Abstract
Background
Anaphase-promoting complex subunit 11 (APC11) plays an important role in gathering E2 and catalyzing ubiquitin-chain formation to support ubiquitination of substrates by acting as a catalytic core subunit of anaphase-promoting complex (APC/C). However, whether APC11 is implicated in the tumorigenesis of lung cancer is never known.
Materials and methods
In this study, we used an online survival analysis software to estimate the prognostic value of APC11 mRNA expression level for lung cancer. Cell Counting Kit-8 assay, colony-forming assay, and transwell assay were used to assess the biological functions of APC11 in lung cancer cells. Then, 107 lung cancer patient tissues were collected to examine the expression level of APC11 by immunohistochemistry staining. Kaplan–Meier method and univariate Cox regression analysis were performed to reveal the prognostic value of APC11 protein expression in lung cancer.
Results
Higher mRNA level of APC11 was significantly associated with worse survival for lung adenocarcinoma, but not for lung squamous cell carcinoma. Knockdown of APC11 by siRNA apparently inhibited cell proliferation and colon formation in both H1299 and H358 cells. In addition, silencing of APC11 decreased cell migrative and invasive abilities. Moreover, immunohistochemical analysis showed that APC11 was highly expressed in lung cancer tissues, and multivariate analysis suggested that APC11 overexpression was an independent prognostic factor in lung adenocarcinoma.
Conclusion
We suggest that APC11 could serve as a prognostic biomarker and a novel target in treating lung adenocarcinoma.
Keywords: APC11, lung cancer, cell proliferation, migration, invasion
Introduction
The ubiquitin–proteasome system (UPS) consists of three essential enzymes, the ubiquitin activating (E1), conjugating (E2), and ligase (E3) enzymes, which can methodically relocate ubiquitin molecules to substrate proteins and subsequently promote substrate degradation by the proteasome.1 The E3 ligases are classified into two groups, the homologous to the E6-AP carboxyl terminus domain containing E3s and the Really Interesting New Gene (RING) domain containing E3s.2 Anaphase-promoting complex (also called the cyclosome or APC/C) is a member of RING E3s which plays a critical role in cell mitosis by controlling the initiation of anaphase and exit from telophase.3 In vertebrates, APC/C is a 1.5 megadalton assembly ubiquitin ligase complex containing at least 15 different proteins including APC8, APC10, APC11, APC12, APC13, APC15, APC16, and the co-activator subunits Cdc20 and Cdh1.4,5 APC11 is a catalytic core subunit of the APC/C, which is responsible for gathering E2 and catalyzing ubiquitin-chain formation to support ubiquitination of substrates.6,7 A dysfunctional APC11 influenced the cell cycle process and mainly caused mitosis arrest.7 Silencing the APC11 gene in nematode embryonic cells resulted in cell cycle arrest during the first meiotic division.8 The nonsense mutations of APC6 and APC8 occasionally occurred in cancer cells from different human tissues, while the deleted mutation of APC11 was rare in cancer samples.9
Human APC11 has been cloned for over 10 years; its role as a catalytic core subunit has been extensively investigated both in vitro and in vivo.10,11 However, to our knowledge, there is no study about whether this gene could be a target for cancer therapy. Lung cancer is the most frequently diagnosed cancer and causes the highest number of cancer-related deaths.12 After exposure to chemotherapeutic stress, lung cancer cells induced a state of dormancy by activating APC/C.13 Whether APC11 was implicated in the development of lung cancer is entirely unknown. In this study, we found that APC11 could be served as a prognostic biomarker for lung adenocarcinoma, knockdown of APC11 significantly inhibited lung adenocarcinoma cell growth, colon formation, migration, and invasion.
Materials and methods
Online survival analysis
An online survival tool, which was established using gene expression data and survival information of 1,715 lung cancer patients downloaded from GEO, were used for survival analysis.14 Briefly, ANAPC11 was entered into the database (http://kmplot.com/analysis/index.php?p=service&cancer=lung) to acquire Kaplan–Meier survival plots. The HR, 95% CIs, and log-rank P were determined and presented on the main plots.
