Abstract
Background
The mechanisms of action of volatile anaesthetics are unclear. Volatile anaesthetics selectively inhibit complex I in the mitochondrial respiratory chain. Mice in which the mitochondrial complex I subunit NDUFS4 is knocked out [Ndufs4(KO)] either globally or in glutamatergic neurons are hypersensitive to volatile anaesthetics. The volatile anaesthetic isoflurane selectively decreases the frequency of spontaneous excitatory events in hippocampal slices from Ndufs4(KO) mice.
Methods
Complex I inhibition by isoflurane was assessed with a Clark electrode. Synaptic function was measured by stimulating Schaffer collateral fibres and recording field potentials in the hippocampus CA1 region.
Results
Isoflurane specifically inhibits complex I dependent respiration at lower concentrations in mitochondria from Ndufs4(KO) than from wild-type mice. In hippocampal slices, after high frequency stimulation to increase energetic demand, short-term synaptic potentiation is less in KO compared with wild-type mice. After high frequency stimulation, both Ndufs4(KO) and wild-type hippocampal slices exhibit striking synaptic depression in isoflurane at twice the 50% effective concentrations (EC50). The pattern of synaptic depression by isoflurane indicates a failure in synaptic vesicle recycling. Application of a selective A1 adenosine receptor antagonist partially eliminates isoflurane-induced short-term depression in both wild-type and Ndufs4(KO) slices, implicating an additional mitochondria-dependent effect on exocytosis. When mitochondria are the sole energy source, isoflurane completely eliminates synaptic output in both mutant and wild-type mice at twice the (EC50) for anaesthesia.
Conclusions
Volatile anaesthetics directly inhibit mitochondrial complex I as a primary target, limiting synaptic ATP production, and excitatory vesicle endocytosis and exocytosis.
Keywords: adenosine, anaesthesia, hypersensitivity
Editor's key points.
-
•
Excitatory synaptic transmission is hypersensitive to volatile anaesthetics in mitochondrial complex I mutants by unclear mechanisms.
-
•
Isoflurane inhibited both respiration and excitatory transmission to a greater extent in complex I mutant compared with wild-type mice.
-
•
This effect appears to involve reduced ATP synthesis and failure of synaptic vesicle endocytosis, which limits high-frequency excitatory synaptic transmission.
-
•
The underlying mechanisms and role of this effect under physiological conditions require further study.
Mitochondrial complex I, the rate-limiting step of electron transport in the nerve terminal,1 is a potentially important direct target of volatile anaesthetics.2, 3 Early studies showed that mitochondrial function was sensitive to volatile anaesthetics and that complex I is the only member of the electron transport chain4 that displays meaningful sensitivity to volatile anaesthetics.5, 6, 7 Across the animal kingdom, complex I deficient organisms are hypersensitive to volatile anaesthetics.8, 9, 10 Complex I is unique in the electron transport chain in its contribution to volatile anaesthetic sensitivity.11, 12
Ligand-gated ion channels were long hypothesised to be critical volatile anaesthetic targets,13, 14 but animal models testing their role by mutating candidate proteins either failed to show an effect, or showed only a modest change in volatile anaesthetic sensitivity.15, 16 In contrast, knockout mice lacking the mitochondrial complex I subunit NDUFS4 [Ndufs4(KO)]17, 18, 19 are remarkably hypersensitive to isoflurane and halothane at postnatal day 25–30 (before developing detectable pathology),9 with an 50% effective concentration (EC50), one-third that of normal animals. Our previous electrophysiological data revealed that the frequency of spontaneous excitatory synaptic currents in the hippocampal CA1 region was selectively hypersensitive to isoflurane in these mice.3 Others have shown that this mouse model is defective in endocytosis because of deficiencies in mitochondrially derived ATP at the synapse.20 In addition, localised knock-down of NDUFS4 in various central nervous system regions led to shifts in anaesthetic sensitivity in otherwise wild-type mice.21 Energetically demanding conditions might further unmask the effects of isoflurane on neuronal function.
Here, we tested the sensitivity of mitochondria to isoflurane, and subjected control and Ndufs4(KO) hippocampal CA1 slices to high frequency stimulation. We recorded field excitatory postsynaptic potentials (fEPSPs) with and without isoflurane under multiple conditions designed to reveal mechanisms underlying anaesthetic sensitivity in both control and Ndufs4(KO) hippocampal slices. Our findings indicate that isoflurane directly inhibits mitochondrial complex I. Downstream effects on synaptic function are most striking under conditions of high energy demand or loss of glycolytic capacity. Thus, complex I function in excitatory neurons is tightly linked to volatile anaesthetic sensitivity.
Methods
Animals
Animal experiments were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Seattle Children's Research Institute. Male and female 23–30 day-old mice of either Ndufs4(KO) or their sibling control genotype (‘wild-type’) were used as described.3 Hippocampal slices prepared as described3 from wild-type mice, GABAergic-specific Ndufs4 KO (Gad2Cre/+::Ndufs4lox/lox), glutamatergic (vGLUT2)-specific Ndufs4 KO (Slc17a6Cre/+::Ndufs4Δ/lox), and cholinergic-specific Ndufs4 KO (ChatCre/+::Ndufs4lox/lox) were compared with slices of their siblings heterozygous for Ndufs4lox and heterozygous for the Cre driver.3
Chemicals
Picrotoxin (Tocris, Bristol, United Kingdom) at 50 μM was used to block GABAA receptors in field recordings. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX; Sigma, St. Louis, MO) at 200 nM was used to inhibit A1 adenosine receptors in field recordings.
Polarography
Mitochondria from whole brain were isolated as described.22 Mitochondrial respiration was measured with a Clark electrode (Oxygraph-K2; Oroboros Instruments, Innsbruck, Austria) in 2000 μl assay medium MiR05 (http://wiki.oroboros.at/index.php/MiPNet14.13_Medium-MiR06) at 30°C. A run consisted of sequential injections of: (i) up to 400 μg mitochondria; (ii) 130 nmol ADP to test for presence of internal electron donors; (iii) complex I-dependent electron donor substrates 10 mM pyruvate plus 5 mM malate, 4 mM ADP for maximal state 3 respiration; (iv) one chamber received two injections with 13.4 mM isoflurane in MiR05, while the other chamber received the same doses of vehicle (MiR05 without anaesthetic). Both assay chambers were then opened for 10 min to remove anaesthetic and replenish oxygen. Recovery of respiration was measured followed by injection of 2 μM rotenone. Complex I-dependent oxidative phosphorylation was calculated as rotenone-sensitive state 3 respiration [pmolO2 s−1 mgprotein−1]. Data were normalised first to the initial state 3 rate before injections of isoflurane/vehicle and then to vehicle treatment. Complex II-dependent respiration was measured using 13 mM succinate in the presence of 2 μM rotenone. At the end of each run 4 μM antimycin A was injected. The antimycin A-sensitive state 3 rates [pmolO2 s−1 mgprotein−1] were used for normalisation.
Field recordings
Field recordings were performed as described.3 Briefly, the stimulating electrode was positioned in the area of Schaffer collateral fibres, and the recording electrode was placed in CA1 stratum radiatum to record fEPSPs. Fibres were stimulated every 30 s for 10 min for baseline activity, and for at least 60 min after high-frequency stimulation (HFS). fEPSPs during and after HFS were normalised to their average values for each recording during the final 10 min before HFS. HFS consisted of three 1-s trains at 100 Hz, delivered at 20 s intervals. In paired-pulse experiments a 60 ms interpulse interval was used. Isoflurane-containing solution was superfused for 40 min before HFS, and for the duration of the experiment. The synaptic input/output curve was constructed by varying stimulation amplitude in the 100–500 μA range (Supplementary Fig. S1).
