Abstract
Background
More than 4 million children are exposed annually to sedatives and general anaesthetics (GAs) in the USA alone. Recent data suggest that common GAs can be detrimental to brain development causing neurodegeneration and long-term cognitive impairments. Challenged by a recent US Food and Drug Administration (FDA) warning about potentially neurotoxic effects of GAs in children, there is an urgent need to develop safer GAs.
Methods
Postnatal Day 7 (P7) rat pups of both sexes were exposed to six (repeated every 2 h) injections of equipotent hypnotic doses of ketamine or the neuroactive steroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) for 12 h. Loss of righting reflex was used to assess hypnotic properties and therapeutic index; quantitative caspase-3 immunohistochemistry was used to assess developmental neuroapoptosis; patch-clamp recordings in acute brain slices were used to assess the effects of 3β-OH on neuronal excitability and synaptic transmission. Cognitive abilities of rats exposed to ketamine, 3β-OH, or vehicle at P7 were assessed in young adulthood using the radial arm maze.
Results
The neuroactive steroid 3β-OH has a therapeutic index similar to ketamine, a commonly used clinical GA. We report that 3β-OH is safe and, unlike ketamine, does not cause neuroapoptosis or impair cognitive development when administered to P7 rat pups. Interestingly, 3β-OH blocks T-type calcium channels and presynaptically dampens synaptic transmission at hypnotically-relevant brain concentrations, but it lacks a direct effect on γ-aminobutyric acid A or glutamate-gated ion channels.
Conclusions
The neurosteroid 3β-OH is a relatively safe hypnotic that warrants further consideration for paediatric anaesthesia.
Keywords: calcium channels, developmental neurotoxicity, neurosteroid
Editor's key points.
-
•
Current general anaesthetics cause developmental neurotoxicity in animal models and possibly humans, creating a need for novel agents devoid of this effect.
-
•
A neuroactive steroid (3β-OH) was shown to possess hypnotic potency without causing neuroapoptosis in neonatal rats or delayed neurocognitive deficits.
-
•
Mechanistic investigations showed that 3β-OH blocks T-type Ca2+ channels and presynaptic transmitter release without affecting major postsynaptic ligand-gated ion channels.
-
•
This provides a promising lead for development of a novel intravenous anaesthetic without developmental neurotoxic effects.
Current research evidence suggests that early exposure to clinically-used general anaesthetics (GAs) can disturb normal brain development leading to permanent cognitive and behavioural impairments in rodents,1, 2, 3, 4 monkeys,5, 6, 7, 8, 9 and possibly in humans as well.10, 11, 12, 13, 14 Currently used GAs are known to modulate two main neurotransmitter systems in the developing brain—γ-aminobutyric acid (GABA) and N-methyl-d-aspartate (NMDA)—which prompted us to propose that GAs with a different cellular mechanism of action might be safer and more promising alternatives. One such alternative is a class of drugs that selectively targets T-type voltage-gated calcium channels (T-channels), known to control neuronal excitability and synaptic transmission.15 Of particular interest for our study is the neuroactive steroid, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH), a potent analgesic and voltage-dependent blocker of neuronal T-currents with minimal effect on voltage-gated Na+ and K+ currents, N-type and L-type Ca2+ currents,16, 17 or recombinant GABAA and NMDA-mediated currents.18
Using a rat pup model, we demonstrate that 3β-OH effectively blocks T-channel-dependent excitability in thalamocortical and subicular neurones, and dampens α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated excitatory transmission acting presynaptically. Furthermore, when compared with ketamine, a commonly used clinical GA, 3β-OH, is an effective hypnotic that does not cause developmental neuroapoptosis or impair cognitive development even during prolonged administration at the peak of synaptogenesis.
Methods
Animals
Most experiments were performed with postnatal day 7 rat pups (P7) (Sprague–Dawley, Envigo, Indianapolis, IN, USA), which is the peak of synaptogenesis and vulnerability to anaesthesia-induced developmental neurotoxicity.19 Pups were housed with their mother and maintained on a 12-h light–dark cycle at a constant temperature of 21(2)°C. For radial arm maze behavioural studies, P45–P70 rats were used. For electrophysiology recordings, rat pups aged P7–P9 were used, except for studies on thalamic neurones, which used P7–P15 rat pups. Animals were housed within accredited animal facilities according to protocols approved by the University of Colorado Anschutz Medical Campus. All animals had ad libitum access to food and water. Treatment of rats adhered to the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering and to use only the number of animals necessary to produce reliable data. All experiments were approved by the Animal Use and Care Committees at the University of Colorado, the Office of Laboratory Animal Resources, Aurora, CO, USA and the Animal Use and Care Committees of the University of Virginia, Charlottesville, VA, USA. Immediately after administration of anaesthesia, pups were reunited with their mothers and allowed to nurse. Details of specific experimental procedures are provided in Supplementary material.
Results
3β-OH is an effective hypnotic
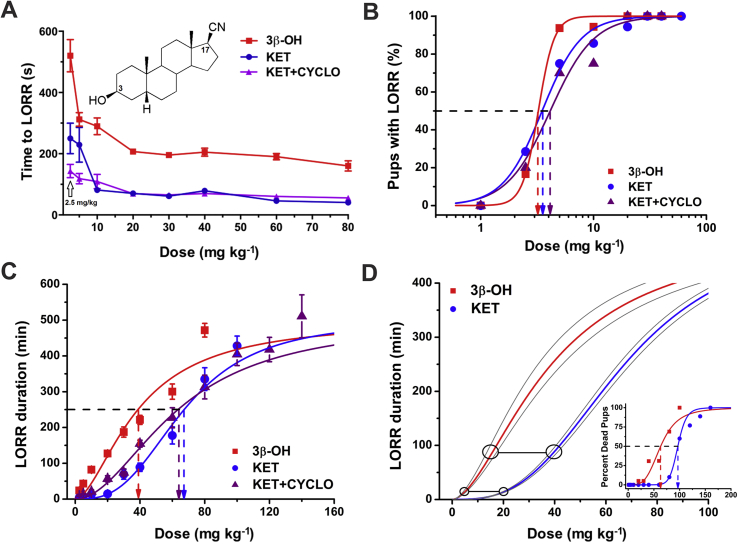
The hypnotic properties of the neuroactive steroid 3β-OH (inset in Fig. 1A) and ketamine were assessed using loss of righting reflex (LORR) in P7 rat pups injected with either agent at doses from 1 to 80 mg kg−1 intraperitoneally (i.p.). Since the vehicles were saline for ketamine and 2-hydroxypropyl-β-cyclodextrin (β-cyclodextrin) for 3β-OH, we included a third experimental group, ketamine+β-cyclodextrin. Rats in each group received only one dose. Neither vehicle, 15% β-cyclodextrin or saline, caused LORR (data not shown). However, 3β-OH, ketamine (KET), and ketamine+β-cyclodextrin (KET+CYCLO) caused dose-dependent shortening of the time to LORR (Fig. 1A). Data are provided as mean (SEM). The estimated ED50 for LORR was 3.2 (0.1) mg kg−1 with 3β-OH, 3.5 (0.2) mg kg−1 with KET and 4.1 (0.4) mg kg−1 with KET+CYCLO (Fig. 1B).
