed a secondary analysis of data from baseline and 9-month family decision-maker interviews and chart reviews.
Setting and Participants: A total of 302 dyads of NH residents and family decision makers in 22 North Carolina NHs were enrolled.
Measurements: In baseline and follow-up interviews, families reported on their and NH staff's primary GOC, and perceived prognosis and goal-concordant care. Chart reviews provided data on treatment orders, hospital transfers, and hospice, which were compared after selection of a primary goal of comfort versus other goals.
Results: Family chose comfort as the primary goal for 66% of residents at baseline, and for nearly 80% by 9 months or death. At baseline, 49% perceived concordance with NH staff on the primary goal, and 69% at follow-up. In multivariate models, choice of comfort as the primary goal, versus other goals, was associated with half as many hospital transfers (0.11 vs. 0.25/90 person-days, confidence interval [−0.2 to −0.01]), but not with hospice or treatment orders.
Conclusions: Most families chose comfort as the primary GOC. Further research is needed to translate this preference into comfort-focused treatment plans for late-stage dementia.
Clinicaltrials.gov: NCT01565642 (3/26/12).
Keywords: : decision making, dementia, palliative care
Introduction
Over 5 million Americans suffer from Alzheimer's disease and other progressive and incurable dementias. Most people with late-stage dementia live and die in nursing homes (NHs).1 Symptom distress is common in advanced dementia, as are burdensome treatments such as hospitalization, emergency room transfers, tube feeding, or parenteral therapies.2–4 Hospital transfers and intensive treatment continue into the final months of life; the rate of intensive treatment for persons with advanced dementia may be increasing over time.5,6
Family decision makers set goals and make major treatment decisions for persons with advanced dementia.7 Although health services research provides detailed insight into treatment use in late-stage dementia, far less is known about whether these treatments are concordant with patient and family goals and preferences. In a national sample of after-death interviews, 23% of family for NH decedents reported an end-of-life care decision was made that was inconsistent with the dying person's preferences.8 Clinicians know that the match between a primary goal of care (GOC) and ultimate treatment is not always straightforward; for example, comfort-focused care might occasionally include hospital transfer when comfort cannot be addressed in the NH setting. However, once a primary GOC is chosen, most treatment should be concordant with that goal.
Since there is no standardized method for measuring the concordance between GOC and treatments received, we measured goal-concordant care in two ways—by asking family decision makers their perceptions of whether treatment plans are concordant with goals and by assessing whether a primary goal of comfort compared with other goals is associated with differences in subsequent treatment.9 We hypothesized that opting for a primary goal of comfort would be associated with fewer hospitalizations and more orders to avoid intensive treatments and to relieve pain or other distressing symptoms. Using data from the GOC clinical trial, we addressed two objectives: to describe family decision makers' preferred GOC and perceptions of goal-concordant care for NH residents with late-stage dementia, and to compare subsequent treatment orders when families chose a primary goal of comfort versus other goals.
Methods
The data used for this study were from the GOC cluster randomized trial, which tested a video decision aid intervention to improve decision making about GOC for NH residents with late-stage dementia. During the trial, we conducted baseline, follow-up (3-, 6-, and 9-month), and after-death interviews with family decision makers. Details on methods and primary outcomes are published elsewhere.10,11 This analysis uses longitudinal data from the clinical trial, adjusted for study arm.
Study participants
Investigators enrolled 302 dyads of residents and their family decision makers in 22 North Carolina NHs, and followed them for 9 months or until the resident's death. Eligible residents were 65 years and older, with dementia staged 5, 6, or 7 on the Global Deterioration Scale (GDS) as affirmed by the primary nurse caring for the resident.12 Family decision makers provided written informed consent for themselves and for the residents, all of whom lacked decisional capacity. The University of North Carolina Institutional Review Board approved all study procedures.