Cell culture
H1299, H358, H520, and H1915 human lung cancer cells were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium with 10% FBS at 37°C in a humidified atmosphere with 5% CO2 in air.
Immunohistochemistry (IHC) staining
The human lung cancer tissues collected from Sir Run Run Shaw Hospital of Zhejiang University were stained with APC11 antibody as described previously.15 Briefly, the tumor tissue sections of 5 microns were dehydrated and blocked by peroxidase. APC11 antibody (CST, D1E7Q, 1:1,000) was incubated overnight at 4°C. The slides were counterstained with hematoxylin (Surgipath, Richmond, IL, USA). The stained slides were observed under microscope (OLYMPUS 1 × 71; Olympus Corporation, Tokyo, Japan) and images were acquired using the software DP controller (Ver. 3.1.1.267; Olympus Corporation).
siRNA transfection
Transfections of siRNAs (synthesized by Dharmacom, Lafayette, CO, USA) were performed using the lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instruction. The target sequences are as follows: APC11: 5′-GACCATTCATGTGACCTTTTGG-3′; si-Control: 5′-AUUGUAUGCGAUCGCAGAC-3′.
Cell Counting Kit-8 (CCK-8) assay
Cells were transfected with si-Control or si-APC11 for 48 hours, then split and seeded into 96-well plates with 3,000 cells per well in quadruplicate. At 24, 48, 72, and 96 hours post cell plating, CCK-8 was added and the OD was measured.
Clonogenic survival assay
Cells were transfected with si-APC11 or si-Control for 72 hours, then split and seeded into six-well plates with 300 cells per well in triplicate, followed by incubation at 37°C for 7 days. The colonies formed were fixed with 10% acidic acid in methanol and stained with 0.05% methylene blue.
Cell migration and invasion assays
The migration and invasion capacities of lung cancer cells were assessed by using transwell assay as described previously.15 Briefly, NCI-H1299 cells transfected with si-Control or si-APC11 were cultured in RPMI-1640 medium for 24 hours and plated on the top surface of the transwell chambers (Corning Inc., Corning, NY, USA) and incubated in humidified environment with 5% CO2 at 37°C for 48 hours. For invasion assay, Matrigel (0.1 mg/mL) was coated on the top of the transwell chambers. The cells passed through polyethylene membrane (migrated cells) or through Matrigel (invaded cells) were fixed by methanol and stained by crystal violet. Then, the staining cells were observed and counted under a light microscopy (Motic, Xiamen, China).
Western blotting analysis
Whole cell lysates were prepared and subjected to immunoblotting (IB) analysis using antibodies against APC11 (D1E7Q Rabbit mAb #14090, CST; dilution 1:1,000). The signals were visualized by exposure to X-ray films by using the ECL system (GE Healthcare UK Ltd, Little Chalfont, UK).
Statistical analysis
The statistical significance of differences between groups was assessed using GraphPad Prism6 software (GraphPad Software, Inc., La Jolla, CA, USA). The unpaired, two-tailed t-test was used for the comparison of parameters between groups. The level of significance was set at P<0.05.
Ethical approval and consent to participate
This study was reviewed and approved by the Ethical Committee of Sir Run Run Shaw Hospital. Written informed consent had been obtained from all the lung cancer patients included in this study.