Fig 1.
(A) (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH), like ketamine, causes dose-dependent loss of righting reflex (LORR). Inset: 3β-OH is a neuroactive steroid analogue based on a progesterone ring where hydrogen bonding groups in positions 3 and 17 were substituted with OH and CN groups, respectively. Rats in each group received only one dose of either ketamine or 3β-OH (as shown on axis x). Vehicles, 15% β-cyclodextrin, or saline, did not cause LORR (n=8 per group). 3β-OH, ketamine, and ketamine+β-cyclodextrin groups induced a dose-dependent shortening of the time to LORR. 3β-OH had slower onset of LORR at each dose. Note that at 1 mg kg−1, none of ketamine (KET), ketamine+β-cyclodextrin (KET+CYCLO) or 3β-OH produced LORR (n=6–26 pups per data point). (B) The percent of rat pups with LORR was used to calculate the ED50 for LORR: 3.2 (0.1) mg kg−1 with 3β-OH, 3.5 (0.2) mg kg−1 with ketamine, and 4.1 (0.4) mg kg−1 with ketamine+β-cyclodextrin (n=6–26 pups per data point). (C) The duration of LORR with 3β-OH, ketamine, or ketamine+β-cyclodextrin was dose-dependent, reaching more than 400 min with the highest doses. Calculated ED50 was 39 (4) mg kg−1 for 3β-OH, 67 (2) mg kg−1 for ketamine, and 63 (2) mg kg−1 for ketamine+β-cyclodextrin, suggesting an almost two-fold higher potency for 3β-OH. (D) The connected circles indicate that the estimated equipotent single dose of 3β-OH, which is comparable with a lower dose of ketamine (20 mg kg−1, i.p.), was 5 mg kg−1, i.p., whereas the estimated equipotent dose of 3β-OH comparable with a higher dose of ketamine (40 mg kg−1, i.p.) was 10 mg kg−1, i.p. The inset shows the mortality data in rat pups treated with 3β-OH or ketamine.
The estimated ED50 based on the duration of LORR that was obtained when either agent was injected at doses from 1 to 140 mg kg−1 i.p. was 39 (4) mg kg−1 for 3β-OH, 67 (2) mg kg−1 for ketamine, and 64 (2) for ketamine+β-cyclodextrin (Fig. 1C). The calculated LD50 for these cohorts yielded values of 63 (4) mg kg−1 for 3β-OH, 97 (0) mg kg−1 for ketamine and 95 (5) for ketamine+β-cyclodextrin (ketamine and 3β-OH LD curves included in the inset, Fig. 1D) with corresponding therapeutic indices of ∼20 and ∼23 for 3β-OH and ketamine+β-cyclodextrin, respectively.
3β-OH, unlike ketamine, does not cause developmental neuroapoptosis
Repeated ketamine administration for 12 h (every 2 h for a total of six doses) at 20 or 40 mg kg−1 induces significant widespread neuroapoptotic degeneration in P7 rats.20 We first determined the equipotent dose of 3β-OH based on the LORR experiments. The estimated equipotent single dose of 3β-OH comparable with the lower dose of ketamine (20 mg kg−1) was 5 mg kg−1, whereas the calculated equipotent dose of 3β-OH comparable with the higher dose of ketamine (40 mg kg−1) was 10 mg kg−1 (Fig. 1D).
Using these equipotent doses injected repeatedly six times every 2 h (total of 12 h), we conducted neurotoxicity studies where ketamine was compared with 3β-OH and vehicle. The general condition of all treated animals appeared normal (skin colour and breathing) and, as shown in Supplementary Table S1, there were no differences in SpO2 values after the last two i.p. injections with either ketamine or 3β-OH compared with respective vehicle controls (P=0.151, ketamine vs saline; P=0.255, 3β-OH vs β-cyclodextrin; two-tailed t-test).
