Abstract
Background
The link between exposure to general anaesthesia and surgery (exposure) and cognitive decline in older adults is debated. We hypothesised that it is associated with cognitive decline.
Methods
We analysed the longitudinal cognitive function trajectory in a cohort of older adults. Models assessed the rate of change in cognition over time, and its association with exposure to anaesthesia and surgery. Analyses assessed whether exposure in the 20 yr before enrolment is associated with cognitive decline when compared with those unexposed, and whether post-enrolment exposure is associated with a change in cognition in those unexposed before enrolment.
Results
We included 1819 subjects with median (25th and 75th percentiles) follow-up of 5.1 (2.7–7.6) yr and 4 (3–6) cognitive assessments. Exposure in the previous 20 yr was associated with a greater negative slope compared with not exposed (slope: –0.077 vs –0.059; difference: –0.018; 95% confidence interval: –0.032, –0.003; P=0.015). Post-enrolment exposure in those previously unexposed was associated with a change in slope after exposure (slope: –0.100 vs –0.059 for post-exposure vs pre-exposure, respectively; difference: –0.041; 95% confidence interval: –0.074, –0.008; P=0.016). Cognitive impairment could be attributed to declines in memory and attention/executive cognitive domains.
Conclusions
In older adults, exposure to general anaesthesia and surgery was associated with a subtle decline in cognitive z-scores. For an individual with no prior exposure and with exposure after enrolment, the decline in cognitive function over a 5 yr period after the exposure would be 0.2 standard deviations more than the expected decline as a result of ageing. This small cognitive decline could be meaningful for individuals with already low baseline cognition.
Keywords: cognitive ageing, cognitive domains, cognitive z-scores, general anaesthesia, older adults
Editor's key points.
-
•
Longitudinal data from 1819 older adults in the Mayo Clinic Study of Aging were analysed to assess the association between exposure to general anaesthesia and surgery and cognitive-decline trajectory.
-
•
Compared with those not exposed, the decline in cognitive function was steeper in those who were exposed to anaesthesia and surgery in the 20 yr before enrolment.
-
•
For those with no prior exposure, the decline in cognitive function was steeper for exposure after enrolment.
-
•
General anaesthesia was associated with accelerated cognitive decline beyond that expected for normal ageing.
Dementia results from a complex interaction of multiple factors that include genes, age, co-morbidities, and environment. Preclinical studies suggest that exposure to inhalational anaesthetics enhances brain neuropathology implicated in Alzheimer dementia.1 Older adults are at risk for transient postoperative cognitive dysfunction2 characterised by impairment of memory, attention, concentration, and executive function. Because these symptoms are also characteristic of dementia, there is concern that this transient postoperative dysfunction is the precursor to permanent cognitive impairment, with evidence to both support3, 4, 5, 6 and refute7, 8, 9, 10, 11 this concern. Given the high frequency of surgery in older adults and the substantial public health implications of dementia, the potential link between exposure to general anaesthesia and surgery (exposure) and cognitive decline is of concern.
Most studies that examine the association between anaesthesia and cognitive decline use a binary time-to-event outcome (e.g. dementia and pre-dementia).3, 6, 10, 12 These diagnostic terms represent demarcations of convenience along the continuum of declining cognition,13 and as such, may not be sensitive enough to detect whether anaesthesia and surgery affect cognition. With the introduction of the diagnosis of mild cognitive impairment (MCI), a clinical entity positioned between cognitively normal and dementia (MCI is also known as pre-dementia),14 the ability to define the effects of anaesthesia and surgery on cognitive impairment has improved. However, the diagnosis of MCI itself has limitations as an outcome measure because of the heterogeneity of its prognosis (i.e. progression to dementia or reversion to normal).15 We recently explored the association between exposure to anaesthesia and incident MCI in older adults.6 Although we did not detect a statistically significant association for exposure after the age of 40, we could not exclude the possibility that more recent exposures (after age 60) are associated with the development of MCI.