Statistical analysis
Electrophysiology traces were analysed with pClamp 10 software (Axon Instruments). Fibre volley (FV) amplitude was measured in relation to baseline potential immediately before the stimulus. fEPSPs were quantified by linear regression applied to their initial slope. FV amplitudes and fEPSP slopes were normalised to their corresponding means obtained during 10 min immediately before HFS. Data during HFS and post-HFS were analysed by fitting generalised least squares linear model with an autoregressive correlation structure followed by Tukey multiple comparisons test. Paired-pulse ratios were analysed with one-way repeated measures analysis of variance with post-Holm–Sidak test multiple comparisons against the paired-pulse ratios before HFS. Decay time constants were obtained by fitting the mono-exponential decay function for each recording to the decaying portion of the fEPSP during each HFS train. Statistical analyses were conducted using R version 3.3.0.23, 24, 25 Figures were generated using ggplot2 package.26 Significance level was selected as 0.05.
Results
Isolated Ndufs4 knockout mitochondria are hypersensitive to isoflurane
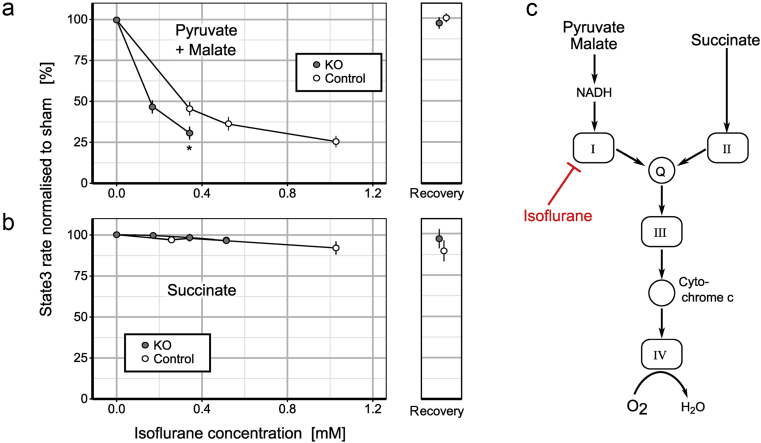
To test whether Ndufs4(KO) mitochondrial complex I is more sensitive to isoflurane than in control mice, we exposed mitochondria from each genotype to isoflurane. Baseline complex I dependent oxygen consumption rates for Ndufs4(KO) and control mitochondria were 0.9(0.3) and 1.7(0.6) nmolO2 s−1 mgprotein−1, respectively. The 50% inhibitory concentration (IC50) for complex I dependent respiration was 0.16 mM isoflurane in Ndufs4(KO) mitochondria and 0.31 mM isoflurane in control (Fig. 1a). Complex II dependent respiration was not appreciably inhibited in either genotype (Fig. 1b). Ndufs4(KO) mitochondria were both less active at baseline and more sensitive to isoflurane inhibition than were control mitochondria. As both complex I and II-dependent respiration require the activities of complexes III–V, the defect in the mutant is in hypersensitivity of complex I to isoflurane (Fig. 1c). Thus, while isoflurane inhibits complex I in normal mice as seen here and by others,6, 7, 27 it causes greater depression of complex I dependent respiration in Ndufs4(KO).
Fig. 1.
Isoflurane reversibly inhibits complex I dependent oxidative phosphorylation. ADP-stimulated respiration (state 3) of mitochondria from whole mouse brain was measured with a Clark electrode. (a) Complex I dependent state 3 respiration powered by pyruvate and malate was inhibited by isoflurane in a dose dependent fashion. The knockout (KO) was hypersensitive to isoflurane compared with wild-type with estimated IC50 values of 0.16 mM for the KO and 0.31 mM for control [* denotes significant difference (P=0.0013) between the genotypes at 0.34 mM isoflurane]. The absolute initial rates for KO and wild-type were significantly different [KO: 0.9(0.3), control: 1.7(0.6) nmolO2 s−1 mgprotein−1, P=0.035]. Inhibition was fully reversible after 10 min of removal from isoflurane. (b) Complex II dependent state 3 respiration was minimally affected for either genotype. In wild-type mitochondria inhibition caused by the highest isoflurane concentration, although small, was not reversible. (c) Diagram of the electron transport chain to illustrate electron flow (arrows) and site of isoflurane inhibition. Data presented as mean (standard deviation), n>4.
HFS induces short-term depression in Ndufs4(KO) slices exposed to isoflurane
As a mitochondrial defect might limit energy production at the synapse, we measured the dependence of CA1 fEPSPs on CA3 presynaptic fibre volleys (FVs, measure of action potential propagation in presynaptic axons). CA1 responses to single pulse stimulation were similar in KO and control slices in the absence of isoflurane (Supplementary Fig. S1) as were paired-pulse responses (see Zimin and colleagues3).
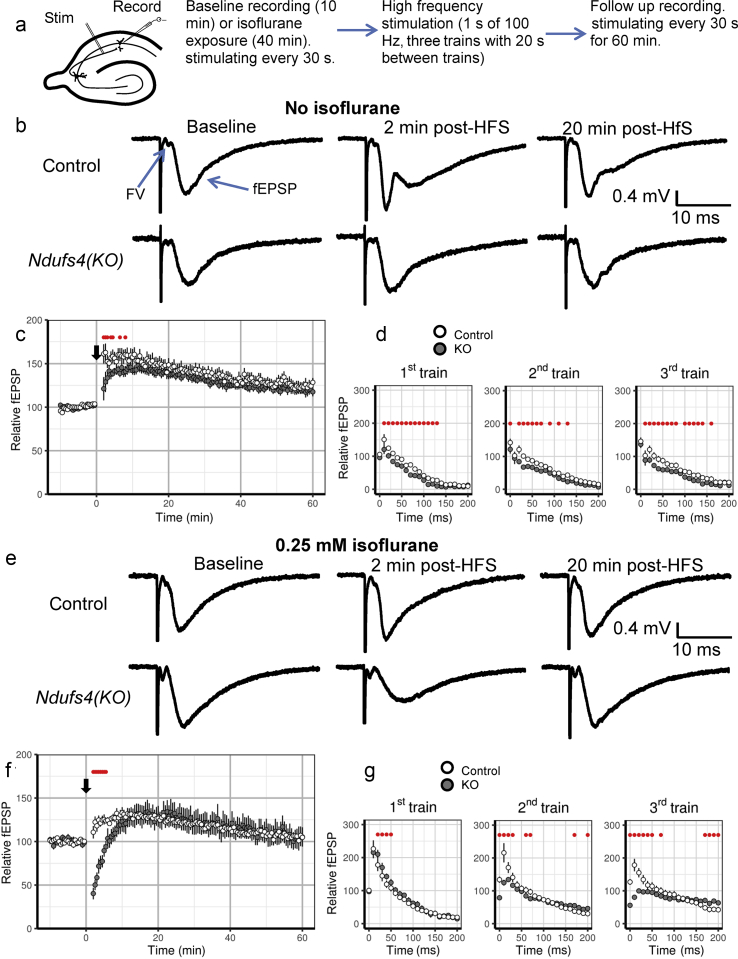
We measured responses of control and Ndufs4(KO) slices to repetitive high frequency stimulation to increase energy demand (Fig. 2a). fEPSPs evoked 2–8 min after HFS were characteristically28 potentiated in control but less potentiated in Ndufs4(KO) slices. fEPSPs from 8.5 min after HFS for up to 1 h after HFS were not significantly different between the genotypes (Fig. 2b and c). Long-term potentiation (1 h after HFS) reached ∼120% of baseline in both genotypes. The FV amplitudes were not affected by HFS in control and Ndufs4(KO) slices (data not shown). We conclude that the decrease in short-term potentiation (STP) of fEPSPs in Ndufs4(KO) synapses is a result of an energetically demanding process downstream from action potential propagation to the presynapse.