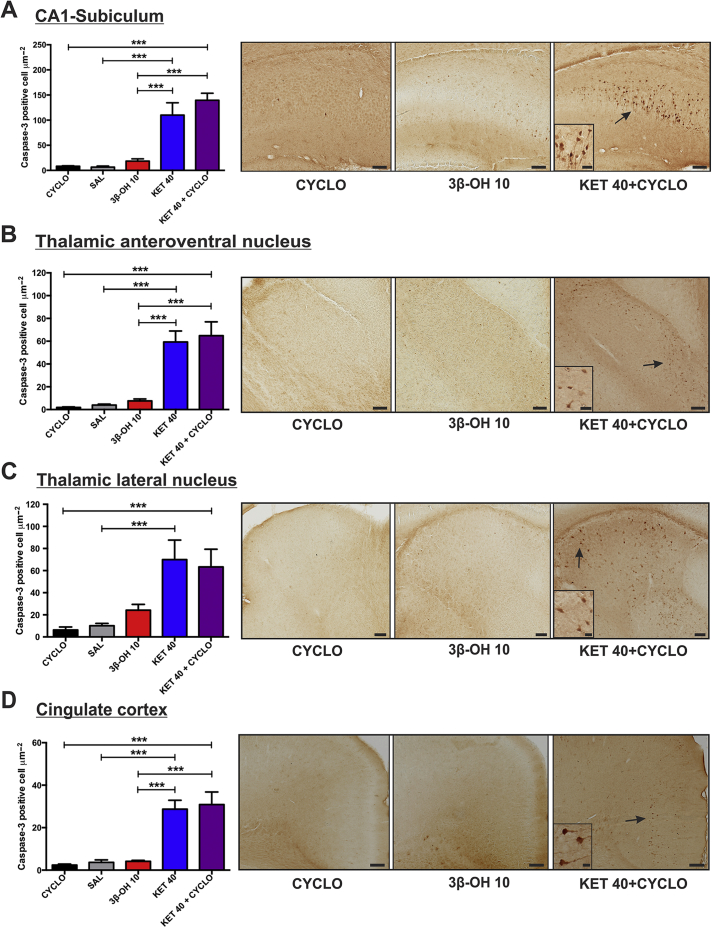
Serial analyses of several of the most vulnerable brain regions (hippocampus-CA1-subiculum junction, two anterior thalamic nuclei, and cingulate cortex) stained with activated caspase-3 revealed that ketamine at 40 mg kg−1 (KET 40) or ketamine at 40 mg kg−1 +β-cyclodextrin (KET 40+CYCLO) compared with vehicle controls [β-cyclodextrin (CYCLO) or saline (SAL)] caused significant upregulation of caspase-3 staining. In the CA1-subiculum region, there was 100- to 125-fold higher density of caspase-3 stained neurones compared with vehicle controls (Fig. 2A). When groups treated with either ketamine 40 mg kg−1 alone or with β-cyclodextrin were compared with those treated with 3β-OH at an equipotent dose of 10 mg kg−1 (3β-OH 10), there was a significant increase in caspase-3 activation. Similar findings were replicated in the thalamic anteroventral nucleus (Fig. 1B), thalamic lateral nucleus (TL) (Fig. 1C), and cingulate cortex (Fig. 1D). Comparable observations were made when lower doses of either ketamine (20 mg kg−1) or 3β-OH (5 mg kg−1) were examined (Supplementary Fig. S1). Thus 3β-OH, in contrast to ketamine, did not cause significant developmental neuroapoptosis compared with its vehicle, β-cyclodextrin.
Fig 2.
(3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH), unlike ketamine, does not cause developmental neuroapoptosis at higher doses. 3β-OH (10 mg kg−1, i.p.), ketamine (40 mg kg−1, i.p.) and ketamine (40 mg kg−1)+β-cyclodextrin were injected in P7 rat pups every 2 h for a total of six injections. Bar graphs on the left show averaged data from multiple experiments; representative images are depicted on the right panels. (A) The CA1-subiculum region exhibits a significant increase in caspase-3 activation when compared with groups treated with 3β-OH (3β-OH 10), ketamine alone (KET 40) or ketamine+β-cyclodextrin (KET 40+CYCLO) (***P<0.001 vs KET 40 and P<0.001 vs KET 40+CYCLO) suggesting that 3β-OH, unlike ketamine, does not cause significant developmental neuroapoptosis. This was confirmed by the finding that activated caspase-3 staining in 3β—OH—treated animals was not significantly upregulated compared with saline (SAL) or β-cyclodextrin (CYCLO) controls (P=0.964 vs saline and P=0.980 vs β-cyclodextrin) (n=6 pups per data point). (B) In thalamic anteroventral nucleus (TAV) there was minimal caspase-3 activation in vehicle groups, whilst there was a significant increase in caspase-3 activation in ketamine or ketamine+β-cyclodextrin groups compared with vehicle controls (***P<0.001). The level of caspase-3 activation in 3β-OH animals was comparable with vehicle controls and significantly lower than in either ketamine or ketamine+β-cyclodextrin (***P<0.001 vs KET 40 and P<0.001 vs KET 40+CYCLO) groups (n=6 pups per data point). (C) In thalamic lateral nucleus (TL) there was minimal caspase-3 activation in vehicle groups, whilst there was significant caspase-3 activation in ketamine or ketamine+β-cyclodextrin groups (**P=0.006 vs SAL and P=0.009 vs CYCLO). Note that the caspase-3 activation in the 3β-OH group is not significant compared with vehicle controls (n=6 pups per data point). (D) In the cingulate cortex, there was no difference in caspase-3 activation in the 3β-OH group compared with vehicle controls (P>0.999 vs SAL; P=0.995 vs CYCLO), but ketamine or ketamine+β-cyclodextrin groups exhibited significant increases in caspase-3 activation compared with vehicle control (***P<0.001 vs SAL and P<0.001 vs CYCLO). 3β-OH does not exhibit neurotoxic potential compared with either ketamine or ketamine+β-cyclodextrin groups (***P<0.001 vs KET 40 and P<0.001 vs KET 40+CYCLO) (n=6 pups per data point). All statistical analyses were done using one-way analysis of variance with Tukey's post hoc test. Representative photomicrographs shown in the right-side panels depict activated caspase-3 staining in ketamine+β-cyclodextrin compared with 3β-OH or a vehicle. Scale bars in the low magnification images in panels (A–C) are 400 μm, and 800 μm in panel (D). Scale bars in all high magnification insets are 100 μm.
Prolonged exposure of rat pups to 3β-OH, unlike ketamine, does not cause learning impairments later in life
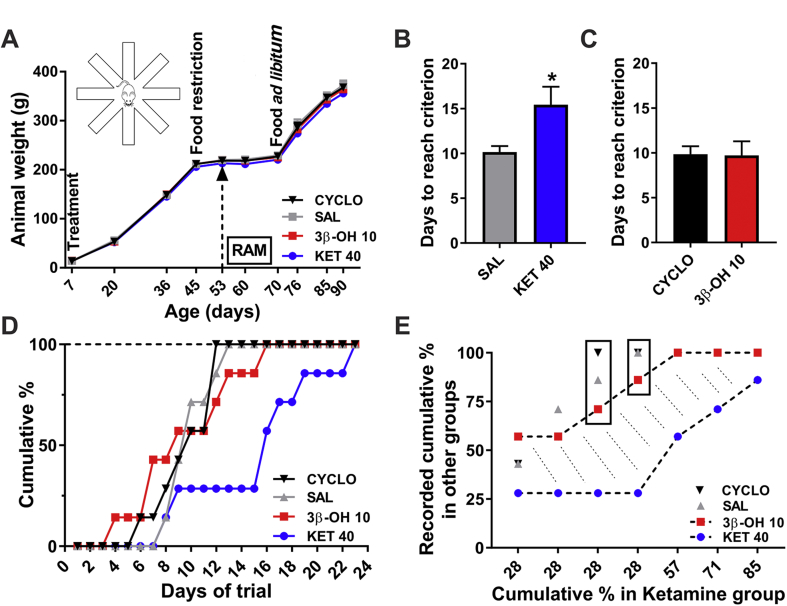
Ketamine exposure during brain development causes cognitive impairment later in life.21, 22 Therefore, we compared the cognitive abilities of young adult rats exposed to saline, β-cyclodextrin, ketamine, or 3β-OH. We assessed their spatial working memory using the eight-arm radial arm maze test. We administered saline, β-cyclodextrin, 3β-OH at 10 mg kg−1 or ketamine at 40 mg kg−1, every 2 h for a total of six doses over 12 h in P7 rat pups, and examined overall appearance and daily weight in each group. There was no significant difference between the rats in the four groups (Fig. 3A).
