Abstract
Background
Socio-emotional development is the expression and management of emotions, which in non-human primates can be examined using responses toward increasing levels of threat. Damage to the limbic system alters socio-emotional development in primates. Thus, neuronal and glial cell loss caused by exposure to general anaesthesia early in infancy might also impact socio-emotional development. We recently reported that repeated sevoflurane exposure in the first month of life alters emotional behaviours at 6 months of age and impairs visual recognition memory after the first year of life in rhesus monkeys. The present study evaluated socio-emotional behaviour at 1 and 2 yr of age in those same monkeys to determine the persistence of altered emotional behaviour.
Methods
Rhesus monkeys of both sexes were exposed to sevoflurane anaesthesia three times for 4 h each time in the first 6 weeks of life. At 1 and 2 yr of age, they were tested on the human intruder task, a well-established mild acute social stressor.
Results
Monkeys exposed to sevoflurane as infants exhibited normal fear and hostile responses, but exaggerated self-directed (displacement) behaviours, a general indicator of stress and anxiety in non-human primates.
Conclusions
Early repeated sevoflurane exposure in infant non-human primates results in an anxious phenotype that was first detected at 6 months, and persists for at least 2 yr of age. This is the first demonstration of such a prolonged impact of early anaesthesia exposure on emotional reactivity.
Keywords: anaesthetic neurotoxicity, cognitive development, general anaesthesia
Editor's key points.
-
•
The long-term impact of early, repeated exposure to anaesthesia on socio-emotional behaviour is unknown.
-
•
In a follow-up study of non-human primates exposed thrice to inhaled sevoflurane as infants, the human intruder test was used to assess socio-emotional behaviours at 1 and 2 yr of age.
-
•
Monkeys exposed to sevoflurane as infants had exaggerated displacement behaviours, an indicator of stress and anxiety in non-human primates, up to 2 yr after exposure.
-
•
Repeated exposure to sevoflurane as infants can have long-term effects on socio-emotional behaviours in rhesus monkeys.
A number of epidemiological studies have reported increased incidence of neurocognitive abnormalities after paediatric surgery and anaesthesia, particularly in children with repeated or prolonged exposure to anaesthesia.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Studies in animal models, both rodents and non-human primates, allow isolation of the effect of general anaesthesia from that of surgery. Exposure to general anaesthesia alone in infant rodents or monkeys causes programmed cell death (apoptosis) of neuronal and glial cells, and long-term alterations in cognitive and socio-emotional behaviour.11, 12, 13, 14, 15
We are studying cognitive and socio-emotional development in rhesus monkeys exposed to sevoflurane inhalation anaesthesia three times in infancy, in comparison with a control group in which infants were briefly separated from their mothers at the same time points. Our model focuses on repeated exposure because this is most reliably associated with neurocognitive impairment in human epidemiological studies.1, 2, 3, 4 Indeed, recent prospective and ambidirectional studies in humans have suggested that single, relatively brief (on the order of 1–2 h) exposures to general anaesthesia in humans do not have substantial impact on neurocognitive development, at least with the measures used and at the time points tested.16, 17 We have previously reported increased displacement (anxiety) behaviour at 6 months of age,14 and delayed-onset mild visual memory deficits that emerge at 2 yr of age18 in rhesus monkeys repeatedly exposed to sevoflurane as infants.
Here, we re-evaluated emotional reactivity in these same monkeys at later developmental time points where memory impairments were evident. At 1 and 2 yr of age, sevoflurane-exposed monkeys exhibited increased self-directed (displacement) behaviours that were stable between the two assessments 1 yr apart. In humans and non-human primates, displacement behaviours are patterns of movements focused on one's own body, such as self-touching, scratching, and self-grooming. Displacement behaviours reflect general anxiety, as they are increased by anxiogenic events, catecholamines, or glucocorticoids, and decreased by anxiolytics19, 20 Therefore, repeated anaesthesia exposure early in life seems to result in an anxious phenotype characterised by different displacement behaviours during infancy and adolescence, specifically increased frequency of anxiety behaviours (e.g. scratching, yawning) during infancy, and increased duration of self-directed behaviours (e.g. self-grooming) as juveniles.