Results
High APC11 mRNA level was associated with worse prognosis in lung adenocarcinoma
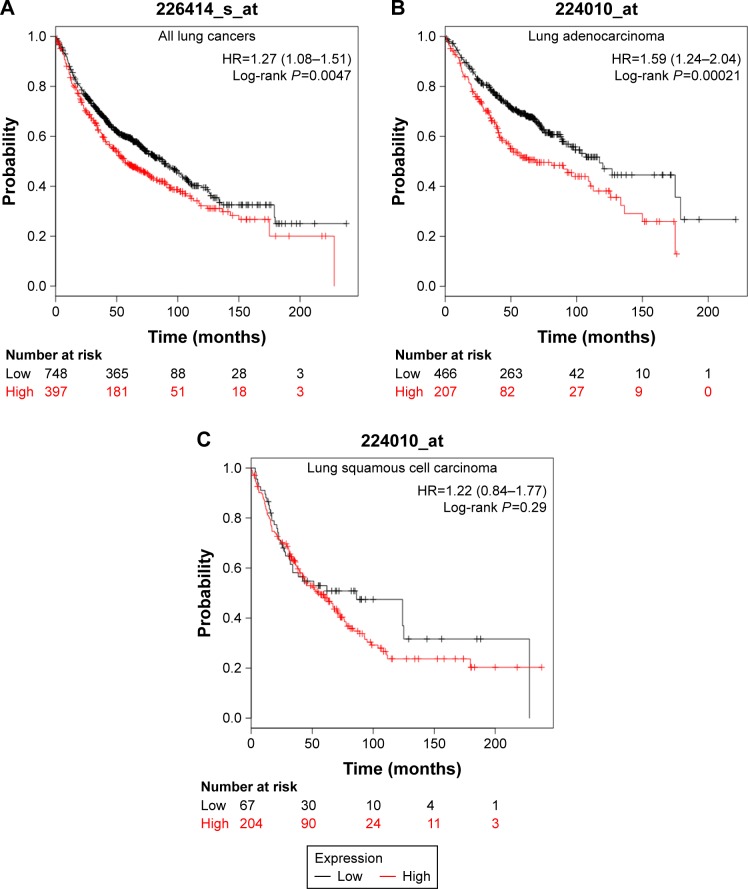
We used an online survival analysis software, which is based on the information of 1,715 lung cancer patients extracted from GEO data sets, to assess the prognostic value of APC11 mRNA expression level for lung cancers.14 As a result, high mRNA expression of APC11 was significantly associated with worse survival in lung cancer patients (Figure 1A). We further analyzed the prognostic roles of APC11 in different pathological types of lung cancers. Interestingly, higher mRNA expression level of APC11 was associated only with poor OS for lung adenocarcinoma, but not for lung squamous cell carcinoma (Figure 1B and C).
Figure 1.
The prognostic value of the mRNA expression of APC11 in www.kmplot.com. (A) Prognostic HRs of APC11 in all lung cancer. Survival curves of APC11 in lung adenocarcinoma (B) and lung squamous cell carcinoma (C).
Detection of APC11 expression in lung cancer cell lines by Western blot analysis
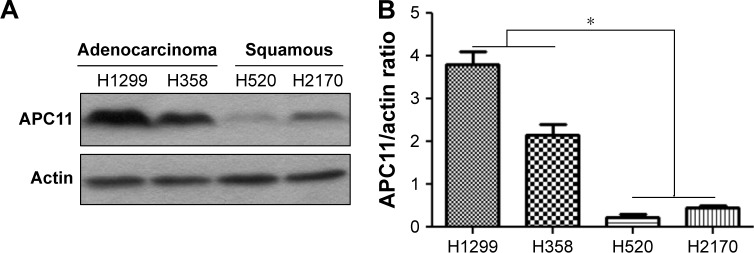
As the online data analysis indicated that high APC11 mRNA expression predicated only worse prognosis for lung adenocarcinoma, but not for lung squamous cell carcinoma. The expression levels of APC11 should be variant in different lung cancer cell lines. We then measured the protein expression of APC11 in several lung cancer cell lines by Western blot. The expression level of APC11 was much higher in lung adenocarcinoma cell lines (H358, H1299) than that in lung squamous cancer cell lines (H520, H2170) (Figure 2A), and densitometric analysis confirmed that lung adenocarcinoma cells (H358, H1299) expressed higher level of APC11 than that in H520 and H2170 cells (Figure 2B), which might suggest that APC11 was mainly exerted its oncogenic role in lung adenocarcinoma cells.
Figure 2.