Fig. 2.
Ndufs4(KO) inhibits short-term potentiation (STP) after high frequency stimulation and leads to short-term depression in the presence of isoflurane. (a) The depiction of the experimental flow. Axons from CA3 are stimulated and postsynaptic potentials in CA1 are recorded. (b) Representative traces recorded during baseline, 2 min after high-frequency stimulation (HFS) and 20 min after HFS in wild-type and Ndufs4(KO) hippocampal slices in the absence of isoflurane. (c) In the absence of isoflurane, compared with wild-type, there is less STP after HFS in Ndufs4(KO) slices [control: n=10 slices, Ndufs4(KO): n=9 slices]. (d) There are small, but statistically significant, differences in field excitatory postsynaptic potentials (fEPSPs) during HFS trains in the absence of isoflurane. We interpret those differences as not biologically substantial. (e) Representative traces recorded during baseline, 2 min after HFS and 20 min after HFS in wild-type and Ndufs4(KO) slices in 0.25 mM isoflurane. (f) Ndufs4(KO) slices pre-exposed to 0.25 mM isoflurane show pronounced short-term depression after HFS [control: n=8 slices, Ndufs4(KO): n=9 slices]. (G) There are no differences between genotypes in fEPSPs during the first HFS train. fEPSPs in Ndufs4(KO) slices do not recover after the first and second HFS train as evidenced by the smaller than baseline initial fEPSPs of the second and third trains. Here and in subsequent figures, the black downward arrow represents the first HFS train, and error bars represent standard error of the mean, unless stated differently. Red dots show statistically significant differences between wild-type and Ndufs4(KO) slices.
Repetitive stimulation, leading to loss of short-term synaptic potentiation as in the mutant, is thought to be due primarily to depletion of synaptic vesicles of the readily releasable pool.29 To determine whether diminished STP observed in Ndufs4(KO) synapses can be explained by differences in the rates of recovery of synaptic vesicles during HFS or inhibition of vesicular release, we examined responses to three trains of HFS (Fig. 2d). While mutant fEPSPs were decreased compared with control pulses during all three trains, there did not appear to be any biologically important differences between genotypes in the magnitudes of fEPSPs (Fig. 2d), decay of pulses during HFS (Supplementary Table S1), or recovery between trains of HFS (Fig. 2d). In the absence of isoflurane, there was no apparent effect on the readily releasable pool of synaptic vesicles during the three trains of HFS. We conclude that, in the absence of isoflurane, only small synaptic differences exist between Ndufs4(KO) and control slices.
Exposure of hippocampal slices to 0.25 mM isoflurane (∼2X the EC50 for anaesthesia in the KO but < EC50 for control) led to ∼20% more fEPSP depression in Ndufs4(KO) slices than in controls (Supplementary Fig. S2a). Following HFS, fEPSPs 30 s to 5.5 min after HFS were dramatically depressed in Ndufs4(KO) slices compared with control slices (Fig. 2e and f). This is most consistent with a loss of the readily releasable pool of synaptic vesicles in neurones in the KO slices. At later time points, from 6 min to 1 h after HFS, there were no differences between genotypes.
Isoflurane could affect the rate of depletion of synaptic vesicles (exocytosis) or the rate of recovery of the readily releasable pool of vesicles after depletion (endocytosis). We therefore examined individual potentials during the three trains of HFS. In both genotypes, slices pre-exposed to 0.25 mM isoflurane (Fig. 2g, Supplementary Table S1) were similar in control and Ndufs4(KO) slices in the first train (Fig. 2g), with a pattern of potentiation followed by similar rates of depression of fEPSPs (Supplementary Table S1). However, the first several pulses of the second train showed fEPSP potentiation in control slices and depression in Ndufs4(KO) slices (Fig. 2g). The mutant was unable to recover 20 s after the initial 1 s stimulation of 100 Hz. The third train never showed potentiation in the KO, completely unlike control slices. This indicates that in the mutant isoflurane inhibited STP after HFS by slowing the rate of recovery of the readily releasable pool of vesicles after depletion. The rate of depression was also slower in Ndufs4(KO) slices in second and third trains compared with control slices (Supplementary Table S1). The cause for the changed slope of decay of fEPSPs in the mutant could represent an additional effect on exocytosis or delayed recovery (endocytosis) such that vesicles are still being delivered to the presynapse during stimulation.
Volatile anaesthetics can enhance GABAergic function, potentially increasing inhibitory neurotransmission.30 To test whether increased inhibitory neurotransmission from anaesthetic exposure contributes to short-term depression of excitatory neurotransmission after HFS, 50 μM picrotoxin (PTX) was added to the bath (Supplementary Fig. S3). Application of PTX in the absence of isoflurane caused HFS-induced short-term depression in the Ndufs4(KO), but not in control slices (Supplementary Fig. S3a). We propose that the loss of GABAergic inhibitory input leads to additional energetic demands in excitatory synapses causing short-term inability to regenerate the readily reversible pool of vesicles.
Isoflurane-induced fEPSP pre-HFS depression was not significantly affected by PTX in either genotype (Supplementary Fig. S2a). PTX did not alleviate HFS-induced short-term and long-term fEPSP responses in the presence of 0.25 mM isoflurane for either genotype (Supplementary Fig. S3b). We conclude that isoflurane-induced enhancement of inhibitory input does not contribute to short-term depression in Ndufs4(KO) after HFS and isoflurane exposure.
Neuronal type-specific loss of NDUFS4
Glutamatergic (vGLUT2)-specific Ndufs4(KO) animals have the same hypersensitivity to isoflurane and halothane as the global Ndufs4(KO), while GABAergic (GAD2) and cholinergic (ChAT)-driven Ndufs4(KO) demonstrate normal sensitivity to those anaesthetics.3 We tested whether loss of NDUFS4 in these cell types recapitulated synaptic findings in the global KO. If isoflurane controls anaesthetic sensitivity by affecting the readily releasable pool of synaptic vesicles, then the effects of isoflurane on STP in cell specific KOs would reveal whether this phenomenon alone predicts anaesthetic behaviour. Glutamatergic (vGLUT2)-specific Ndufs4(KO), GABAergic (GAD2)-specific Ndufs4(KO), and cholinergic (ChAT)-specific Ndufs4(KO) hippocampal slices were subjected to HFS. In the absence of isoflurane, each of the three transmitter-specific Ndufs4(KO) lines had reduced STP compared with control slices after HFS (Supplementary Figs S4a, S5a and S6a). The patterns of fEPSPs within trains of HFS were similar between mutant and control slices (Supplementary Figs S4b, S5b and S6b, Supplementary Table S2).
Exposure of all three transmitter-specific Ndufs4(KO) line hippocampal slices to 0.25 mM isoflurane led to fEPSP depression in the absence of HFS that was not different from the controls (Supplementary Fig. S2f–h). Exposure to 0.25 mM isoflurane led to short-term depression after HFS in the GAD2-specific and vGLUT2-specific Ndufs4(KO) slices, but not in the ChAT-specific Ndufs4(KO) slices (Supplementary Figs S4c, S5c and S6c). First fEPSPs of the second and third trains of the vGLUT2-specific and GAD2-specific KO strains also showed depression from baseline similar to the global KO, while control and ChAT-specific KO slices showed recovery from HFS in each train (Supplementary Figs S4d, S5d and S6d). Decreased STP is not sufficient to predict the anaesthetised state as the GAD2-specific KO is not anaesthetic hypersensitive yet shows decreased STP during isoflurane exposure. It is possible that the mechanism of HFS-induced decrease of STP in GAD2-specific KOs is different from that in the global KO. In the GAD2-specific KO, isoflurane may decrease output of GABAergic neurons after HFS. This, in turn, could lead to relatively increased input to excitatory neurones resulting in a short-term energy deficit and reduced STP, explaining a mismatch between the STP decrease in slices and isoflurane sensitivity in whole animals. However, we do interpret the results to indicate that energy failure does occur in the presence of isoflurane after HFS.