Methods
All animal procedures were approved by the Yerkes National Primate Research Center and the Emory University Institutional Animal Care and Use Committee, and were conducted in full compliance with United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. Subject descriptions and anaesthetic procedures have been published.14 Briefly, 20 newborn rhesus monkeys of Indian origin (Macaca mulatta) were born in two cohorts in the breeding colony at the Yerkes National Primate Research Center field station. In the first cohort, six females and four males were born in the 2012 birth season, and in the second, four females and six males were born in 2013. All infants were delivered vaginally without veterinary intervention in their natal group compounds. Infants were born to middle-ranking dams and were housed in large social groups of 50–100 individuals comprising several family groups. Infants were assigned as they were born and with consideration to balancing for sex and weight to either the control group or the anaesthesia group. A power analysis before beginning the study indicated that 10 animals per group would give 80% power to detect an effect size of partial eta squared (ηp2)=0.25 in a comparison of group means,14 hypothesising cognitive impairment after early anaesthesia exposure. That effect size and number of subjects is also reasonable for detecting group differences in freezing (ηp2=0.13, ηp2=0.26)21, 22 and anxiety behaviours (ηp2=0.22)22 after other developmental perturbations in the human intruder paradigm.
Monkeys received either three anaesthetic exposures to sevoflurane (anaesthesia group), or three brief maternal separations (control group) on or about postnatal Day 7 (range Day 6–10), that was repeated at 2-week intervals for a total of three exposures/separations between postnatal Days 7–35. After removal from the dam, all infants received a brief neurological exam.14 At this point, monkeys in the anaesthetic group were mask-induced with sevoflurane (from 2% to effect, maximum 8% in 100% O2), intubated and catheterised for i.v. fluids. Sevoflurane was administered for 4 h with monitoring of vital signs, depth of anaesthesia, and blood gases. Physiological monitoring during anaesthesia was consistent with normal physiology, with no indication of hypoxaemia, hypercapnia, or hypotension (Table 1). After complete recovery, lasting on average 20–30 min, the infant was returned to its dam. Subjects in the control group experienced maternal separation at the same ages, which comprised the neurological exam and a period of handling that matched in total duration the separation consciously experienced by the experimental group. Thus, on average, control infants experienced 30–40 min of maternal separation and were returned to the dam. Mother-infant interactions after these separations did not differ between groups,23 indicating that the separations involved in anaesthesia exposures did not cause alterations in mother-infant bonding that might have impacted later cognitive or socio-emotional behaviour.
Table 1.
Physiological measures during sevoflurane exposure. P6–10, postnatal days 6–10. Reprinted with modifications from Raper and colleagues,14 with permission from the publisher, American Society of Anesthesiologists Inc
| First anaesthetic (P6–10) |
Second anaesthetic (14 days after first) |
Third anaesthetic (28 days after first) |
|
|---|---|---|---|
| Mean (sd) | |||
| End-tidal carbon dioxide (kPa) | 5.01 (0.47) | 5.30 (0.42) | 5.03 (0.24) |
| Respiration rate (bpm) | 52 (1) | 45 (8) | 43 (10) |
| Oxygen saturation of haemoglobin (%) | 97 (2) | 97 (2) | 98 (1) |
| Pulse rate (beats min−1) | 152 (22) | 159 (15) | 160 (19) |
| Rectal temperature (°C) | 37.1 (0.4) | 37.2 (0.3) | 37.1 (0.3) |
| Inspired sevoflurane (%) | 2.48 (0.17) | 2.61 (0.16) | 2.66 (0.27) |
| Expired sevoflurane (%) | 2.46 (0.15) | 2.59 (0.15) | 2.64 (0.28) |
| Blood pressure (mm Hg) | 56 (20) | 52 (18) | 55 (14) |
| Blood pH | 7.38 (0.03) | 7.39 (0.05) | 7.41 (0.03) |
For behavioural testing in the human intruder paradigm at 1 and 2 yr of age, monkeys were separated from their social group, transported to a novel testing room, and transferred to a stainless steel testing cage (53×53×55 cm) with one wall made of clear plastic to allow for unobstructed viewing. The human intruder paradigm consisted of three conditions (alone, profile, stare) presented in the same order to all monkeys which lasted for a total of 30 min. The experimenter wore a rubber mask depicting a male face and a different mask was used at each age, such that every monkey saw the same novel human intruder at 1 and 2 yr of age across both cohorts. The monkey first remained alone in the cage for 9 min (alone condition) to acclimatise to the environment and obtain a baseline level of behaviour. Then the intruder entered the room and sat 2 m from the test cage while presenting his/her profile to the animal for 9 min (profile condition). The intruder then left the room while the monkey remained in the cage for a 3 min period, then the intruder re-entered the room, and sat 2 m from the cage while making direct eye contact for 9 min (stare condition). Emotional behaviour responses to the intruder were assessed using Observer XT 10 software (Noldus Inc., Wageningen, The Netherlands) and a detailed ethogram (Table 2). Three experimenters with a high degree of interrater reliability Cohen's κ=0.90 and an average intrarater reliability of Cohen's κ=0.97 coded all the video recordings.