Fig 3.
(3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH), unlike ketamine, does not impair spatial learning and memory. P7 rat pups were exposed to saline, β-cyclodextrin, or higher doses of ketamine or 3β-OH at P7 and tested for their spatial working memory using the eight-arm radial arm maze test (RAM) in young adulthood. (A) The overall appearance and daily weight of rats in each group (saline, β-cyclodextrin at 15%, 3β-OH at 10 mg kg−1 or ketamine at 40 mg kg−1, administered every 2 h for a total of six doses) were examined. Daily weight did not differ between groups. The time points when animals were treated (P7), food restricted (from P45), tested by RAM (from P53), and permitted to eat ad libitum (from P70, except one ketamine-treated rat) are indicated on the graph. (B) Ketamine-treated rats (KET) were compared with saline treated controls (SAL); there was a significant increase in the number of days required to reach learning criterion (unpaired t-test: t(12)=2.495, *P=0.028). (C) Number of days to reach criterion was similar for 3β—OH—treated rats compared with the β-cyclodextrin vehicle (CYCLO) treated animals (unpaired t-test: t(12)=0.079, P=0.938). (D) The cumulative percentage of rats reaching 100% as a function of days of trials was compared for the four groups. The acquisition rate of the ketamine-treated group (KET) was slower than that of saline controls (SAL) by Day 9 and remained substantially slower for the remainder of training. In contrast, rats in the 3β-OH group (closed squares) initially exhibited slightly faster acquisition than the vehicle control groups (SAL and CYCLO). However, by Day 16, their learning was identical to the learning curve of vehicle controls. Unlike rats in the ketamine group, all 3β—OH—treated rats completed the task in a manner practically indistinguishable from vehicle controls (n=7 rats per data point in panels A–D). (E) A percent-percent plot analysis shows that when only 28% of ketamine-treated rats (KET) had reached criterion, roughly half of 3β—OH—treated and vehicle control (SAL, CYCLO) rats had mastered the task. Whilst the learning curve of ketamine-treated rats remained flat, both 3β—OH—treated and vehicle-treated controls showed steady improvement enabling animals to reach criterion (100%), whilst about 85% of ketamine animals managed to master the task in the allotted time. The learning behaviour of 3β—OH—treated rats was similar to that of vehicle controls, resulting in a large gap (shaded area) in learning ability between ketamine-treated and 3β—OH—treated animals.
Ketamine-treated rats were significantly impaired relative to controls treated with saline (*P=0.028) in terms of days required to reach a criterion demonstrating learning (Fig. 3B). In contrast, 3β—OH—treated rats were indistinguishable from vehicle treated animals (β-cyclodextrin) (Fig. 3C).
The cumulative percentage analysis (Fig. 3D) showed that the acquisition rate of the ketamine-treated group (blue circles) began to slow compared with saline controls (grey triangles) by the 9th day and remained substantially slower for the remainder of training with significant decreases for Days 13–15 (P=0.021, Fisher's exact test). In contrast, rats in the 3β-OH group (red squares) displayed learning curves similar to vehicle controls (black triangles).
The cumulative percent scores for ketamine-treated animals relative to recorded cumulative percent scores for other groups were examined using a percent-percent plot (Fig. 3E), which revealed a large gap (shaded area) in learning ability between ketamine-treated (lower black dotted line and blue symbols) and 3β—OH—treated animals (upper black dotted line and red symbols).
Pharmacokinetic studies of 3β-OH in rat pups
In order to conduct proper mechanistic studies in vitro, we measured the plasma and brain concentrations of 3β-OH. For these pharmacokinetic studies, we used a dose of 10 mg kg−1 as that was the higher dose used to conduct morphological studies of developmental neuroapoptosis (Fig. 2) and behavioural studies (Fig. 3). The plasma and brain concentrations of 3β-OH in P7 rat pups were determined over the course of 120 min after a single 3β-OH injection. 3β-OH was detected as early as 2 min in both plasma and brain homogenate with the peak occurring at 5 and 15 min, respectively, followed by a fairly rapid decrease in plasma levels (Supplementary Fig. S2). The calculated plasma and brain tissue half-lives were 8.2 and 29 min, respectively (Supplementary Table S2).
3β-OH blocks native CaV3.1 channels in immature brain
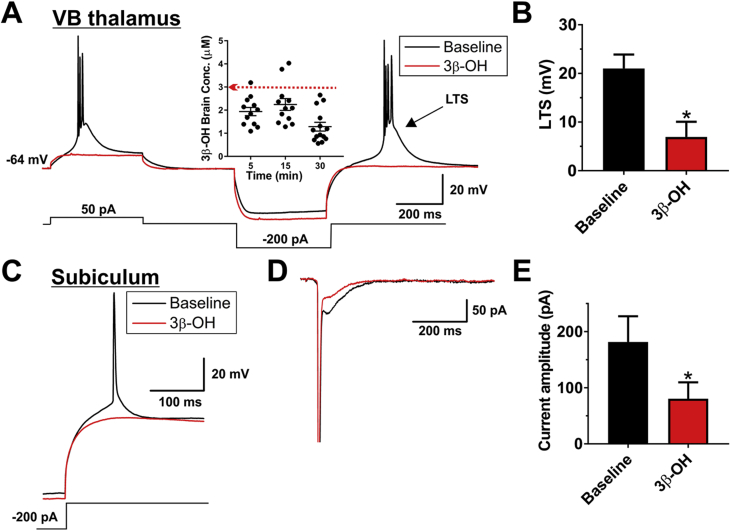
Considering our findings that 3β-OH has a similar therapeutic index and produces less neuroapoptosis than ketamine, we examined whether 3β-OH has a unique cellular target compared with currently available anaesthetics known to be neurotoxic. Since low-voltage activated T-type Ca2+ channels are rapidly emerging as promising therapeutic targets and important modulators of neuronal excitability, they were the initial focus of our mechanistic studies. Molecular studies have identified three isoforms of neuronal T-channels based on their pore-forming α subunits: CaV3.1 (α1G), CaV3.2 (α1H), and CaV3.3 (α1I).23 3β-OH Effectively blocks native CaV3.2 currents in rat dorsal root ganglion cells with an IC50 of 3 μM and native CaV3.3 currents in the reticular thalamic nucleus with an IC50 of 2 μM.16, 17 We further examined the cellular targets of 3β-OH using two in vitro systems, known both to express abundant CaV3.1 T-channel isoform,24 and to be vulnerable to anaesthesia-induced developmental neurotoxicity: (i) thalamocortical neurones in the ventrobasal (VB) nucleus, and (ii) pyramidal neurones in the subiculum.