Effects of isoflurane concentrations normalised to behavioural effects for each genotype
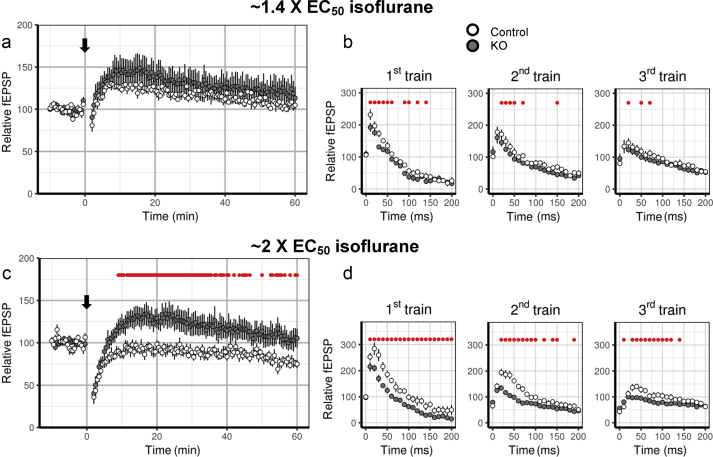
Isoflurane at 0.25 mM was ∼2 EC50 for Ndufs4(KO) mice but ∼0.7 EC50 for control mice in the data above. Exposure of control and Ndufs4(KO) hippocampal slices to their respective EC50 isoflurane concentrations for anaesthesia in the whole animal [EC50 (KO) ∼0.12 mM; EC50 (CTRL) ∼0.35 mM] led to depression of fEPSP responses in the absence of HFS (Supplementary Fig. S2b). Responses after HFS were similar at ∼1.4 EC50 (0.17 mM isoflurane for the KO and 0.5 mM isoflurane for control) isoflurane in both genotypes (Fig. 3a). Slices from both genotypes had similar short-term depression followed by potentiation and return to baseline levels by 1 h after HFS (Fig. 3a). Responses during all three trains of HFS were similar at ∼1.4 EC50 in both genotypes (Fig. 3b, Supplementary Table S1).
Fig. 3.
Control and Ndufs4(KO) slices exhibit similar high-frequency stimulation (HFS)-induced short-term depression when exposed to behaviourally equipotent isoflurane concentrations. (a) Short-term and long-term changes after HFS are similar in wild-type and Ndufs4(KO) slices in presence of ∼1.4 EC50 isoflurane for each genotype. (b) No pronounced differences in field excitatory postsynaptic potentials (fEPSPs) during HFS trains between wild-type and Ndufs4(KO) slices at 1.4 EC50 isoflurane [wild-type: n=6 slices, Ndufs4(KO): n=5 slices]. (c) Short-term changes are similar in wild-type and Ndufs4(KO) slices in the presence of 2 EC50 isoflurane for each genotype, however long-term fEPSP responses are diminished in the wild-type slices [wild-type: n=7 slices, Ndufs4(KO): n=9 slices]. (D) Ndufs4(KO) slices show diminished facilitation, but similar rates of depression in the three trains of HFS. Red dots show statistically significant differences between wild-type and Ndufs4(KO) slices.
Exposure to ∼2 EC50 (0.25 mM isoflurane for KO and 0.74 mM isoflurane for control) led to greater depression of fEPSPs before HFS in control slices than in Ndufs4(KO) slices (Supplementary Fig. S2c). HFS in slices exposed to ∼2 × EC50 led to similar fEPSP responses in STP (from 2 to 8.5 min after HFS, Fig. 3c) in both genotypes. Long-term fEPSP responses (8.5 min after HFS) were not potentiated in control slices, but were potentiated to ∼130% at 15 min after HFS in the KO. This effect gradually decayed over 60 min (Fig. 3c). fEPSPs during HFS in the Ndufs4(KO) slices showed less initial facilitation in the first train than in control, while initial fEPSPs of the second and third train were similarly depressed in both genotypes (Fig. 3d). Both genotypes displayed a decrease in the readily releasable pool by the second train. Facilitation of subsequent pulses of the second and third trains was smaller in the KO (Fig. 3d), although rates of depression were similar in both genotypes (Supplementary Table S1). These experiments show that, when exposed to isoflurane at equipotent concentrations, both genotypes demonstrate similar loss of STP and decreases in the readily releasable vesicle pool.
Effect of isoflurane on paired-pulse facilitation
Paired-pulse facilitation (PPF) results from residual Ca2+ in the presynapse after an initial depolarisation. Increases in PPF generally result from decreased Ca2+ influx for the second pulse (moving leftward on the release vs calcium curve),31 while decreases in PPF result from decreased synaptic vesicle availability. As reported,3, 32 before HFS slices of both genotypes demonstrate PPF (Fig. 4a, left panel), which increased in the presence of 0.25 mM isoflurane (compare Fig. 4a with Fig. 4b left panels). Therefore, isoflurane reduces pre-HFS Ca2+-dependent glutamate release similarly in both genotypes.
Fig. 4.
Paired-pulse ratios, first field excitatory postsynaptic potential (fEPSP) and second fEPSP plots of wild-type and Ndufs4(KO) synapses after high frequency stimulation. (a) In the absence of isoflurane, control and Ndufs4(KO) paired-pulse ratios decrease from corresponding baseline values between 2 and 3 min after high-frequency stimulation [HFS; wild-type: n=7 slices, Ndufs4(KO): n=7 slices]. (b) In the presence of isoflurane, wild-type paired-pulse ratios decrease between 2 and 28 min after HFS, while the KO paired-pulse ratios do not change from baseline values between 2 and 60 min after HFS [wild-type: n=7 slices, Ndufs4(KO): n=6 slices]. Red dots show statistically significant changes from baseline values.
Slices from both genotypes were then subjected to HFS. HFS initially decreased PPF in both genotypes, with subsequent recovery to pre-HFS levels (Fig. 4a, left panel). The short-term decrease in PPF was primarily the result of a failure to increase the magnitude of the second fEPSP (Fig. 4a, middle, right panels). As presynaptic Ca2+ should increase before the second pulse compared with the first, these results suggest that the availability of readily releasable vesicles is diminished after HFS in both genotypes.
Isoflurane did not change the effect of HFS on PPF in the Ndufs4(KO) (Fig. 4b, left panel). Control slices showed a short-term decrease in PPF followed by recovery by 60 min after HFS (Fig. 4b, left panel). In the presence of isoflurane, the magnitudes of both the first and second pulses was decreased after HFS compared with those without isoflurane in both genotypes (Fig. 4b, middle, right panels), consistent with reduced availability of releasable vesicles. The short-term decrease of both the first and second fEPSP of Ndufs4(KO) synapses in the presence of 0.25 mM isoflurane is consistent with reduced availability of releasable vesicles after HFS. We interpret these data as saturation of the sigmoidal Ca2+/vesicular release curve by HFS. Vesicle availability becomes limiting in the presence of isoflurane, more so in the KO than control. The first pulse in the control can recover to baseline in 30 s, but not for the second pulse. The KO cannot recover for either pulse, resulting in PPF ratios that are about the same as before HFS.