(A) Different expression levels of APC11 in several lung cancer cell lines were detected by Western blot. (B) The densitometric analysis of Western blot in (A).
Note: *P<0.05.
Knockdown of APC11 inhibited lung cancer cell proliferation and metastasis
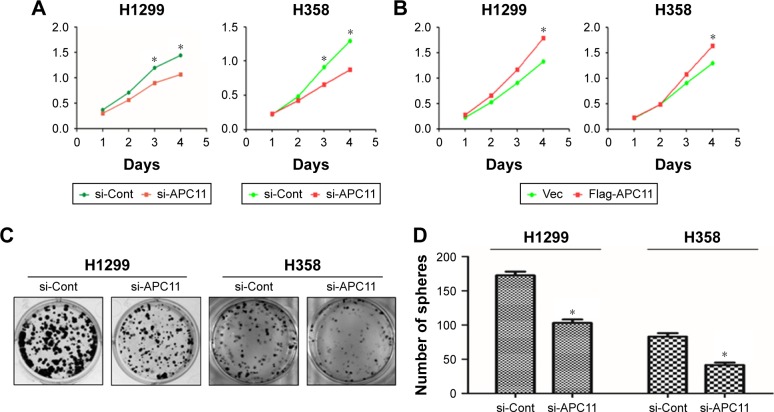
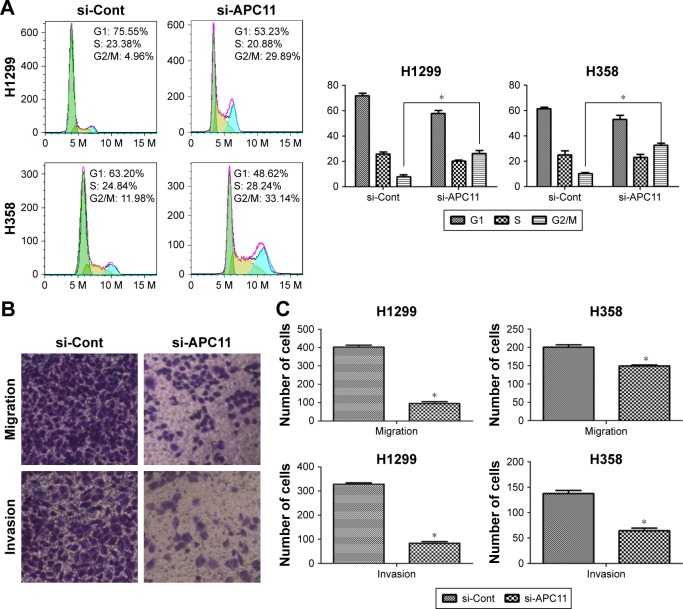
We next evaluated the role of APC11 in regulating cell proliferation and metastasis of lung cancer cells. Knockdown of APC11 by siRNA significantly reduced the cell proliferation in both H1299 and H358 cells (Figure 3A) while overexpression of APC11 could promote cell proliferation (Figure 3B). In addition, knockdown of APC11 inhibited colony forming ability of both H1299 and H358 cells (Figure 3C and D). As a catalytic core subunit of APC/C, APC11 is critical for accurate cell cycle process. Therefore, we examined whether downregulation of APC11 influenced the cell cycle distribution of lung cancer cells. As expected, silencing of APC11 caused mitosis arrest in both H1299 and H358 cells (Figure 4A). We next wanted to determine whether APC11 would affect the cancer cell metastasis. Of note, the results of Transwell assay showed that knockdown of APC11 caused an apparent reduction of migrative and invasive capacity in both H1299 and H358 cells (Figure 4B and C).
Figure 3.
(A) Growth rates were measured at the indicated times in si-Cont and si-APC11 lung cancer cells using Cell Counting Kit-8 assay. (B) Growth rate curves of Vector and Flag-APC11 transfected lung cancer cells. (C) Colony formation assay results of lung cancer cells transduced with si-Cont or si-APC11. (D) The results of colony formation assay are expressed as mean ± SD from triplicate experiments.