We found that 3 μM 3β-OH decreased the average amplitude of the low-threshold Ca2+ spike by ∼70% in thalamic neurones in the VB nucleus, thus abolishing burst firing (Fig. 4A and B), without significantly changing the resting membrane potential [−62.4 (1.8) vs −62.0 (1.3) mV]. This in vitro concentration corresponds well to the total brain concentration achieved 5–30 min after acute injection of 10 mg kg−1 of 3β-OH (inset of Fig. 4A).
Fig 4.
The effects of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) on excitability of thalamic and subicular neurones. (A) Original traces from a representative thalamic neurone in control pre-drug conditions (black trace) portraying the loss of burst firing pattern as a response to both depolarising and hyperpolarising stimulus and in the presence of 3 μM 3β-OH (red trace). The inset shows concentrations of 3β-OH in rat brain achieved 5, 15, and 30 min after i.p. injection of 10 mg kg−1 3β-OH. (B) Application of 3 μM 3β-OH also significantly decreased the average rebound low-threshold spike (LTS), which underlies bursting in these neurones (paired t-test: t4=3.14, P=0.035; n=5 neurones, three rats). (C) Original traces from a representative subicular neurone depicting control (black trace) and the effect of 3 μM 3β-OH (red trace) on the rebound firing pattern to a 200 pA hyperpolarising stimulus. On average 3 μM 3β-OH decreased the number of rebound action potentials from 1.41 (0.26) to 0.76 (0.21) (paired t-test: t(5)=3.81, P=0.013; n=6 neurones, three rats). (D) Original traces from a representative subicular neurone showing that 3β-OH reduced the amplitude of inward calcium currents (evoked using Vh of −90 mV and Vt of −40 mV). (E) Bar graphs showing averages from multiple experiments similar to panel (D) of this figure, which demonstrate that 3β-OH reduced the amplitude of calcium current by approximately 50% compared with baseline (pre-drug) control in the same cells (paired t-test: t3=4.30, P=0.023; n=4 neurones, one rat), *P<0.05 vs baseline pre-drug conditions.
Next, we examined the effects of 3β-OH on membrane firing of pyramidal neurones in the rat subiculum. Original traces depicted in Figure 4C show that 3β-OH abolished rebound action potentials generated after a brief hyperpolarising stimulus used to de-inactivate T-channels. This correlated well with inhibition of the amplitude of T-currents at −40 mV as shown in the original traces in Figure 4D and the average bar graph in Figure 4E. These data confirm that T-channels play a crucial role in regulating neuronal excitability of the immature rat subiculum.25
3β-OH decreases evoked AMPA-mediated, but not NMDA-mediated excitatory synaptic currents acting presynaptically
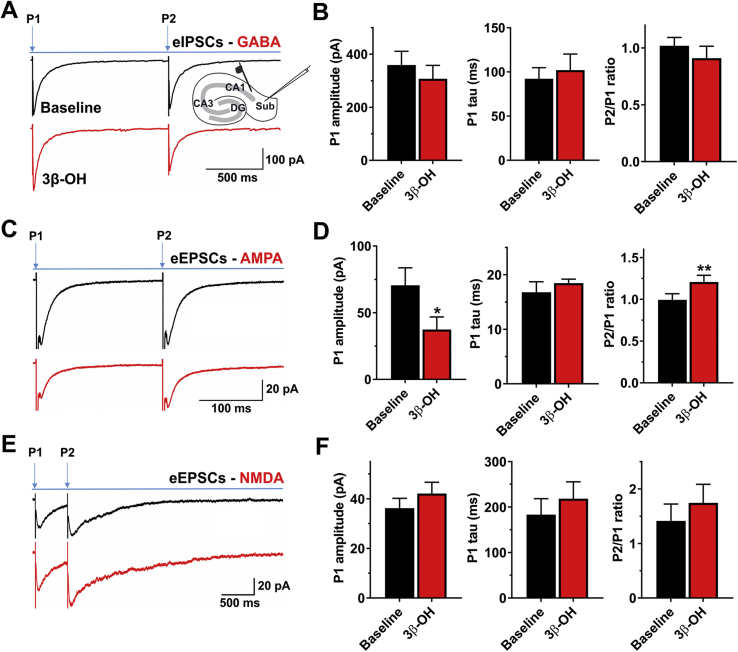
Next, we recorded evoked inhibitory postsynaptic currents (eIPSCs) and evoked excitatory postsynaptic currents (eEPSCs) in subicular neurones using a paired-pulse protocol (Fig. 5). We found that 3 μM 3β-OH had very little effect on eIPSC amplitude, decay tau or paired-pulse ratio (PPR) (Fig. 5A and B). In contrast, 3β-OH decreased the amplitude of AMPA-mediated eIPSCs by ∼50%, with minimal effect on decay kinetics (Fig. 5C). The PPR was significantly increased, suggesting a lower release probability of glutamate from presynaptic terminals (Fig. 5C and D). Interestingly, this finding was not replicated when we pharmacologically isolated NMDA receptor-mediated eEPSCs (Fig. 5E and F), which suggests that immature subicular neurones have differential synaptic expression of AMPA and NMDA receptors.
Fig 5.