Short-term depression is mediated by A1 adenosine receptors
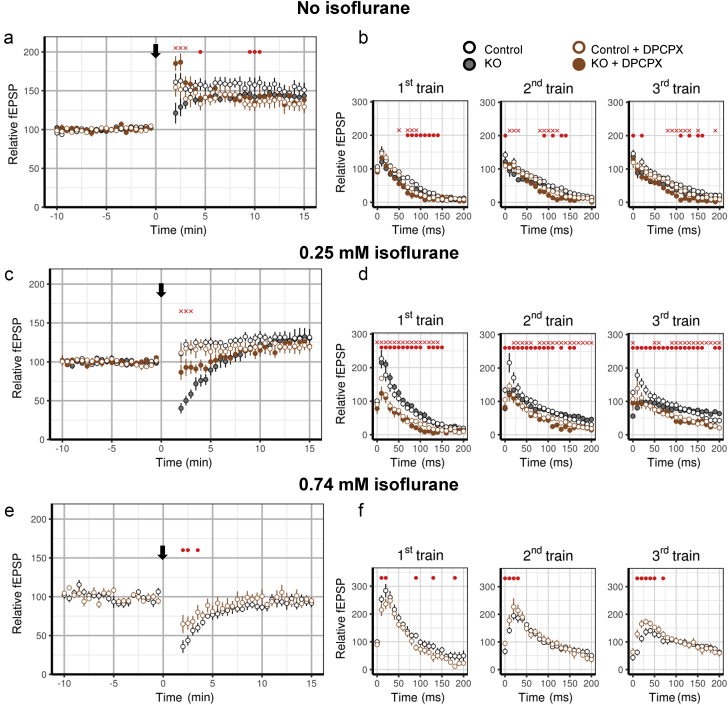
Adenosine has depressant effects on Ca2+ influx and synaptic function33; it is released during HFS34, 35, 36 or high metabolic demand.36 We used 200 nM DPCPX, a selective adenosine A1 receptor antagonist, to test whether adenosine contributes to short-term depression in Ndufs4(KO) slices after HFS. In the absence of isoflurane, DPCPX treatment did not affect fEPSPs of either genotype before HFS (data not shown), but increased HFS-induced STP compared with control in Ndufs4(KO) slices (Fig. 5a). DPCPX treatment did not affect HFS-induced STP in wild-type synapses (Fig. 5a). No substantial differences were seen in responses during the three trains of HFS in either the wild-type or KO slices after exposure to DPCPX (Fig. 5b). These results indicate that increased adenosine signalling contributes to the decrease in STP in Ndufs4(KO) slices after HFS.
Fig. 5.
Role of A1 adenosine receptor signalling in short-term depression after high frequency stimulation. (a) In the absence of isoflurane, DPCPX increases short-term potential after high-frequency stimulation (HFS) in the Ndufs4(KO) without a pronounced effect on wild-type [DPCPX wild-type: n=5 slices, DPCPX Ndufs4(KO): n=5 slices]. (b) While statistically significant changes were detected (red dots and crosses), DPCPX exposure did not substantially affect field excitatory postsynaptic potentials (fEPSP) responses during the three trains of HFS in either the wild-type or Ndufs4(KO). (c) DPCPX partially alleviated HFS-induced short-term depression in the Ndufs4(KO) in 0.25 mM isoflurane, but did not affect wild-type [DPCPX wild-type: n=5 slices, DPCPX Ndufs4(KO): n=5 slices]. (d) In Ndufs4(KO) slices, DPCPX decreased responses in the first and second train of HFS and eliminated depression of the first fEPSP of the third train of HFS. (e) DPCPX lessened HFS-induced short-term depression in wild-type slices in 0.74 mM isoflurane (DPCPX wild-type: n=7 slices, wild-type without DPCPX: n=7 slices). (f) No profound changes were observed in fEPSP responses during HFS trains between DPCPX-treated and DPCPX-untreated wild-type slices in the presence of 0.74 mM isoflurane. Red dots represent statistically significant differences between wild-type vehicle and wild-type DPCPX slices. Red crosses represent statistically significant differences between Ndufs4(KO) vehicle and Ndufs4(KO) DPCPX slices. Error bars in the anaesthetic behaviour experiments represent standard deviations.
To test if adenosine signalling mediates HFS-induced short-term depression by isoflurane, HFS was applied in the presence of DPCPX and 0.25 mM isoflurane. DPCPX reduced short-term depression in Ndufs4(KO) slices (Fig. 5c, solid brown circles), but not in wild-type slices (Fig. 5c, open brown circles). Before HFS, DPCPX reduced isoflurane-induced synaptic depression in Ndufs4(KO) but not in wild-type slices (Supplementary Fig. S2d) even at high doses (Supplementary Fig. S2e). These results indicate that isoflurane depression of fEPSPs before HFS has an adenosine-independent component in both genotypes (Supplementary Fig. S2d and e) and an additional adenosine-dependent component in Ndufs4(KO).
As adenosine appears to partially mediate the HFS-induced short-term depression in Ndufs4(KO) slices, we examined individual pulses of HFS. DPCPX did not affect the rate of depression in Ndufs4(KO) slices during the first HFS train, but significantly increased the rate of depression during the second and third HFS trains (Fig. 5d, Supplementary Table S2). These results suggest that, in the presence of isoflurane, adenosine inhibits Ca2+ influx in Ndufs4(KO) slices, and this slows the rate of depletion of synaptic vesicles. Wild-type slices exposed to 0.74 mM isoflurane (equipotent to 0.25 mM isoflurane in Ndufs4(KO) mice) and DPCPX showed only a small effect on HFS-induced short-term depression (Fig. 5e) compared with without DPCPX. There were no marked differences between DPCPX treated and untreated slices in fEPSPs during HFS trains (Fig. 5f). These results are most consistent with the interpretation that, in wild-type slices, adenosine plays only a small role in synaptic depression induced by HFS trains in isoflurane-exposed synapses.
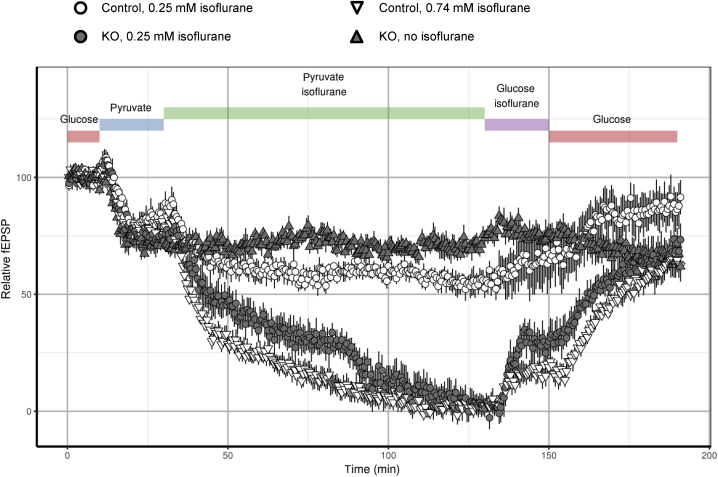
Complete synaptic depression in the Ndufs4 knockout by isoflurane with pyruvate as sole energy substrate
ATP can be produced by mitochondria-independent glycolysis and mitochondria-dependent oxidative phosphorylation. In neurones using pyruvate as the sole metabolic substrate, synaptic vesicle endocytosis was interrupted when mitochondrial complex I was inhibited by rotenone (with a resulting decrease in synaptic ATP).20 In the presence of supraphysiological concentrations of glucose (30 mM as used in culture media), glycolysis is likely to play a more important role in ATP production than at physiological glucose concentrations (1–1.5 mM), where oxidative phosphorylation is likely to be the more important contributor to energy production. Isoflurane also inhibits complex I dependent oxidative phosphorylation, and does so at lower concentrations in Ndufs4(KO) than in wild-type mice (Fig. 1). We tested excitatory synaptic function under conditions favouring oxidative phosphorylation by substituting 10 mM pyruvate for 30 mM glucose as energy substrate. Pyruvate led to ∼20% reductions in fEPSPs in both wild-type and Ndufs4(KO) slices compared with baseline (Fig. 6). We interpret this change to reflect the partial reduction in ATP production caused by bypassing glycolysis to generate pyruvate for mitochondrial respiration. Isoflurane 0.25 mM completely eliminated fEPSPs in Ndufs4(KO) slices, and reduced fEPSPs to ∼60% of baseline in wild-type (Fig. 6). This effect was not because of prolonged exposure to pyruvate as Ndufs4(KO) slices failed to show progressive synaptic depression in the absence of isoflurane (Fig. 6). Wild-type slices exposed to pyruvate and 0.74 mM isoflurane also demonstrated complete elimination of fEPSPs. These results support the model that isoflurane inhibits mitochondrial ATP production, resulting in synaptic silencing at doses approximating the EC50 for anaesthesia in each genotype.