Data collection and measures
At baseline, trained research staff collected demographic information from family decision makers about themselves and the NH resident with dementia, whether the resident had advance directives, and family perception of the resident's 6-month prognosis (During the next 6 months, what do you expect may happen to [resident], based on what you know about [his/her] health? Response options: get better, stay about the same, get worse, and get much worse or possibly even die). Chart reviews provided data at baseline to calculate the Advanced Dementia Prognostic Tool. This prognostic score ranges from 1 to 32.5, with higher scores indicating higher mortality risk.13
To meet the first objective, family decision makers responded to two items on the primary GOC to guide treatment at baseline, and at 3, 6, and 9 months of follow-up, or after a resident's death. First, family decision makers reported their choice of the “best goal to guide the resident's care and medical treatment.” Then, they reported their perception of the goal they believed was the NH staff's “top priority for the resident's care and medical treatment.” Response options were prolonging life (prolonging life as much as possible with medical treatment), supporting function (maintaining or improving function with treatments, while avoiding care that would worsen function), or improving comfort (improving the level of comfort with treatments as much as possible). Family perception of goal-concordant care was considered present when the family reported that their primary GOC was the same goal as the goal used by staff to guide actual treatment.
To further describe family decision makers' perceptions of whether any treatment was given inconsistent with residents' preferences, they also responded to the three-item Advance Care Planning (ACP) problem score, within which the final item addresses goal-concordant treatment.14 The ACP problem score ranges from 0 to 3, with lower values indicating treatment more consistent with preferences (Cronbach's alpha = 0.58–0.87). Respondents answered three yes/no items, for which “yes” responses indicate treatment more consistent with preferences:
-
1.
To the best of your knowledge, did [resident]'s doctor or the NH staff speak to you about, or review with you [resident]'s wishes about medical treatment in the past 3 months?
-
2.
Did [his/her] doctor or the NH staff speak to you, or review with you, about making sure [resident]'s care was consistent with [his/her] wishes in the past 3 months?
-
3.
In the past 3 months, was there any medical procedure or treatment that happened to [resident] that was inconsistent with [his/her] previously stated wishes?
To meet the second objective, structured chart reviews at 3, 6, and 9 months provided data on treatment orders for do-not-resuscitate (DNR), do not hospitalize, and orders to forego use of tube feeding or antibiotics, and data on hospice use, hospital transfer (hospital admission or emergency room visit), and treatment plans for physical and psychological symptoms. A treatment plan for physical and psychological symptoms was considered present if there was chart documentation of (1) physical and (2) psychological symptom assessment and treatment. Investigators created a Palliative Care Treatment Plan Domain score to capture the overall palliative care content of residents' treatment plans. Scores range from 0 to 10, with 1 point assigned when a specific domain was documented explicitly in the medical record in orders, progress notes, or treatment administration records: prognosis, GOC, assessment and treatment for physical symptoms, emotional needs, spiritual needs, and decisions to use or avoid use of five treatments: resuscitation, artificial feeding, intravenous fluids, antibiotics, and hospitalization.
Statistical analysis
Resident–family dyads were the primary unit of analysis. All analyses controlled for clustering effects at the NH level, and for study assignment to intervention or control. Demographic characteristics, GOC, and treatment experience were described in mean and standard deviation for continuous variables, and in frequency and percentage for categorical variables.
To describe temporal change in GOC, family report of the primary goal was compared at baseline and at the final time point of either 9 months or resident death through a t test for the time indicator in a generalized linear mixed effects model (GLME) after controlling for clustering effects of NHs and study assignments to intervention or control with a random intercept. All temporal changes were described in adjusted odds ratio (OR) and its 95% confidence interval (CI) using the baseline time point as the reference.
To describe families' perceptions of care concordant with preferences, we examined agreement between the family decision maker's primary GOC and their perception of the NH staff's primary goal guiding treatment for the resident and tested its temporal change using the same approach.
To compare treatments and orders between family decision makers who chose a primary goal of comfort versus other goals at baseline, we used a t test to test the difference between the two groups in a GLMM with a random intercept controlling for clustering effects and study assignments, with further adjustment for resident race, gender, age, and family-perceived prognosis (dichotomized to get better/stay about the same vs. get worse/get much worse and possibly even die). Orders not to resuscitate (DNR), hospitalize, tube feed, or use antibiotics were treated as binary outcomes, as was an indicator for the presence of a management plan for both physical and psychological symptoms, and hospice enrollment. Frequency of hospital transfers and Palliative Care Domain Score were normally distributed variables. Adjusted OR and its 95% CI were used to describe the percentage difference between two groups of dyads for binary outcomes; beta coefficient and its 95% CI were used to describe the mean difference in the normal outcomes. Analyses were implemented using SAS 9.4 (Cary, NC). All of the statistical tests were two sided. p Values <0.05 were considered statistically significant.