Note: *P<0.05.
Figure 4.
(A) Cell cycle analysis was performed in si-Cont and si-APC11 lung cancer cells using fluorescence-activated cell sorter (FACS). (B) Transwell migration and invasion assays were performed in lung cancer cells with or without APC11 silencing. (C) The results are expressed as mean ± SD from triplicate experiments.
Note: *P<0.05.
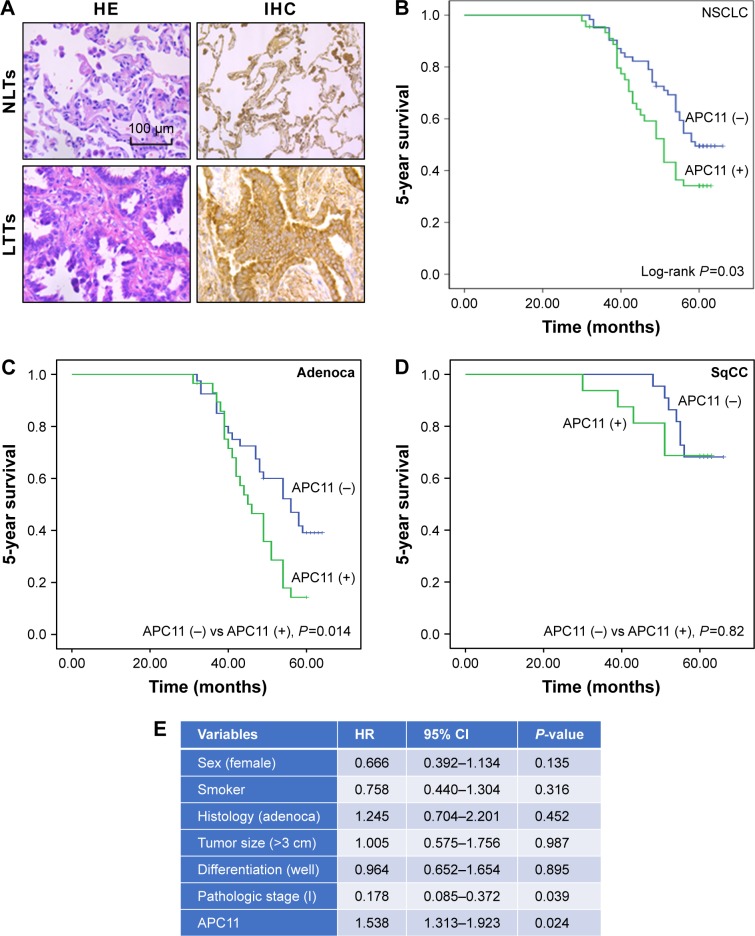
APC11 serves as an independent factor for the prediction of worse survival in lung adenocarcinoma patients
The in vitro experiments revealed that silencing of APC11 significantly inhibited lung adenocarcinoma cell proliferation, migration, and invasion. The online survival data analysis also indicated that APC11 would be a prognostic biomarker in lung adenocarcinoma. In order to further confirm that APC11 could be an independent risk factor of worse outcome in lung adenocarcinoma patients, we then measured the expression of APC11 in 10 normal tissues and 107 lung adenocarcinoma tissues by IHC staining. We could see that APC11 was expressed weakly or moderately in lung normal tissues but was overexpressed in lung tumor tissues (Figure 5A). We further divided patients into two groups, the weak and moderate staining of APC11 as negative expression group and the strong staining as positive expression group. As shown in Table 1, APC11-positive expression did not correlate with any clinical parameters of lung adenocarcinoma, except for a trend in association with tumor differentiation (P=0.054). We then employed Kaplan–Meier analysis to evaluate the relationship between APC11 expression and OS in non-small-cell lung cancer (NSCLC) patients. As shown in Figure 5B, high APC11 levels apparently affected the OS of all NSCLC patients. Furthermore, APC11-positive expression indicated a survival disadvantage in lung adenocarcinoma patients (Figure 5C), but not in lung squamous cell carcinoma patients (Figure 5D). Importantly, multivariate COX analysis suggested that over-expression of APC11 was an independent risk factor in the prediction of OS of lung cancer patients, the HR is 1.538 (95% CI 1.313–1.923, P=0.024) (Figure 5E).