Fig. 6.
Ndufs4(KO) synaptic function is completely depressed by isoflurane in conditions favouring oxidative phosphorylation. Replacement of glucose with pyruvate (blue bar) leads to similar synaptic depression in Ndufs4(KO) and wild-type synapses. Addition of 0.25 mM isoflurane (green bar) leads to complete loss of synaptic function in Ndufs4(KO) synapses (closed circles), while causing ∼30% depression in wild-type synapses (open circles). Addition of 0.74 mM isoflurane (green bar) with pyruvate leads to complete loss of synaptic function in wild-type slices (open triangles). Prolonged incubation of slices in pyruvate solution without isoflurane did not lead to additional depression of field excitatory postsynaptic potentials (fEPSPs) in Ndufs4(KO) synapses (closed triangles). Changing solution to glucose solution with isoflurane (purple bar) led to partial recovery of fEPSPs.
Discussion
The potency of isoflurane for inhibition of complex I dependent respiration was significantly greater in mutant mitochondria than in controls. As Ndufs4(KO) complex I activity at baseline is about half that of wild-type, isoflurane produces a marked decrease in the absolute complex I activity in Ndufs4(KO) mitochondria compared with wild-type, which supports the hypothesis that mitochondrial complex I is a significant target for volatile anaesthetics. Mitochondrial complex I deficiency decreases HFS-induced STP in the absence of isoflurane. However, in the presence of isoflurane at anaesthetising doses in each genotype, there is a profound short-term depression both in Ndufs4(KO) and wild-type slices after HFS, which is not because of enhanced GABAergic signalling from isoflurane exposure. The depression in fEPSPs by isoflurane is not seen during the first train of HFS, but becomes evident during the second and third trains of HFS. This time course suggests that synaptic release of neurotransmitter is not initially inhibited strongly by isoflurane. These data are most consistent with a resultant critical inability to recycle synaptic vesicles at lower concentrations of anaesthetic. However, both wild-type and mutant are not able to recover from stimulated release in the presence of equipotent doses of isoflurane suggesting that inhibition occurs at lower anaesthetic doses in the KO because of the pre-existing complex I defect. Isoflurane, itself a proven complex I inhibitor, adds to the genetic defect caused by Ndufs4(KO) to complex I.
During and shortly after HFS, synaptic depression is generally attributed to the rate of recovery of available synaptic vesicles, which is slower than that demanded by release during HFS.37, 38 We previously have shown that intrinsic membrane properties and synaptic function are not changed at baseline in Ndufs4(KO) slices.3 Thus, the changes seen in the field recordings in the presence of isoflurane, the Ndufs4(KO) mutation, or both, probably occur as a result of the increased energetic requirements caused by HFS. Similar results have been seen using fluorescent markers of vesicle recycling in Ndufs4(KO) neurones using rotenone as the mitochondrial inhibitor20 and when glycolysis is inhibited.39
Synaptic vesicle cycling energetics
Synaptic depression caused by HFS is frequently associated with depletion of synaptic vesicles from the readily releasable pool.29 Our data are consistent with a model in which, in the absence of isoflurane, HFS leads to the depletion of synaptic vesicles in the readily releasable pool in Ndufs4(KO) and recovery is delayed. HFS depletes ATP as a result of increased use and mitochondrial deficiency reduces resynthesis. This phenomenon is further exacerbated by inhibition of complex I by isoflurane, which further limits replenishment of ATP at the synapse. This is not simply a feature of this mutant model, as the same phenomenon occurs in normal mice at an anesthetising dose of isoflurane.
Pathak and colleagues20 recently showed that in Ndufs4(KO) neurones ATP concentrations decreased when stressed by HFS with concurrent inhibition of synaptic vesicle endocytosis, while exocytosis was not affected. The dependence of synaptic vesicle recovery from strong presynaptic demands on oxidative phosphorylation was further demonstrated by Sobieski and colleagues40 who found that inhibition of glycolysis did not affect the ability of synaptic vesicles to restore the readily releasable pool, while inhibition of oxidative phosphorylation slowed down recovery substantially. However, Rangaraju and colleagues39 showed that synaptic vesicle endocytosis was inhibited by either a loss of glycolysis or of oxidative phosphorylation. Our results are most consistent with a partial loss of synaptic function in the absence of glycolysis (dependence on pyruvate) and complete loss of synaptic function when oxidative phosphorylation is severely inhibited (addition of isoflurane). The relative contributions of glycolysis and oxidative phosphorylation to synaptic function likely depend on the concentration of glucose20 and potential upregulation of glycolytic41 and oxidative capabilities42 during ATP consumption driven by high neuronal activity.39 We are undertaking studies to correlate ATP concentrations with failure of endocytosis in synapses using isoflurane as the mitochondrial inhibitor.
A distinct mechanism of isoflurane effect on synaptic vesicle cycling was proposed by Baumgart and colleagues,43 who showed that isoflurane inhibits exocytosis in neuronal cultures by decreasing Ca2+ influx without affecting the coupling of Ca2+ influx to exocytosis. These results were consistent with other studies indicating that synaptic vesicle release is a target for volatile anaesthetics, with no effect on endocytosis.44, 45 In contrast, a study in PC12 pheochromocytoma cells showed a direct inhibitory effect of isoflurane on the vesicle release machinery.46 Both studies used higher isoflurane concentrations than those in our experiments. If exocytosis is strongly inhibited at the higher concentrations, limitations on endocytosis might not be detected. However, our results also indicate that Ca2+ influx is affected by a relatively low concentration of isoflurane through an energy dependent mechanism, which might be represented by the depression seen in fEPSPs in both genotypes.
Our paired pulse experiments in slices from wild-type animals before HFS confirm our previous results3 and those of MacIver and colleagues.32 HFS increases Ca2+ concentrations in the presynapse increasing release of available synaptic vesicles.47 The smaller fEPSPs seen in the values of both the first and second EPSPs in the Ndufs4(KO) in isoflurane, compared with in the absence of isoflurane, are consistent with a decrease in availability of vesicles. These results suggest that HFS causes a short-term decrease in synaptic vesicle availability in isoflurane-exposed wild-type and Ndufs4(KO) slices after HFS. The simplest model is that isoflurane causes mitochondrial inhibition to selectively inhibit neurotransmitter recycling in excitatory neurones.
Adenosine is released in response to neuronal activity and energy depletion.48, 49 Upon binding to A1 adenosine receptors, adenosine reduces Ca2+ influx through N- and P/Q-type Ca2+ channels,50, 51, 52 which suppresses synaptic function by reducing vesicle release probability.53, 54 Our data suggest that increased ATP consumption (caused by HFS) combined with decreased ATP production (Ndufs4 mutation, isoflurane), increases ADP and adenosine. As mitochondrial complex I in the KO is hypersensitive to isoflurane, isoflurane causes ATP concentrations to decrease more in the KO than in wild-type. The decrease in fEPSPs in KO slices is alleviated by blocking A1 receptors indicating that an energy deficit already exists before HFS in the KO. Adenosine concentrations increase sufficiently to partially dampen fEPSPs after HFS in the absence of isoflurane in Ndufs4(KO). In the KO in the presence of isoflurane, the adenosine effect is maintained, with a small effect of adenosine in wild-type slices exposed to isoflurane.50, 53 Baumgart and colleagues43 showed that isoflurane inhibits synaptic vesicle exocytosis in hippocampal neurones through inhibition of presynaptic Ca2+ influx. Our results suggest that this effect might be partially mediated by the effect of adenosine and the result of an isoflurane-induced energy deficit. Further experiments studying the effects of inhibition of adenosine signalling on the behaviour of Ndufs4(KO) animals are planned.