Effects of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) on the evoked synaptic currents in the subiculum of P7−P9 rat pups. (A) Average original evoked inhibitory postsynaptic currents (eIPSC) traces in the absence (black trace) or presence (red trace) of 3 μM 3β-OH recorded using the paired-pulse protocol. The illustration depicts the placements of stimulatory and recording electrodes in CA1 and subiculum, respectively. (B) Column graphs show very little effect of 3 μM 3β-OH on the three parameters measured after the first pulse: eIPSC amplitude, decay time constant, and the paired-pulse (P2/P1) ratio (n=8 neurones, five rats). (C) Average original evoked excitatory postsynaptic currents (eEPSC) [α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)] traces in the absence (black trace) or presence (red trace) of 3β-OH recorded using the same protocol described above. (D) Addition of 3 μM 3β-OH resulted in a significant reduction of AMPA-mediated eEPSC amplitude after the first pulse (paired t-test: t4=3.75, P=0.020; n=5 neurones, two rats) without changing the decay time constant (t4=0.93, P=0.403). The far-right bar graph shows that steroid had increased the paired-pulse ratio (t4=5.28, P=0.006). (E) Average original eEPSC [N-methyl-d-aspartate (NMDA)-mediated] traces in the absence (black) or presence of 3β-OH (red trace) recorded using a paired-pulse protocol. (F) Bar graphs show minimal and non-significant effect of 3 μM 3β-OH on NMDA current amplitude and decay time constant after the first pulse, and on the paired-pulse ratio (n=5 neurones, three rats).
3β-OH decreases spontaneous GABA release
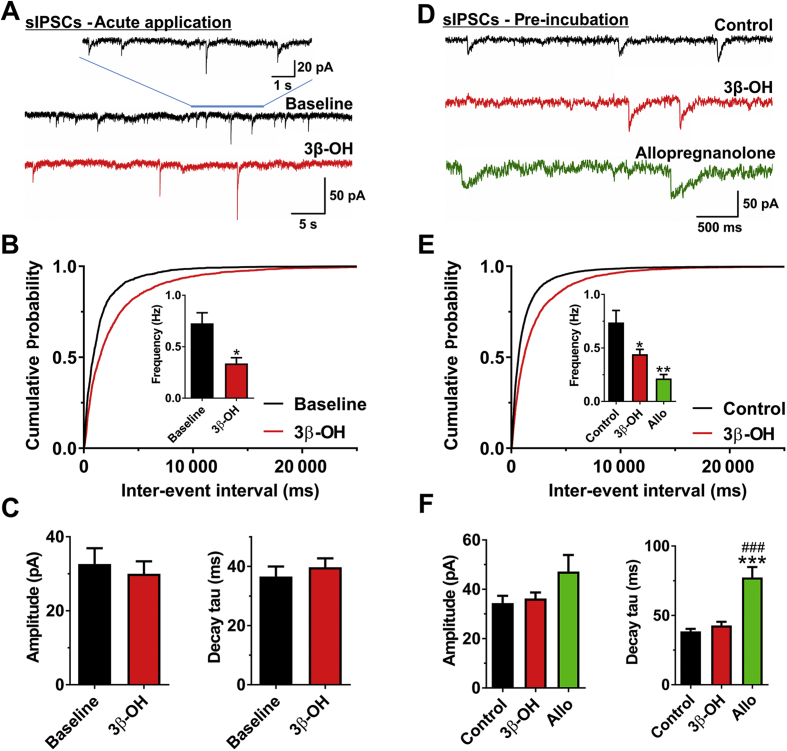
We next studied spontaneous GABAA-mediated IPSCs (sIPSCs) in pyramidal neurones of rat subiculum. Any changes in frequency of sIPCSs would indicate presynaptic effects, whilst alterations of amplitude or decay kinetics of sIPSCs would indicate postsynaptic effects on GABAA receptors. Representative traces of sIPSCs before (black) and after (red) bath application of 10 μM 3β-OH for 10 min in the same neurone are shown in Figure 6A. We found that acutely applied 3β-OH decreased the frequency sIPSCs by >50% [0.73 (0.10) vs 0.34 (0.06) Hz], which was also evident by the longer inter-event intervals represented by cumulative probability plots (Fig. 6B). In contrast, 3β-OH induced minimal changes in amplitude and decay tau of sIPSCs (Fig. 6C).
Fig 6.
Effects of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) on spontaneous GABAA-mediated inhibitory postsynaptic currents (sIPSCs) in the subiculum of P7–P9 rat pups. (A) Original traces from a representative subicular neurone in the absence (black) or presence (red trace) of 3β-OH. (B) Cumulative probability plots for baseline (1682 events) and 3β-OH (2232 events) demonstrate longer inter-event intervals after the acute application of 10 μM 3β-OH. This finding is confirmed by the lower sIPSC frequency presented in the inset (paired t-test: t8=3.32, P=0.011; n=9 neurones, five rats). (C) 3β-OH is devoid of effects on both amplitude (upper) and decay (lower) of sIPSCs, even at a relatively high concentration of 10 μM. (D) Original traces from representative subicular neurones in the control group (black trace), and groups pre-incubated with 3β-OH (red trace) or allopregnanolone (Allo, green trace). (E) Neurones pre-incubated with 10 μM 3β-OH (red) had longer inter-event intervals of sIPSCs than the control group (black), as shown by cumulative probability plots (5168 and 7341 events, respectively). The column graph in the inset shows lower frequency for groups treated with 3β-OH (n=19 neurones, nine rats) and Allo (green) (n=6 neurones, four rats) compared with control (n=19 neurones, nine rats). One-way analysis of variance (ANOVA): F2,41=7.37, P=0.002; Tukey's post hoc test: P=0.017 and P=0.005, respectively. (F) The left bar graph shows that the amplitude of sIPSC events was not affected by different treatments (one-way ANOVA: F2,41=2.44, P=0.100). Conversely, the right bar graph shows 500 nM Allo significantly prolonged the decay time constant (tau) compared with both the control and 3β-OH groups (one-way ANOVA: F2,41=27.62, P<0.001; Tukey's post hoc test: P<0.001 vs both groups). *P<0.05, **P<0.01, and ***P<0.001 vs control group; ###P<0.001 vs 3β-OH group.