Results
Resident and family decision-maker characteristics by baseline GOC
Of the 302 dyads enrolled, residents with advanced dementia were 87 (SD 7.2) years old on average, and 82% (n = 246) were female. Half of the enrolled residents had GDS stage 6 dementia (n = 152), and one quarter had GDS stage 7 (n = 76) dementia. Family decision makers had a mean age of 63 years, 68% (n = 204) were female, and most were adult children of the person with dementia (n = 238, 79%). Additional resident and family decision-maker characteristics are reported in Table 1.
Table 1.
Resident and Family Characteristics at Baseline
| Resident characteristics | Total (n = 302) | Primary goal comfort at baseline (n = 200) | Other primary goal at baseline (n = 102) | p value |
|---|---|---|---|---|
| Age, mean (SD) | 86.5 (7.2) | 86.5 (7.1) | 86.5 (7.4) | 0.96 |
| Female, n (%) | 246 (81.5) | 163 (81.5) | 83 (81.4) | 0.93 |
| Race, n (%) | ||||
| White | 257 (85.4) | 180 (90.5) | 77 (75.5) | <0.01* |
| African American | 39 (13.0) | 15 (7.5) | 24 (23.5) | |
| Other | 5 (1.6) | 4 (2.0) | 1 (1.0) | |
| Hispanic or Latino, n (%) | 2 (0.7) | 2 (1.0) | 0 (0.0) | NA |
| GDS dementia stage, n (%) | ||||
| 5 | 74 (24.5) | 42 (21.0) | 32 (31.4) | <0.01* |
| 6 | 152 (50.3) | 94 (47.0) | 58 (56.9) | |
| 7 | 76 (25.2) | 64 (32.0) | 12 (11.7) | |
| ADEPTa prognostic score at baseline, mean (SD) | 8.9 (2.7) | 9.1 (2.6) | 8.5 (2.9) | 0.10 |
| Survival from enrollment, median days (range) | 274 (8–308) | 274 (8–308) | 275 (26–294) | 0.30 |
| Mortality at 9 months, n (%) | 60 (19.9) | 37 (18.5) | 23 (22.5) | 0.39 |
| Family decision-maker characteristic | ||||
| Age, mean (SD) | 62.9 (10.6) | 62.8 (10.2) | 63.0 (11.6) | 0.87 |
| Female, n (%) | 204 (67.6) | 130 (65.0) | 74 (72.6) | 0.23 |
| Race, n (%) | ||||
| White | 261 (86.7) | 182 (91.5) | 79 (77.5) | <0.001* |
| African American | 38 (12.6) | 15 (7.5) | 23 (22.5) | |
| Other | 2 (0.7) | 2 (1.0) | 0 (0.0) | |
| Hispanic or Latino | 2 (0.7) | 2 (1.0) | 0 (0.0) | NA |
| Relationship to resident, n (%) | ||||
| Daughter | 161 (53.3) | 107 (53.5) | 54 (52.9) | 0.52 |
| Son | 77 (25.5) | 55 (27.5) | 22 (21.6) | |
| Spouse | 40 (13.2) | 25 (12.5) | 15 (14.7) | |
| Other | 24 (8.0) | 13 (6.5) | 11 (10.8) | |
| Family-perceived 6-month prognosis | ||||
| Get better | 7 (2.4) | 2 (1.0) | 5 (5.2) | <0.01* |
| Stay about the same | 135 (47.2) | 78 (41.1) | 57 (59.4) | |
| Get worse | 116 (40.6) | 87 (45.8) | 29 (30.2) | |
| Get much worse and possibly even die | 28 (9.8) | 23 (12.1) | 5 (5.2) | |
ADEPT, derived from standardized variables in NH records. ADEPT scores range from 1 to 32.5, with higher scores indicating higher mortality risk.
Statistically significant at p > 0.05.
ADEPT, Advanced Dementia Prognostic Tool; GDS, Global Deterioration Scale; NA, not applicable; NH, nursing home.
Residents whose families prioritized the goal of comfort during baseline interviews were more often white (p < 0.001) with later-stage dementia (p = 0.001). Although families who opted for comfort were also more likely to expect death in the next six months (p < 0.01), median survival did not differ based on the selection of comfort as the primary GOC (p = 0.39; Table 1).