Conclusions
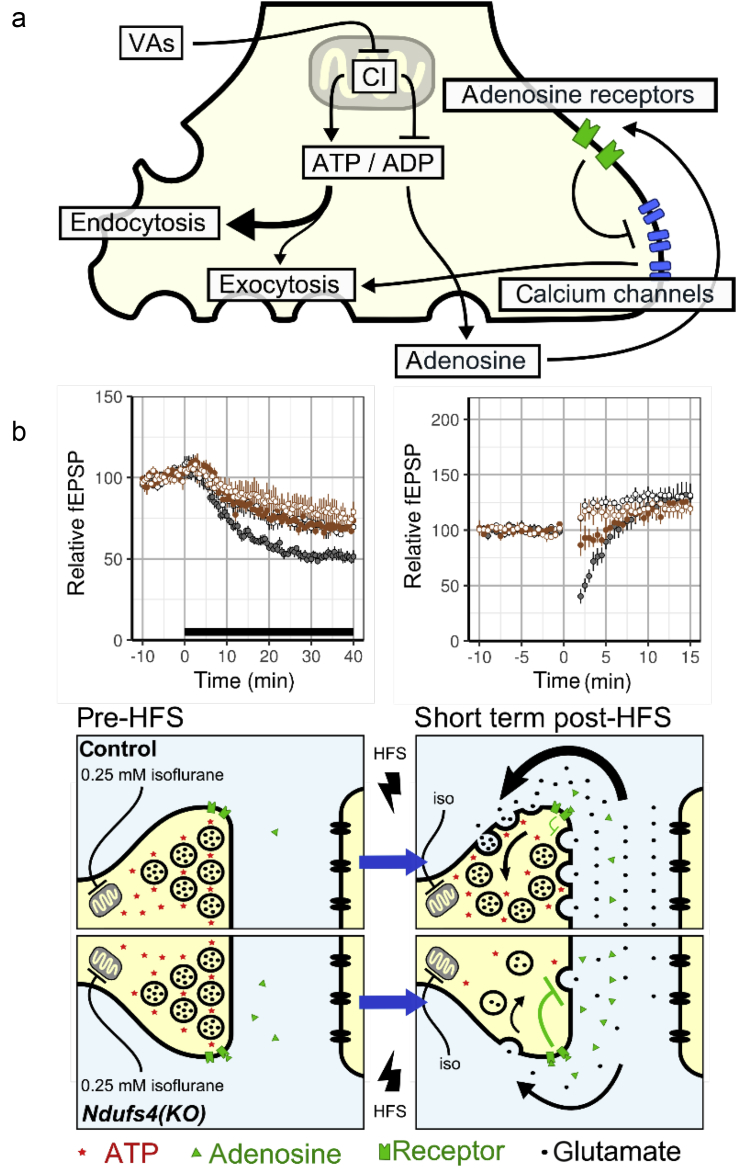
We propose a model in which anaesthetic- and genetically-induced mitochondrial dysfunction reduce ATP production, which inhibits synaptic vesicle endocytosis. This effect is independent of GABAergic effects and partially mediated by A1 adenosine receptors. We propose a model in which isoflurane inhibits mitochondria-dependent ATP production. This leads to energy failure at excitatory synapses causing a primary failure in synaptic vesicle endocytosis and indirect inhibition of exocytosis. Ndufs4(KO) is hypersensitive to isoflurane because of the additive effects of isoflurane and a pre-existing genetic defect in complex I. As isoflurane also inhibits STP after high frequency stimulation in normal animals at anaesthetising doses, we postulate that mitochondrial inhibition by isoflurane is not just a pathological phenomenon. Rather mitochondria could be a crucial target for anaesthetic action in normal animals (Fig. 7). Proof of the role of mitochondria in isoflurane sensitivity awaits identification of resistant mutants affecting the mitochondria.
Fig. 7.
Proposed model for volatile anaesthetic mechanism of action at excitatory synapses. (a) Volatile anaesthetics (VAs) directly inhibit complex I function to reduce ATP in the presynapse which, in turn, inhibits synaptic vesicle endocytosis. A decrease in the ATP/ADP ratio leads to an increase in adenosine, which, through A1 adenosine receptors, inhibits Ca2+ influx. The decreased Ca2+ influx, in turn, inhibits synaptic vesicle exocytosis. (b) The left lower panels [wild-type and Ndufs4(KO)] represent the mechanisms underlying the findings depicted immediately above the panels. In the presence of 0.25 mM isoflurane, mitochondrial function is inhibited in both genotypes, more so in the KO. This inhibition leads to more adenosine release (green dots) in the KO. Before high-frequency stimulation (HFS), control synapses balance ATP synthesis with ATP use, while Ndufs4(KO) synapses show larger isoflurane-induced depression because of increased adenosine signalling. The right lower panels represent the mechanisms underlying the findings depicted immediately above those panels. After HFS, ATP concentrations decrease (red dots) more so in the KO than wild-type synapses. This leads to decreased synaptic vesicle endocytosis in the KO and increased release of adenosine. Immediately after HFS in Ndufs4(KO), ATP synthesis fails to keep up with demand and the readily releasable pool of synaptic vesicles is depleted because of impaired endocytosis. This is depicted by black arrows in extracellular space and by less release of glutamate. The small black arrows in the intracellular spaces depict the recycling of synaptic vesicles into the readily releasable pools. At 0.75 mM isoflurane, synaptic dynamics would be the same in the wild-type as in the KO at 0.25 mM isoflurane. CI, complex I; iso, isoflurane.
Authors' contributions
Study design: all authors.
Electrophysiology experiments: P.Z., C.W.
Biochemical experiments: E.-B.K.
Data analysis: P.Z., C.W., E.-B.K., P.M.
Manuscript preparation: all authors.
Declaration of interest
None.
Funding
The research described was supported by US National Institutes of Health (R01GM105696 to P.G.M., M.M.S., J.M.R. and T32GM086270 to P.Z.). The laboratory was also supported in part by the NW Mitochondrial Research Guild.
Acknowledgements
We thank Drs Alfredo Garcia, Aguan Wei, Michele Shaffer for invaluable discussions and Dr Beatrice Predoi for excellent technical assistance.