Table 2.
Behavioural ethogram. List of all behaviours scored, how they were measured, and a brief definition. Ø indicates a behaviour that was not observed
| Category and specific behaviours | Measurement | Brief definition |
|---|---|---|
| Freezing | Duration | Rigid, tense, motionless posture except slight head movement |
| Hostile behaviours | Cumulative frequency | |
| Threat bark vocalisation | Frequency | Low pitch, high intensity, rasping, guttural |
| Threat (facial expression) | Frequency | Any of the following: open mouth (no teeth exposed), head-bobbing, or ear flapping |
| Cage aggression | Frequency | Vigorously slaps, shakes, or slams body against cage |
| Lunge | Frequency | A quick, jerky movement toward the intruder |
| Vocalisations | Cumulative frequency | |
| Coo | Frequency | Clear soft, moderate in pitch and intensity, usually ‘oooooh’ sounding |
| Scream | Frequency | High pitch, high intensity screech, or loud chirp |
| Displacement behaviours | ||
| Anxiety | Cumulative frequency | |
| Scratch | Frequency | Rapid scratching of body with hands or feet |
| Body shake | Frequency | Whole body or just head and shoulder region shakes |
| Tooth grind | Frequency | Repetitive, audible rubbing of upper and lower teeth |
| Yawn | Frequency | Open mouth widely, exposing teeth |
| Self-directed | Cumulative duration | |
| Self-grooming | Duration | Use of hands or mouth to smooth or pick through fur |
| Self-clasping | Duration | Non-manipulatory enclosing or holding of a limb or body part with arms |
| Other self-directedØ | Duration | Sucking thumb, eye poke |
Statistical analysis
Before analysis, Kolmogorov–Smirnov tests were performed to determine that the behavioural data were normally distributed. There were no hypotheses regarding sex differences with early anaesthesia exposure, yet exploratory repeated measures analysis of variance (ANOVA) with group (control, anaesthesia) and sex as between subjects factors, and condition (alone, profile, stare), and age (1 and 2 yr) as repeated factors, were initially conducted to determine whether any effects of sex were present. Species-typical sex differences were detected for vocalisations and hostile behaviours, such that females vocalise more than males and hostile behaviours decreased with age for females and increased with age for males. However, as there were no interactions between the sex and anaesthesia groups and sex did not interact with any overall group differences, it was not included as a between subjects factor in the final analysis. Therefore, the potential impact of early repeated anaesthesia exposure on emotional behaviours was examined using repeated measures ANOVA with group as the between subjects factors, and condition and age as the within subjects factors with repeated measures. Correlations between displacement behaviours, anxiety and self-directed, were also explored using Pearson's correlations. All analyses were conducted using SPSS 24 for Windows (IBM Corporation, Armonk, New York, USA), P<0.05 was considered significant, and effect sizes were calculated using partial eta squared (ηp2).