To exclude possible run-down of sIPSCs or inadequate diffusion of steroid through the tissue slice, we performed a population study of sIPSCs in subicular neurones after slice incubation (for at least 1 h) with either vehicle (control) or 10 μM 3β-OH. We also compared effects of 3β-OH to allopregnanolone (Allo), an endogenous steroid that also blocks T-channels,26 and that is also a positive allosteric modulator of GABAA receptors.27 Representative traces of neurones are depicted in Figure 6D showing vehicle-treated control (black trace), 3β-OH pre-incubated slice (red trace), and Allo pre-incubated slice (green trace). Consistent with their presynaptic effects, both steroids decreased sIPSC frequency [0.77 (0.11) vs 0.44 (0.05) Hz for 3β-OH; and 0.21 (0.04) Hz for Allo, inset of Fig. 6E]. Importantly, 3β-OH had no effect on either amplitude or decay kinetics of sIPSCs, whilst Allo significantly prolonged the decay time constant of sIPSCs ∼2-fold [40 (1.9) vs 77 (7.5) ms] (Fig. 6F). Thus, it appears that 3β-OH reduces presynaptic GABA release, but unlike Allo, it is devoid of direct effects on postsynaptic GABAA receptors, even at a relatively high concentration of 10 μM. We conclude that 3β-OH inhibits presynaptic AMPA-mediated eEPSCs and GABA-mediated sIPSCs, whilst NMDA-mediated eEPSCs are generally spared at relevant brain concentrations.
Discussion
Here we show that the neuroactive steroid 3β-OH is a promising injectable hypnotic with suitable pharmacokinetic properties. 3β-OH is a distinct non-selective T-channel blocker devoid of GABA potentiating or NMDA antagonistic properties at hypnotically-relevant brain concentrations, and has a safety profile superior to the commonly used injectable anaesthetic ketamine. Specifically, at equipotent doses, 3β-OH does not exhibit evident neurotoxic properties when used in rat pups at the peak of brain development and does not impair subsequent cognitive development. We propose that a novel class of anaesthetic agents with different cellular targets might be safe and promising alternatives to traditional GAs currently used in paediatric medicine.
The neuroactive steroids modulate neuronal activity whilst causing a variety of behavioural and neuroendocrine changes (e.g. general anaesthesia, analgesia, cognitive, and mood alterations).28 These changes are mediated primarily by actions at ligand-gated ion channels, with much attention focused on the modulation of GABAA receptors by 5α-reduced steroids like Allo.28, 29, 30 Because all GABAergic GAs share neurotoxic potential, we identified a novel steroid analogue with similar hypnotic potency that is an effective inhibitor of neuronal T-channels, but lacks GABA-mimetic properties.
Prevailing concepts are that common GAs that potentiate inhibitory GABA-gated and/or inhibit NMDA-gated ion channels cause neurodegeneration and possibly other long-term changes in the developing mammalian brain.31 We reasoned that other relevant targets, including ion channels that regulate neuronal excitability, must be considered in designing new and safer GAs. For example, a family of T-channels play important roles in generating low-threshold spike in the thalamus and other central nervous system regions, as well as supporting excitatory synaptic transmission.15 For example, neuronal T-channels regulate sleep and wakefulness32 and are inhibited by relevant concentrations of some GAs.33, 34, 35, 36 Here we report that 3β-OH, a potent T-channel blocker with no direct effect on either GABA- or NMDA-mediated currents, displays good hypnotic properties in rat pups in vivo. Furthermore, unlike ketamine, a commonly used agent in paediatric anaesthesia and sedation, 3β-OH caused neither apoptosis in P7 rats nor long-lasting impairment in neurocognitive development, even after a 12 h exposure.
It is generally accepted that GAs affect multiple cellular targets (e.g. GABAA, NMDA, background potassium channels) that act in concert to induce both acute hypnotic and lasting cognitive effects on the brain.37, 38, 39 Studies have also shown the common GA isoflurane at clinically-relevant concentrations inhibits not only native and recombinant T-type currents, but also CaV2.3 R-type voltage-gated Ca2+ currents expressed in the thalamus (reviewed by Orestes and Todorovic36). Therefore, the potential utility of voltage-gated Ca2+ channels as targets for the action of GAs remains an important issue in understanding cellular mechanisms of anaesthetic action. It remains to be determined if other cellular targets besides T-channels contribute to the hypnotic properties of 3β-OH. Nevertheless, our present work has the potential to shift the focus to underappreciated targets such as neuronal T-channels for development of novel and safer GAs. We anticipate that specific targeting of neuronal voltage-gated Ca2+ channels with neurosteroids can be developed in the near future into an effective therapeutic approach and might overcome problems that have been associated with the use of traditional GAs in the young and most vulnerable patient populations.
An ideal GA should be a good analgesic as well, thus enabling minimal use of opioids or non-steroidal analgesics which have considerable side effects of their own. T-channel antagonists in general, and T-channel-blocking steroid analogues in particular (including 3β-OH), are very effective analgesics, not only for acute nociception,16, 26 but also for alleviation of chronic pain conditions caused by mononeuropathies40 and systemic neuropathies41 (e.g. diabetic painful neuropathy).
The vehicle used to dissolve 3β-OH (β-cyclodextrin) improves water solubility of lipophilic drugs42 and might provide targeted encapsulation of endogenous neuroactive steroids that are positive modulators of GABAA receptors.43, 44 Hence, we were concerned that diminishing the activity of these or other potential lipophilic endogenous modulators of ion channels by β-cyclodextrin encapsulation could contribute to the safety profile of 3β-OH. However, ketamine+β-cyclodextrin had similar hypnotic properties and neurotoxic profiles to ketamine alone, such that 3β-OH's favourable safety was likely not as a result of an encapsulating effect by β-cyclodextrin of endogenous ion channel modulators.
Currently used GAs impair developmental synaptogenesis, but are a medical necessity in daily clinical practice. Although protective strategies have been reasonably successful in acutely reducing apoptotic activation,19, 45 repairing synapse integrity,4 and mending mitochondrial morphogenesis,46 the issues with protective strategies remain numerous: limited protection, questionable long-lasting benefits, and added complexity to anaesthesia protocols with potential for unpredictable drug interactions. Thus, addition of protective agents has not provided a ‘quick’ solution to anaesthetic-induced neurotoxicity. Based on the recent public warning by the FDA regarding the safety of GAs in very young children, rational and systematic development of safer GAs based on previously unrecognised cellular targets47 such as T-channels is justified and could be the best strategy for addressing the current conundrum, considering GA is a necessity that often cannot be avoided in children.
Authors' contributions
Performed experiments and analysed the data: N.A., S.M.J., A.O., D.M., J.K., P.E., K.E.
Wrote part of the manuscript: S.M.J.
Designed the experiments: D.F.C.
Designed the studies, supervised the overall project, and performed final manuscript preparation: S.M.T., V.J-T.