Handling editor: H.C. Hemmings Jr
Editorial decision: February 09, 2018
Footnotes
This article is accompanied by an editorial: Bioblasts, anaesthesia, and power failure: rein in the excitement by Perouansky & Hemmings, Br J Anesth 2018:120:891–895, doi: 10.1016/j.bja.2018.02.018.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2018.01.036.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Telford J.E., Kilbride S.M., Davey G.P. Complex I is rate-limiting for oxygen consumption in the nerve terminal. J Biol Chem. 2009;284:9109–9114. doi: 10.1074/jbc.M809101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayser E.B., Morgan P.G., Sedensky M.M. GAS-1: a mitochondrial protein controls sensitivity to volatile anesthetics in the nematode Caenorhabditis elegans. Anesthesiology. 1999;90:545–554. doi: 10.1097/00000542-199902000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Zimin P.I., Woods C.B., Quintana A., Ramirez J.M., Morgan P.G., Sedensky M.M. Glutamatergic neurotransmission links sensitivity to volatile anesthetics with mitochondrial function. Curr Biol. 2016;26:2194–2201. doi: 10.1016/j.cub.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 5.Cohen P.J. Effect of anesthetics on mitochondrial function. Anesthesiology. 1973;39:153–164. doi: 10.1097/00000542-197308000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Harris R.A., Munroe J., Farmer B., Kim K.C., Jenkins P. Action of halothane upon mitochondrial respiration. Arch Biochem Biophys. 1971;142:435–444. doi: 10.1016/0003-9861(71)90507-8. [DOI] [PubMed] [Google Scholar]
- 7.Kayser E.B., Suthammarak W., Morgan P.G., Sedensky M.M. Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg. 2011;112:1321–1329. doi: 10.1213/ANE.0b013e3182121d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk M.J., Kayser E.B., Morgan P.G., Sedensky M.M. Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol. 2006;16:1641–1645. doi: 10.1016/j.cub.2006.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintana A., Morgan P.G., Kruse S.E., Palmiter R.D., Sedensky M.M. Altered anesthetic sensitivity of mice lacking Ndufs4, a subunit of mitochondrial complex I. PLoS One. 2012;7:e42904. doi: 10.1371/journal.pone.0042904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan P.G., Hoppel C.L., Sedensky M.M. Mitochondrial defects and anesthetic sensitivity. Anesthesiology. 2002;96:1268–1270. doi: 10.1097/00000542-200205000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Suthammarak W., Yang Y.Y., Morgan P.G., Sedensky M.M. Complex I function is defective in complex IV-deficient Caenorhabditis elegans. J Biol Chem. 2009;284:6425–6435. doi: 10.1074/jbc.M805733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suthammarak W., Morgan P.G., Sedensky M.M. Mutations in mitochondrial complex III uniquely affect complex I in Caenorhabditis elegans. J Biol Chem. 2010;285:40724–40731. doi: 10.1074/jbc.M110.159608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franks N.P., Lieb W.R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 14.Forman S.A., Chin V.A. General anesthetics and molecular mechanisms of unconsciousness. Int Anesthesiol Clin. 2008;46:43–53. doi: 10.1097/AIA.0b013e3181755da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao M., Sonner J.M., Jurd R. Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg. 2005;101:412–418. doi: 10.1213/01.ANE.0000154196.86587.35. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Sonner J.M., Werner D.F., Elsen F.P. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology. 2007;106:107–113. doi: 10.1097/00000542-200701000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Petruzzella V., Vergari R., Puzziferri I. A nonsense mutation in the NDUFS4 gene encoding the 18 kDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum Mol Genet. 2001;10:529–535. doi: 10.1093/hmg/10.5.529. [DOI] [PubMed] [Google Scholar]
- 18.Scacco S., Petruzzella V., Budde S. Pathological mutations of the human NDUFS4 gene of the 18-kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J Biol Chem. 2003;278:44161–44167. doi: 10.1074/jbc.M307615200. [DOI] [PubMed] [Google Scholar]
- 19.Vogel R.O., van den Brand M.A., Rodenburg R.J. Investigation of the complex I assembly chaperones B17.2L and NDUFAF1 in a cohort of CI deficient patients. Mol Genet Metab. 2007;91:176–182. doi: 10.1016/j.ymgme.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Pathak D., Shields L., Mendelsohn B.A. The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem. 2015;290:22325–22336. doi: 10.1074/jbc.M115.656405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramadasan-Nair R., Hui J., Zimin P.I., Itsara L.S., Morgan P.G., Sedensky M.M. Regional knockdown of NDUFS4 implicates a thalamocortical circuit mediating anesthetic sensitivity. PLoS One. 2017;12:e0188087. doi: 10.1371/journal.pone.0188087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayser E.B., Sedensky M.M., Morgan P.G. Region-specific defects of respiratory capacities in the Ndufs4(KO) mouse brain. PLoS One. 2016;11:e0148219. doi: 10.1371/journal.pone.0148219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing. [Google Scholar]
- 24.Pinheiro J., Bates D., DebRoy S., Sarkar D., Team R.C. 2016. nlme: linear and nonlinear mixed effects models. [Google Scholar]
- 25.Lenth R.V. Least-squares means: the R package lsmeans. J Stat Software. 2016;69:1–33. [Google Scholar]
- 26.Wickham H. Springer–Verlag; New York: 2009. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 27.Hanley P.J., Ray J., Brandt U., Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung L.S., Fu X.W. Factors affecting paired-pulse facilitation in hippocampal CA1 neurons in vitro. Brain Res. 1994;650:75–84. doi: 10.1016/0006-8993(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 29.Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 30.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 31.Xu-Friedman M.A., Regehr W.G. Structural contributions to short-term synaptic plasticity. Physiol Rev. 2004;84:69–85. doi: 10.1152/physrev.00016.2003. [DOI] [PubMed] [Google Scholar]
- 32.MacIver M.B., Mikulec A.A., Amagasu S.M., Monroe F.A. Volatile anesthetics depress glutamate transmission via presynaptic actions. Anesthesiology. 1996;85:823–834. doi: 10.1097/00000542-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Dunwiddie T.V., Hoffer B.J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980;69:59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sciotti V.M., Park T.S., Berne R.M., Van Wylen D.G. Changes in extracellular adenosine during chemical or electrical brain stimulation. Brain Res. 1993;613:16–20. doi: 10.1016/0006-8993(93)90448-v. [DOI] [PubMed] [Google Scholar]
- 35.Dale N., Pearson T., Frenguelli B.G. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526(Pt 1):143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd H.G., Lindström K., Fredholm B.B. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- 37.Sakaba T., Schneggenburger R., Neher E. Estimation of quantal parameters at the calyx of Held synapse. Neurosci Res. 2002;44:343–356. doi: 10.1016/s0168-0102(02)00174-8. [DOI] [PubMed] [Google Scholar]
- 38.Hanse E., Gustafsson B. Factors explaining heterogeneity in short-term synaptic dynamics of hippocampal glutamatergic synapses in the neonatal rat. J Physiol. 2001;537:141–149. doi: 10.1111/j.1469-7793.2001.0141k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangaraju V., Calloway N., Ryan T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobieski C., Fitzpatrick M.J., Mennerick S.J. Differential presynaptic ATP supply for basal and high-demand transmission. J Neurosci. 2017;37:1888–1899. doi: 10.1523/JNEUROSCI.2712-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang S., Nelson J.C., Bend E.G. Glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron. 2016;90:278–291. doi: 10.1016/j.neuron.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun T., Qiao H., Pan P.Y., Chen Y., Sheng Z.H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgart J.P., Zhou Z.Y., Hara M. Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proc Natl Acad Sci U S A. 2015;112:11959–11964. doi: 10.1073/pnas.1500525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemmings H.C., Yan W., Westphalen R.I., Ryan T.A. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005;67:1591–1599. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- 45.Hawasli A.H., Saifee O., Liu C., Nonet M.L., Crowder C.M. Resistance to volatile anesthetics by mutations enhancing excitatory neurotransmitter release in Caenorhabditis elegans. Genetics. 2004;168:831–843. doi: 10.1534/genetics.104.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Z., McMillan K., Pike C.M. Interaction of anesthetics with neurotransmitter release machinery proteins. J Neurophysiol. 2013;109:758–767. doi: 10.1152/jn.00666.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacIver M.B. Anesthetic agent-specific effects on synaptic inhibition. Anesth Analg. 2014;119:558–569. doi: 10.1213/ANE.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halassa M.M., Florian C., Fellin T. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovatt D., Xu Q., Liu W. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L.G., Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 51.Wu L.G., Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 52.Gundlfinger A., Bischofberger J., Johenning F.W., Torvinen M., Schmitz D., Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J Physiol. 2007;582:263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunwiddie T.V., Haas H.L. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scanziani M., Capogna M., Gähwiler B.H., Thompson S.M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.