Family decision makers' GOC and perceptions of goal-concordant care
At baseline, comfort was the primary GOC chosen by 66% of family decision makers, supporting function was the primary goal for 22%, and prolonging life was the primary goal for 5%. By the time of the final interview, at 9-month follow-up or after the resident's death, nearly 80% of family decision makers reported that the primary goal was comfort (Table 2).
Table 2.
Family Goals of Care and Perceptions of Goal-Concordant Care
| Baseline (n = 302) | 9-Month follow-up/bereavement (n = 295) | Adjusted p value | |
|---|---|---|---|
| Family primary goal of carea, n (%) | |||
| Prolonging life | 16 (5.3) | 9 (3.0) | 0.16 |
| Supporting function | 65 (21.5) | 39 (13.2) | 0.01 |
| Improving comfort | 200 (66.2) | 235 (79.7) | <0.001 |
| Multiple/other | 21 (7.0) | 12 (4.1) | 0.13 |
| Perception of agreement on the primary goal of care, n (%) | |||
| Family-perceived agreement with staff | 149 (49.3) | 202 (68.5) | <0.001 |
| Family decision maker's goal is comfort | 200 (66.2) | 235 (79.7) | |
| Family perception of NH staff's goal is comfort | 177 (58.6) | 216 (73.2) | |
| ACP problem score itemsb, n (%) | |||
| ≥1 ACP problem | 196/290 (67.6) | ||
| Doctor or staff spoke about resident's treatment wishes | 118/293 (40.3) | ||
| Doctor or staff spoke about care consistent with resident's treatment wishes | 116/292 (39.7) | ||
| Any treatment inconsistent with resident's prior wishes | 9/293 (3.1) | ||
Adjusted for only clustering effect due to nonconvergence of the full model.
Denominators account for observations lost to follow-up.
ACP, Advance Care Planning.
At baseline, 49% of family decision makers perceived goal-concordant care, defined as perceiving that their chosen GOC was also the goal guiding the NH staff treatment plan. By the time of the final interview, 69% of family decision makers perceived concordance between their primary goal and the NH staff GOC. The most common pattern of perceived lack of agreement was when the family decision maker prioritized comfort, but perceived that staff used a goal other than comfort to guide treatment plans (Table 2).
On the ACP problem score, two-thirds of family decision makers perceived a problem during subsequent months of follow-up (68%). However, examining the three individual items composing the ACP problem score, families primarily reported that there were gaps in communication about resident preferences. Despite the lack of communication, only 3% perceived that the resulting treatment was inconsistent with residents' wishes (Table 2).
Treatment concordance with baseline goal of comfort versus other goals
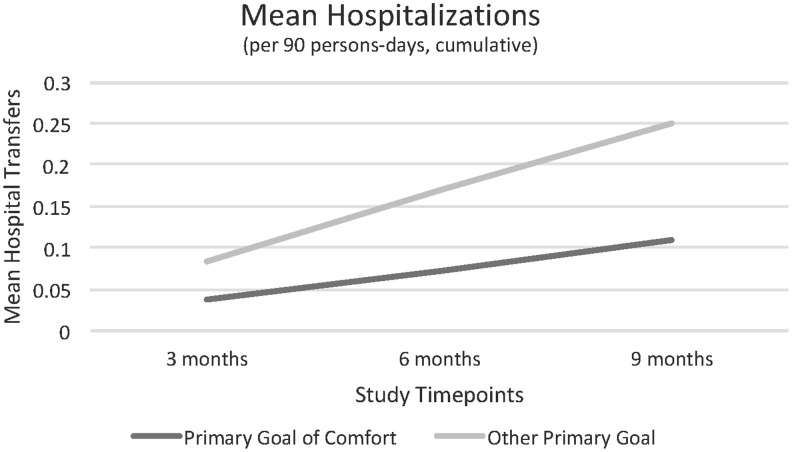
Compared with other residents with late-stage dementia, NH residents whose families chose a primary goal of comfort at baseline were half as likely to be hospitalized over the subsequent 9 months (0.11 vs. 0.25 hospitalizations per 90 person-days, β = −0.1, CI 1.3–9.5; Fig. 1). Orders regarding major treatments, enrollment in hospice, symptom management, and overall palliative care content of treatment plans did not differ significantly based on GOC (Table 3). In multivariate models, the GOC intervention was also associated with fewer hospital transfers (data reported elsewhere). African American race was associated with a decreased use of DNR and do not hospitalize orders and more hospital transfers. Later-stage dementia was associated with more do not hospitalize orders.