Funding
Department of Anesthesiology at the University of Colorado Anschutz Medical campus. National Institute of Health (GM102525 to S.M.T., R0144517, R0144517-S, R01 GM118197, R21 HD080281, and March of Dimes National Award, USA to V.J.-T.), University of Colorado Medicine Endowment (to V.J.-T.), and funds from the Taylor Family Institute for Innovative Psychiatric Research (to D.F.C.).
Declarations of interest
None declared.
Editorial decision: January 2, 2018
Handling editor: H.C. Hemmings Jr
Footnotes
This article is accompanied by an editorial: Quest for new drugs: a way to solve anaesthesia neurotoxicity? By Vutskits & Sneyd, Br J Anesth 2018:120:619–621, doi: 10.1016/j.bja.2018.01.024
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2017.12.039.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loepke A.W., Istaphanous G.K., McAuliffe J.J., 3rd The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 3.Rizzi S., Carter L.B., Ori C., Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal Guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head B.P., Patel H.H., Niesman I.R., Drummond J.C., Roth D.M., Patel P.M. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambrink A.M., Evers A.S., Avidan M.S. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creeley C.E., Dikranian K.T., Dissen G.A., Back S.A., Olney J.W., Brambrink A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(Suppl 1):i29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenning K.J., Noguchi K.K., Martin L.D. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder R.T., Flick R.P., Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprung J., Flick R.P., Katusic S.K. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block R.I., Thomas J.J., Bayman E.O., Choi J.Y., Kimble K.K., Todd M.M. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 13.Ing C.H., DiMaggio C.J., Malacova E. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary J.D., Warner D.O. What do recent human studies tell us about the association between anaesthesia in young children and neurodevelopmental outcomes? Br J Anaesth. 2017;119:458–464. doi: 10.1093/bja/aex141. [DOI] [PubMed] [Google Scholar]
- 15.Leresche N., Lambert R.C. T-type calcium channels in synaptic plasticity. Channels (Austin) 2017;11:121–139. doi: 10.1080/19336950.2016.1238992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todorovic S.M., Pathirathna S., Brimelow B.C. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 17.Joksovic P.M., Covey D.F., Todorovic S.M. Inhibition of T-type calcium current in the reticular thalamic nucleus by a novel neuroactive steroid. Ann N Y Acad Sci. 2007;1122:83–94. doi: 10.1196/annals.1403.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang M., He Y., Eisenman L.N. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yon J.H., Carter L.B., Reiter R.J., Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Zou X., Patterson T.A., Sadovova N. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredriksson A., Archer T., Alm H., Gordh T., Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–376. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Fredriksson A., Ponten E., Gordh T., Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 24.Talley E.M., Cribbs L.L., Lee J.H., Daud A., Perez-Reyes E., Bayliss D.A. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joksimovic S.M., Eggan P., Izumi Y. The role of T-type calcium channels in the subiculum: to burst or not to burst? J Physiol. 2017;595:6327–6348. doi: 10.1113/JP274565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathirathna S., Brimelow B.C., Jagodic M.M. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Paul S.M., Purdy R.H. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 28.Zorumski C.F., Mennerick S., Isenberg K.E., Covey D.F. Potential clinical uses of neuroactive steroids. Curr Opin Investig Drugs. 2000;1:360–369. [PubMed] [Google Scholar]
- 29.Covey D.F., Evers A.S., Mennerick S., Zorumski C.F., Purdy R.H. Recent developments in structure-activity relationships for steroid modulators of GABA(A) receptors. Brain Res Brain Res Rev. 2001;37:91–97. doi: 10.1016/s0165-0173(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 30.Lambert J.J., Belelli D., Hill-Venning C., Peters J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 31.Soriano S.G., Vutskits L., Jevtovic-Todorovic V., Hemmings H.C., 2016 BJA Neurotoxicology and Neuroplasticity Study Group Thinking, fast and slow: highlights from the 2016 BJA seminar on anaesthetic neurotoxicity and neuroplasticity. Br J Anaesth. 2017;119:443–447. doi: 10.1093/bja/aex238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crunelli V., David F., Leresche N., Lambert R.C. Role for T-type Ca2+ channels in sleep waves. Pflugers Arch. 2014;466:735–745. doi: 10.1007/s00424-014-1477-3. [DOI] [PubMed] [Google Scholar]
- 33.Joksovic P.M., Brimelow B.C., Murbartian J., Perez-Reyes E., Todorovic S.M. Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant Ca(v)3.3 channels. Br J Pharmacol. 2005;144:59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckle V.S., Digruccio M.R., Uebele V.N., Renger J.J., Todorovic S.M. Inhibition of T-type calcium current in rat thalamocortical neurons by isoflurane. Neuropharmacology. 2012;63:266–273. doi: 10.1016/j.neuropharm.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Todorovic S.M., Perez-Reyes E., Lingle C.J. Anticonvulsants but not general anesthetics have differential blocking effects on different T-type current variants. Mol Pharmacol. 2000;58:98–108. doi: 10.1124/mol.58.1.98. [DOI] [PubMed] [Google Scholar]
- 36.Orestes P., Todorovic S.M. Are neuronal voltage-gated calcium channels valid cellular targets for general anesthetics? Channels (Austin) 2010;4:518–522. doi: 10.4161/chan.4.6.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 38.Akeju O., Brown E.N. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol. 2017;44:178–185. doi: 10.1016/j.conb.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 40.Pathirathna S., Todorovic S.M., Covey D.F., Jevtovic-Todorovic V. 5alpha-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain. 2005;117:326–339. doi: 10.1016/j.pain.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Latham J.R., Pathirathna S., Jagodic M.M. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes. 2009;58:2656–2665. doi: 10.2337/db08-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welliver M., McDonough J. Anesthetic related advances with cyclodextrins. ScientificWorldJournal. 2007;7:364–371. doi: 10.1100/tsw.2007.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chisari M., Eisenman L.N., Krishnan K. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shu H.J., Eisenman L.N., Jinadasa D., Covey D.F., Zorumski C.F., Mennerick S. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L.X., Yon J.H., Carter L.B., Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–1615. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 46.Boscolo A., Starr J.A., Sanchez V. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2012;45:1031–1041. doi: 10.1016/j.nbd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sneyd J.R. Thiopental to desflurane—an anaesthetic journey. Where are we going next? Br J Anaesth. 2017;119(Suppl 1):i44–i52. doi: 10.1093/bja/aex328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.