Results
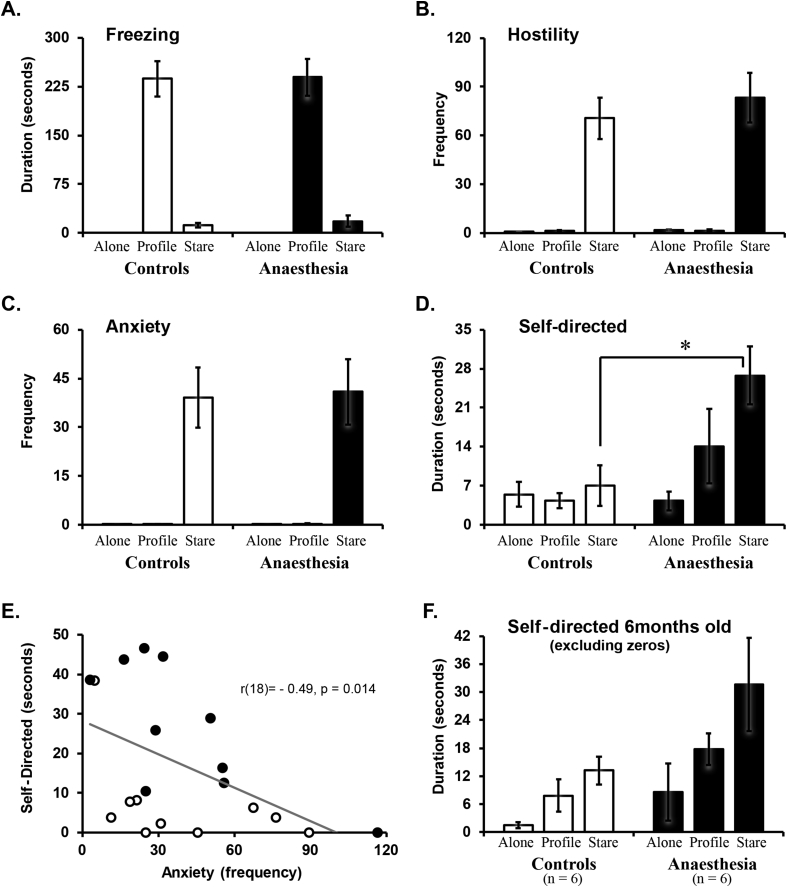
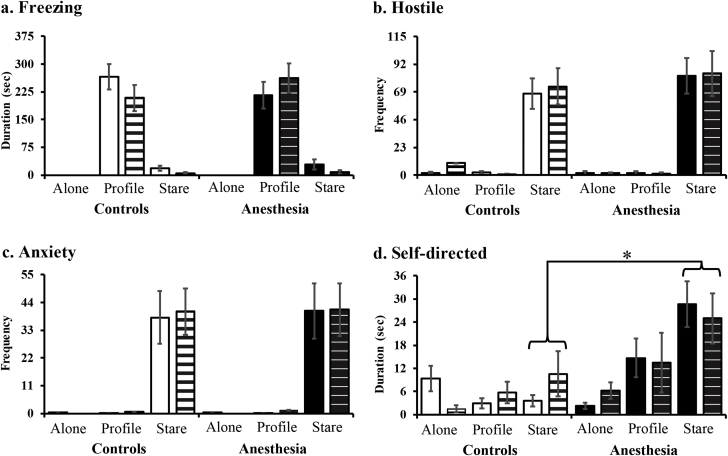
At 1–2 yr of age, monkeys exposed to repeated anaesthetics early in life exhibited similar behaviour to controls on all but one behavioural measure (Fig. 1; see also Supplementary Fig. S1). Species-typical increased freezing in the profile [condition: F(2,36)=147.01, P<0.001, ηp2=0.89] and increased vocalisations and hostile behaviours in the stare [condition: F(2,36)=8.99, P=0.001, ηp2=0.33; F(2,36)=58.88, P<0.001, ηp2=0.77, respectively], with no differences between groups [group: F(1,18)=0.04, P=0.85, ηp2=0.002; F(1,18)=0.004, P=0.95, ηp2=0.001; F(1,18)=0.45, P=0.51, ηp2=0.02, respectively], age [age: F(1,18)=0.48, P=0.49, ηp2=0.03; F(1,18)=1.54, P=0.23, ηp2=0.08; F(1,18)=0.04, P=0.83 ηp2=0.002, respectively], or interactions. Interestingly, differences in displacement behaviours between groups were specific to the type of behaviour, such that there was no group difference in anxiety [group: F(1,18)=0.02, P=0.90, ηp2=0.001], but monkeys with repeated neonatal anaesthesia exposure exhibited more self-directed behaviours during the stare condition compared with controls [condition X group: F(2,36)=3.88, P=0.03, ηp2=0.18] with no difference across age [age: F(1,18)=0.04, P=0.84, ηp2=0.002; F(1,18)=0.003, P=0.96, ηp2=0.001, respectively]. We observed a negative correlation between self-directed and anxiety-related behaviours at 1–2 yr [r(18)=−0.488, P=0.014; see Fig. 1e), suggesting that self-directed behaviours could be a type of stress coping strategy, which is further supported by negative correlations between self-directed behaviours and self-reported experience of stress in humans.20, 24, 25
Fig 1.