Figure 5.
(A) Hemotoxylin and eosin (HE) staining of normal lung tissues (NLTs) and lung tumor tissues (LTTs). Immunohistochemical analysis of APC11 in negative (−) and positive (+) normal lung NLTs and LTTs. (B) Kaplan–Meier OS analysis of APC11 expression for NSCLC. (C) Kaplan–Meier OS analysis of APC11 expression for lung adenocarcinoma patients. (D) Survival curves of APC11 expression for lung squamous cell carcinoma patients. (E) Multivariate COX analyses of APC11 for OS in lung adenocarcinoma patients.
Table 1.
Relationship between APC11 overexpression and clinicopathologic characteristics in NSCLCs
| Variables | APC11 (−) |
APC11 (+) |
P-value |
|---|---|---|---|
| Sex | 0.443 | ||
| Female | 31 | 21 | |
| Male | 31 | 24 | |
| Age | 53+9.8 | 54.9+10.4 | 0.533 |
| Smoker | 0.086 | ||
| Former or current | 25 | 25 | |
| Never | 37 | 20 | |
| Tumor location | 0.744 | ||
| Right upper lobe | 25 | 13 | |
| Right middle lobe | 8 | 7 | |
| Right lower lobe | 12 | 12 | |
| Left upper lobe | 9 | 8 | |
| Left lower lobe | 8 | 5 | |
| Tumor size, mean±SD | 3.06+0.86 | 3.13+0.85 | 0.881 |
| Histology | 0.509 | ||
| Adenoca | 41 | 21 | |
| Seqcc | 21 | 16 | |
| Differentiation | 0.054 | ||
| Well | 22 | 22 | |
| Moderate | 21 | 18 | |
| Poor | 19 | 5 | |
| Pathologic stage | 0.640 | ||
| I | 23 | 13 | |
| II | 27 | 21 | |
| IIIA | 12 | 11 |
Discussion
The APC/C is an E3 ubiquitin ligase with critical functions in controlling cell cycle. It ubiquitinates the anaphase inhibitor securin to initiate sister chromatid separation and ubiquitinates the CyclinB1 to exit from mitosis.16 Here, we found that APC11, the catalytic core subunit of the APC/C, was much highly expressed in lung cancer tissues than that in normal lung tissues. APC11 silencing arrested lung cancer cells in M phase and inhibited cell proliferation and colon formation. Furthermore, knockdown of APC11 decreased cell migrative and invasive capacities. Importantly, APC11 appeared to be a promising biomarker for predicting the prognosis of lung cancer patients.
The APC/C includes four-part enzyme complex, a structural complex (APC1, APC4, APC5), a tetratricopeptide repeat arm (APC3, APC6-8), an activator that recruits substrates (Cdc20/Cdh1), and a catalytic arm that houses the E2-binding sites (APC22, APC11, APC10).17 Mutations in several subunits of APC/C had been reported in cancer samples and were implied as the cause for carcinogenesis.9,18 Here, we first used the online survival analysis tool to show that higher mRNA expression of APC11 was significantly associated with worse survival in lung adenocarcinoma patients, but not in lung squamous cell cancer patients. We further investigated the prognostic value of APC11 protein expression in lung cancer tissues. As expected, overexpression of APC11 in protein level also predicted poor prognosis in lung adenocarcinoma patients. The activity of APC/C oscillates during the cell cycle, resulting in different regulatory proteins undergoing degradation sequentially. Targeting some subunits of APC/C with siRNA causes cell cycle arrest and apoptosis in cancer cells.7,19,20 In the present study, we found that silencing of APC11 significantly impaired the cell proliferative and colon forming abilities. Furthermore, APC11 silencing induced cell apoptosis and cell cycle arrest. APC/C was also involved in cell cycle-independent functions, for example, APC/C regulates cellular motility by controlling Rho GTPase activity via ubiquitination of p190.21 Interestingly, we also found that knockdown of APC11 inhibited lung cancer cell migration and invasion, which implicated the role of APC11 in controlling cell motility. To our knowledge, there is no study about the prognostic significance of APC11 in cancers. In the current study, our multivariate COX analysis suggested that APC11 overexpression was an independent prognostic factor in lung adenocarcinoma.