FIG. 1.
Mean number of hospitalizations per 90 person-days at each time point (cumulative).
Table 3.
Treatment Orders for Residents with a Primary Goal of Comfort versus Other Goals
| Treatment orders (cumulative) | Baseline goal of comfort (n = 200) | Baseline goal of supporting function, prolonging life, or other (n = 102) | OR (95% CI) |
|---|---|---|---|
| Do-not-resuscitate order | 182 (91.0%) | 83 (81.4%) | 2.4 (0.97 to 6.0) |
| Do not hospitalize order | 84 (42.0%) | 25 (24.5%) | 1.8 (0.8 to 4.3) |
| Do not tube feed order | 77 (38.5%) | 38 (37.3%) | 0.9 (0.4 to 2.1) |
| MOST for comfort-focused care | 36 (18.0%) | 12 (11.8%) | 1.5 (0.3 to 8.0) |
| Do not use antibiotics | 41 (20.5%) | 20 (19.6%) | NA |
| Hospice enrollment | 34 (17.0%) | 16 (15.7%) | NA |
| Treatment plan for physical and psychological symptoms | 145 (72.5%) | 69 (67.6%) | 1.04 (0.4 to 2.5) |
| Beta coefficient (CI) | |||
| Hospital transfers | 0.11 (0.26) | 0.25 (0.56) | −0.1 (−0.2 to −0.01)* |
| Mean (SD) per 90 person-daysa | |||
| Palliative care domain score (0–10)a | 6.1 (1.9) | 6.2 (1.9) | −0.06 (−0.7 to 0.6) |
These results are from a model including intervention arm, clustering, primary goal, resident age, race, gender, and perceived prognosis.
Four residents do not report follow-up data, two from each group.
African American race was associated with decreased use of DNR and do not hospitalize orders, and with more hospital transfers.
Beta coefficient with 95% CI.
Statistically significant.
CI, confidence interval; DNR, do-not-resuscitate; MOST, Medical Orders for Scope of Treatment; OR, odds ratio.
Discussion
Most families prioritized comfort as the primary GOC for NH residents with late-stage dementia, even when most did not expect that death was imminent. Early selection of comfort as the primary goal was associated with subsequent reductions in hospital transfers, but not with hospice or treatment plans for pain or other symptoms. Furthermore, many family decision makers recognized a lack of concordance between their primary goal and the NH treatment plan. Findings show support among family decision makers for comfort-focused dementia care, with opportunity to improve concordance between this GOC and comfort-focused treatment plans.
This study elicited family perspectives about concordance between their preferred goals and the NH staff goals and treatments for persons with late-stage dementia. Family perceptions are important when considering quality of care for dementia because families make all major treatment decisions once their relative loses capacity. At baseline, only half of family decision makers perceived concordance between their primary GOC and the goal used by NH staff, although their perception of concordance improved over time. Despite challenges in communication and in agreement on GOC, only 3% of family decision makers believed that actual treatments were discordant with preferences. Still, engaging families in comunication about treatments, prognosis, and goals may help align all sides and improve preparation for advanced dementia care. Furthermore, our finding that a primary goal of comfort was not associated with use of Medical Orders for Scope of Treatment (MOST in some states, Physician Orders for Life-Sustaining Treatment [POLST]) sets or treatment plans for pain suggests that communication between family decision makers and NH staff is likely an important mechanism by which treatment pathways can change.
When measuring care concordant with goals, it is important to acknowledge that specific treatments do not uniquely correlate with specific goals. For example, although hospital transfers are stressful and may promote discomfort, they may at times be necessary to promote the goal of comfort. Indeed, do not hospitalize orders are typically conditional; hospitalizations to promote comfort—such as treatment of a hip fracture—are considered consistent with this GOC. Other empirical research on use of Physician Orders for Life-Sustaining Treatment (POLST) and do not hospitalize orders is consistent with our finding that hospitalization is reduced but not eliminated by a primary goal of comfort.15,16
This study is strengthened by prospective data collection and use of validated outcome measures, yet some limitations should be considered. This study is a secondary analysis of data from a large clinicial trial; the trial was not powered for our outcomes, and thus these findings may be considered exploratory. Because data included in this study are from individuals enrolled in the GOC trial in one state, generalizability is limited, although existing evidence suggests North Carolina NHs are relatively representative of the national climate.17 Our chart reviews may not capture all clinical treatments and decisions, and other differences in care for comfort may have been used but not documented. However, orders and other information documented in the medical records largely drive major treatment experienced by residents, even when undocumented communication and decision making ocurs.