Behavioural responses to an acute stressor as juveniles (n=10 per group). Freezing (a), Hostile (b), Anxiety (c), and Self-directed behaviours (d) across the three conditions (Alone, Profile, Stare) of the task. Because no effect of Age was observed, results are averaged across one and two years of age (see Supplemental Fig. 1 for data by age). Correlation between self-directed and anxiety behaviours as juveniles (e). Self-directed behaviours at 6 months (f), excludes subjects that did not express any self-directed behaviours. Monkeys with neonatal exposure to sevoflurane anaesthesia are represented by filled bars or circles and control monkeys are represented by open bars or circles. Bar height in A–D and F represents the mean and standard error of the mean. * Indicates a significant difference between groups (P<0.05).
Our investigation of emotional behaviour at 6 months of age in these same monkeys14 did not include self-directed behaviour as a category because a number of monkeys at this age (seven out of 19) did not exhibit behaviours in this category. Consistent with this, ANOVA on this behavioural category at this age revealed no effect of treatment [group: F(1,16)=2.55, P=0.13, ηp2=0.14). Yet, among the monkeys who showed self-directed behaviours at 6 months of age, these were higher in monkeys that were exposed to sevoflurane as infants (control range: 3.05–12.02; anaesthesia range: 4.72–43.35; see Fig. 1f). Our data suggest that self-directed displacement behaviours emerge later in development in all monkeys.
Discussion
Juvenile rhesus monkeys who received multiple sevoflurane exposures during the first month of life exhibited increased self-directed (displacement) behaviours at 1 and 2 yr of age. In human and non-human primates, displacement behaviours are exhibited during situations of social tension, social ambiguity, unavoidable stress, or all three,20 and thus are regarded as a general indicator of stress and anxiety. In fact, anxiogenic drugs, catecholamines, or glucocorticoids cause increased self-grooming.26 The acute stressor used in the current study creates both unavoidable stress and social tension from the human intruder. Therefore, the current finding of increased self-directed behaviours together with our previous finding of increased anxious behaviours at 6 months of age,14 suggest that early anaesthesia exposure results in an anxious phenotype that persists at least into the second year of life, but that the specific displacement behaviours expressed change with age.
In humans, a negative correlation between displacement behaviours (i.e. self-touch) and self-reported experience of stress has been reported in many studies,24, 25, 27 but also see Villada and colleagues.28 Some studies suggest that displacement behaviours are coping strategies, such that there is a negative relationship between displacement behaviours and physiological stress measures (e.g. heart rate).27, 28 Therefore, juvenile monkeys exposed to repeated anaesthesia may exhibit more self-directed behaviours as an attempt to decrease their stress. However, further studies will need to combine behaviour and physiological measures of stress to truly address this.
Our findings of an anxious phenotype are consistent with a previous report of increased freezing, indicative of behavioural inhibition, in 2-yr-old monkeys that received repeated isoflurane exposure as infants.29 Although the monkeys in our study did not differ from controls in freezing during the mild profile threat, the differences in the anxiety profiles found between studies might be related to differences in genetic background of the animals. Among rhesus macaques, the degree of Chinese- or Indian-origin ancestry influences emotional reactivity, especially freezing, in the human intruder task.30 It is also notable that juvenile monkeys exposed to repeated sevoflurane as infants did not differ from controls in hostile behaviour expression. In contrast, monkeys with neonatal hippocampal lesions exhibit decreased freezing and hostility, but increased anxiety and self-directed behaviours.31 Therefore, the emotional alterations after repeated sevoflurane exposure are mild and perhaps more subtle in comparison, highlighting the need for sensitive tests to detect behavioural differences in children after early anaesthesia exposure.