In summary, our data indicated that overexpression of APC11 was significantly associated with unfavorable outcome, and it was also suggested that overexpression of APC11 could be an independent prognostic factor for lung adenocarcinoma patients. In addition, knockdown of APC11 could significantly inhibit lung cancer cell proliferation, colon formation, migration, and invasion by in vitro study. Taken together, overexpression of APC11 played an important role in the development of lung adenocarcinoma, and it could be a novel target for lung cancer therapy.
Footnotes
Author contributions
JZ, ZC, and SZ proposed, designed, and revised the article. GF and ZH designed, summarized data, and revised this article. YX and WY collected articles, summarized data, did statistical work, and drafted the manuscript. All authors reviewed this manuscript and approved the final draft.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 2.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10(1):29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan DO. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1(2):E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Atomic structure of the APC/C and its mechanism of protein ubiquitination. Nature. 2015;522(7557):450–454. doi: 10.1038/nature14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang LF, Zhang Z, Yang J, McLaughlin SH, Barford D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature. 2014;513(7518):388–393. doi: 10.1038/nature13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, He M, Shah AA, Wan Y. Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div. 2016;11:9. doi: 10.1186/s13008-016-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang TS, Jeong W, Lee DY, Cho CS, Rhee SG. The RING-H2-finger protein APC11 as a target of hydrogen peroxide. Free Radic Biol Med. 2004;37(4):521–530. doi: 10.1016/j.freeradbiomed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Moore R, Boyd L. Analysis of RING finger genes required for embryo-genesis in C. elegans. Genesis. 2004;38(1):1–12. doi: 10.1002/gene.10243. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Moyret-Lalle C, Couzon F, et al. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene. 2003;22(10):1486–1490. doi: 10.1038/sj.onc.1206224. [DOI] [PubMed] [Google Scholar]
- 10.Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci U S A. 2000;97(16):8973–8978. doi: 10.1073/pnas.97.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Z, Li B, Bharadwaj R, et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell. 2001;12(12):3839–3851. doi: 10.1091/mbc.12.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 13.Dai Y, Wang L, Tang J, et al. Activation of anaphase-promoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stress. Oncotarget. 2016;7(18):25478–25492. doi: 10.18632/oncotarget.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8(12):e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Zhang S, Shan J, et al. Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett. 2016;376(2):284–292. doi: 10.1016/j.canlet.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13(16):2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 17.Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20(22):3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- 18.Park KH, Choi SE, Eom M, Kang Y. Downregulation of the anaphase-promoting complex (APC)7 in invasive ductal carcinomas of the breast and its clinicopathologic relationships. Breast Cancer Res. 2005;7(2):R238–R247. doi: 10.1186/bcr978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16(4):347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi YJ, Huo KK. Knockdown expression of Apc11 leads to cell-cycle distribution reduction in G2/M phase. Genet Mol Res. 2012;11(3):2814–2822. doi: 10.4238/2012.August.24.6. [DOI] [PubMed] [Google Scholar]
- 21.Naoe H, Araki K, Nagano O, et al. The anaphase-promoting complex/cyclosome activator Cdh1 modulates Rho GTPase by targeting p190 RhoGAP for degradation. Mol Cell Biol. 2010;30(16):3994–4005. doi: 10.1128/MCB.01358-09. [DOI] [PMC free article] [PubMed] [Google Scholar]