This study demonstrates that comfort is a high priority for most family of NH residents with dementia, yet opportunities exist to improve comfort-focused care in the NH setting. Improving comfort may include factors not captured by this study, such as interpersonal time staff spend with the resident, spiritual care, or innovative approaches, such as aromatherapy or music therapy.18–20
Conclusion
Most family decision makers for NH residents with late-stage dementia identified comfort as their primary GOC. Only half of families perceived that their primary GOC was concordant with the goals of NH staff guiding the treatment plan. Although this choice was associated with fewer hospital transfers, simply selecting the goal of comfort did not assure treatments for comfort or access to hospice. Further research is needed to translate family GOC into comfort-focused treatment plans for late-stage dementia.
Acknowledgment
This work was supported by the National Institutes of Health R01AG037483. Dr. Mitchell is supported by NIH-NIA K24AG033640.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B: Deaths: Final data for 2007. Natl Vital Stat Rep 2010;58:1–136 [PubMed] [Google Scholar]
- 2.Gozalo P, Teno JM, Mitchell SL, et al. : End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med 2011;365:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell SL, Teno JM, Kiely DK, et al. : The clinical course of advanced dementia. N Engl J Med 2009;361:1529–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson EL, Candy B, Davis S, et al. : Living and dying with advanced dementia: A prospective cohort study of symptoms, service use and care at the end of life. Palliat Med 2018;32:668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloane PD, Zimmerman S, Williams CS, Hanson LC: Dying with dementia in long-term care. Gerontologist 2008;48:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Gozalo P, Khandelwal N, et al. : Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med 2016;176:1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudore R, Lum HD, You J, et al. : Outcomes that define successful advance care planning: A delphi panel consensus. J Pain Symptom Manage 2018;55:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandelwal N, Curtis JR, Freedman VA, et al. : How often is end-of-life care in the United States inconsistent with patients' goals of care?. J Palliat Med 2017;20:1400–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unroe KT, Hickman SE, Torke AM: Care consistency with documented care preferences: Methodologic considerations for implementing the “measuring what matters” quality indicator. J Pain Symptom Manage 2016;52:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson LC, Song MK, Zimmerman S, et al. : Fidelity to a behavioral intervention to improve goals of care decisions for nursing home residents with advanced dementia. Clin Trials 2016;13:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson LC, Zimmerman S, Song MK, et al. : Effect of the goals of care intervention for advanced dementia: A randomized clinical trial. JAMA Intern Med 2017;177:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisberg B, Ferris SH, de Leon MJ, Crook T: The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982;139:1136–1139 [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SL, Miller SC, Teno JM, et al. : The advanced dementia prognostic tool: A risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manage 2010;40:639–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teno JM, Clarridge B, Casey V, et al. : Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage 2001;22:752–758 [DOI] [PubMed] [Google Scholar]
- 15.Cohen AB, Knobf M, Fried TR: Do-Not-Hospitalize orders in nursing homes: “Call the family instead of calling the ambulance.” J Am Geriatr Soc 2017;65:1573–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman SE, Nelson CA, Moss AH, et al. : The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc 2011;59:2091–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services, Centers for Medicare and Medicaid Services: Nursing Home Data Compendium: 2015 Edition. Retrieved April 2018. 2015
- 18.Ballard CG O B.rien JT, Reichelt K, Perry EK: Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: The results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry 2002;63:553–558 [DOI] [PubMed] [Google Scholar]
- 19.vansdottir HB, Snaedal J: Music therapy in moderate and severe dementia of Alzheimer's type: A case–control study. Int Psychogeriatr 2006;18:613–621 [DOI] [PubMed] [Google Scholar]
- 20.Ernecoff NC, Curlin FA, Buddadhumaruk P, White DB: Health care professionals' responses to religious or spiritual statements by surrogate decision makers during goals-of-care discussions. JAMA Intern Med 2015;175:1662–1669 [DOI] [PubMed] [Google Scholar]