The US Food and Drug Administration recently implemented a warning for common sedative and anaesthetic drugs specifically for children under the age of 3 experiencing repeated exposures or single exposures longer than 3 h, based on a combination of clinical and preclinical work (www.fda.gov/Drugs/DrugSafety/ucm532356.htm).32 Our findings support that precautionary warning and indicate that changes in emotional behaviour after repeated anaesthesia exposure in infancy could persist for years. Based on the correspondence between human and rhesus monkey lifespan, a 2-yr-old rhesus monkey is similar to a 6-yr-old human,33 the age at which children are beginning formal schooling. Disturbances in emotional behaviour at this age could have a profound impact on the adjustment to school.
Our findings do not elucidate the possible mechanisms of long-term effects of repeated anaesthesia exposure in infancy. However, recent reports confirm that early anaesthesia exposure results in neuro- and glio-apoptosis, whether that exposure occurs on postnatal day 6,34, 35, 36 20, or 40 in infant rhesus monkeys exposed to isoflurane,11, 37 which were the ages of repeated exposure in the present study. Loss of neurones and oligodendrocytes was observed in amygdala and hippocampus,37 structures involved in the development of emotional behaviour. Early loss of neurones and oligodendrocytes could alter the developmental trajectory of the central nervous system in a number of ways, as early lesions have different consequences to lesions later in life as their effects interact with the developmental trajectory of cognitive and emotional processes.22, 31, 38 It is also possible that surviving neurones function abnormally, as adult rodents that were exposed to anaesthesia in early development have identified electrophysiological and cell morphological abnormalities.13, 39, 40 Interestingly, hippocampal damage also leads to increased anxiety-related behaviours in humans and animals31, 41, 42; monkeys with neonatal hippocampal lesions exhibit increased self-directed behaviours in infancy and adulthood.31 In conjunction with impaired visual recognition memory in these monkeys at 2 yr of age,18 these findings suggest a persistent abnormality in hippocampal function after early anaesthesia exposure that results in both heightened anxiety and impaired memory.
Now that effects of early anaesthesia exposure that persist for years have been identified, future work to elucidate the mechanisms of these long-term effects will dictate whether strategies to mitigate these impairments should focus on protection at the time of anaesthetic exposure, remediation of neuronal function once impairments have manifested themselves, or perhaps both. In this way, infants and young children with conditions that require surgical management can undergo safe surgery permitted by modern anaesthetic procedures, while allaying concerns that surgery and anaesthesia might have unintended long-term consequences on the function of the central nervous system.
Authors' contributions
Responsible for study design/planning: M.G.B., M.C.A., K.L.M.
Helped in conducting the study and collection of data: all authors.
Conducted data analysis: J.R., J.C. de B., M.C.A., M.G.B.
Writing of the manuscript: J.R., M.C.A., M.G.B.
Helped to edit manuscript: all authors.
Acknowledgements
We thank J. Johnson for technical assistance with behavioural testing and video coding and S. Deiner for comments on a draft of this manuscript. We would also like to thank the veterinary, colony management, and animal care staff at the Yerkes National Primate Research Centre Field Station for their support for this project.
Editorial decision: January 2, 2018
Handling editor: H.C. Hemmings Jr
Footnotes
This article is accompanied by an editorial: Monkey business: the importance of mounting behavioural evidence for anaesthesia-induced developmental neurotoxicity by V. Jevtovic-Todorovic, Br J Anesth 2018:120:617–619, doi: 10.1016/j.bja.2018.02.001
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2018.01.014.
Declaration of interest
None of the authors have any conflicts of interest to disclose.
Funding
National Institutes of Health (NIH)/National Institute of Child Health and Development (grant R01-HD068388). Yerkes National Primate Research Centre is supported by NIH/Office of the Director P51-OD011132.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
figs1.
References
- 1.Flick R.P., Katusic S.K., Colligan R.C. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu D., Flick R.P., Zaccariello M.J. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population based birth cohort. Anesthesiology. 2017;127:227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprung J., Flick R.P., Katusic S.K. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder R.T., Flick R.P., Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMaggio C., Sun L.S., Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gano D., Andersen S.K., Glass H.C. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr Res. 2015;78:323–329. doi: 10.1038/pr.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ing C., DiMaggio C., Whitehouse A. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 8.Ing C.H., DiMaggio C.J., Malacova E. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 9.Glatz P., Sandin R.H., Pedersen N.L., Bonamy A.K., Eriksson L.I., Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2016.3470. [DOI] [PubMed] [Google Scholar]
- 10.Ing C., Sun M., Olfson M. Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg. 2017;125:1988–1998. doi: 10.1213/ANE.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brambrink A.M., Evers A.S., Avidan M.S. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman K., Robertson N.D., Dissen G.A. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anesthetic agents causes widespread neurodengeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satomoto M., Satoh Y., Terui K. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 16.Davidson A.J., Disma N., de Graaff J.C. Neurodevelopmental outcome at 2 years of age after general anaesthesis and awake-regional anaesthesia in infancy (GAS): an international multicenter, randomized controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L.S., Li G., Miller T.L.K. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarado M.C., Murphy K.L., Baxter M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119:517–523. doi: 10.1093/bja/aew473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schino G., Perretta G., Taglioni A.M., Monaco V., Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- 21.Howell B.R., Godfrey J., Gutman D.A. Social subordination stress and serotonin transporter polymorphisms: associations with brain white matter tract integrity and behavior in juvenile female macaques. Cerebal Cortex. 2014;24:3334–3349. doi: 10.1093/cercor/bht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raper J., Wallen K., Sanchez M.M. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm Behav. 2013;63:646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raper J., Bush A., Murphy K.L., Baxter M.G., Alvarado M.C. Multiple sevoflurane exposures in infant monkeys do not impact the mother-infant bond. Neurotoxicol Teratol. 2016;54:46–51. doi: 10.1016/j.ntt.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohiyeddini C., Bauer S., Semple S. Displacement behaviour is associated with reduced stress levels among men but not women. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohiyeddini C., Semple S. Displacement behaviour regulates the experience of stress in men. Stress. 2013;16:163–171. doi: 10.3109/10253890.2012.707709. [DOI] [PubMed] [Google Scholar]
- 26.Ninan P.T., Insel T.M., Cohen R.M., Cook J.M., Skolnick P., Paul S.M. Benzodiazepine receptor-mediated experimental “anxiety” in primates. Science. 1982;218:1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- 27.Mohiyeddini C., Bauer S., Semple S. Public self-consciousness moderates the link between displacement behaviour and experience of stress in women. Stress. 2013;16:384–392. doi: 10.3109/10253890.2012.755171. [DOI] [PubMed] [Google Scholar]
- 28.Villada C., Hidalgo V., Almela M., Mastorci F., Sgoifo A., Salvador A. Coping with an acute psychosocial challenge: behavioral and physiological responses in young women. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brambrink A.M., Coleman K., Robertson N., Martin L.D., Dissen G., Neuringer M. American Society for Anesthesiologists; Chicago, IL: 2016. Isoflurane anesthesia during the first 2 weeks of life alters long-term behavioral development in rhesus macaques. [Google Scholar]
- 30.Jiang J., Kanthaswamy S., Capitanio J.P. Degree of Chinese ancestry affects behavioral characteristics of infant rhesus macaques (Macaca mulatta) J Med Primatol. 2013;42:20–27. doi: 10.1111/jmp.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raper J., Wilson M., Sanchez M., Payne C., Bachevalier J. Increased anxiety-like behaviors, but blunted cortisol stress response after neonatal hippocampal lesions in monkeys. Psychoneuroendocrinology. 2017;76:57–66. doi: 10.1016/j.psyneuen.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andropoulos D.B., Greene M. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med. 2017;376:905–907. doi: 10.1056/NEJMp1700196. [DOI] [PubMed] [Google Scholar]
- 33.Tigges J.G.T., McClure H.M., Hall E.C., Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 34.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paule M.G., Li M., Allen R.R. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110:i29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenning K.J., Noguchi K.K., Martin L.D. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachevalier J., Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Curr Opin Neurobiol. 2005;15:168–174. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Amrock L.G., Starner M.L., Murphy K.L., Baxter M.G. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122:87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M.Q., Ji M.H., Zhao Q.S. Neurobehavioural abnormalities induced by repeated exposure of neonatal rats to sevoflurane can be aggravated by social isolation and enrichment deprivation initiated after exposure to the anaesthetic. Br J Anaesth. 2015;115:752–760. doi: 10.1093/bja/aev339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchanan T.W., Tranel D., Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav. 2009;56:44–50. doi: 10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado C.J., Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]