Longitudinal measurements of cognitive function provide an opportunity to evaluate the trajectory of cognitive decline. Compared with binary outcomes, the analysis of longitudinally measured cognitive scores is a potentially more sensitive approach to detect declines in cognition without relying on diagnostic thresholds. Cognitive scores are commonly expressed as z-scores, which denote how many standard deviations are between a given score and the mean value of a set of scores derived from reference data. Prior studies have examined cognitive trajectories before and after surgery and anaesthesia,7, 16, 17 but systematic, repeated assessments of cognitive trajectories, as conducted in the present study, have not been used to study the long-term consequences of anaesthesia and surgery on cognition.
In this study, we used the resources of the Mayo Clinic Study of Aging (MCSA), a large population-based cohort of older participants in Olmsted County, MN, who undergo prospective longitudinal cognitive assessments approximately every 15 months.18 We tested the hypothesis that exposure to anaesthesia and surgery is associated with an accelerated decline in cognitive function. The primary outcome of interest was the change in global cognitive domain score; the secondary outcomes were the domains of memory, attention/executive function, language, and visuospatial skills.
Methods
This study conformed to the requirements of the Strengthening the Reporting of Observational Studies in Epidemiology Statement, and was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center, Rochester, MN, USA. At enrolment, all subjects provided written informed consent to participate in the study.
Selection of subjects
Study subjects were enrolled in the MCSA, an epidemiological study of the prevalence, incidence, and risk factors for MCI and dementia amongst Olmsted County residents.18 The present study included non-demented participants (cognitively normal or MCI) aged 70 through 91 years at baseline who had assessments in the MCSA study from October 1, 2004, through December 31, 2014, and had at least one follow-up MCSA visit.
Assessments
Details of the clinical evaluation at baseline and at each 15 monthly follow-up involved three evaluators and have been described.18 Briefly, the evaluation by a study coordinator or a nurse included assessment of patient-characteristic information, medical history, years of education, memory (self-report), and family history of dementia. A study partner (informant) completed the Clinical Dementia Rating Scale19 to assess dementia severity when present, and the functional activities questionnaire to assess the participant's functioning. Apolipoprotein ε4 (APOE ε4) allele (apolipoprotein E genotype; APOE) genotyping was performed using standard methods.
The neurological evaluation by a physician included the Short Test of Mental Status,20 modified Hachinski Ischemic Scale,21, 22 modified Unified Parkinson's Disease Rating Scale,23 and a neurological examination. The neuropsychological evaluation included assessment of four cognitive domains using nine tests in a cognitive testing battery: (i) attention/executive domain (Trail Making Test B and Digit Symbol Substitution Test),24, 25 (ii) language (Boston Naming Test26 and Category Fluency),27 (iii) memory domain (Wechsler Memory Scale–Revised Logical Memory II [delayed recall], Wechsler Memory Scale–Revised Visual Reproduction-II (delayed recall),28 and Auditory Verbal Learning Test (delayed recall),29 and (iv) visuospatial skills (Wechsler Adult Intelligence Scale–Revised Picture Completion Test and Wechsler Adult Intelligence Scale–Revised Block Design Test).24 To compute the domain z-scores and overall global cognitive z-score, the means and standard deviations of raw test scores for subjects who were cognitively normal in the MCSA 2004 enrolment cohort (n=1969) were used as the reference distribution when computing z-scores for individual tests.18, 30 Scores within a domain were averaged and scaled using the reference cohort to create domain z-scores. A global cognitive summary z-score was estimated by averaging and scaling the four domain z-scores as a measure of global cognitive impairment.18, 30
All data for each subject were reviewed by the study coordinator or nurse and a physician, and a neuropsychologist reviewed the neuropsychological testing data for a diagnosis of normal cognitive function, MCI, or dementia according to published criteria.18, 31, 32 MCSA procedures avoid testing in the temporal vicinity of procedures and acute illness to avoid the effects of transient postoperative cognitive decline. All surgery or procedures performed under anaesthesia were retrospectively identified through the Rochester Epidemiology Project.10, 12, 33
Statistical analyses
We use exposure to denote ‘exposures to surgeries and procedures performed under general anaesthesia’. The primary outcomes were baseline global z-score and the change (slope and rate of decline) in global z-score during follow-up in subjects with and without anaesthesia and surgery exposure. All analyses were performed to compare the MCSA study participants according to exposure status using two explanatory variables. One dichotomous variable was used to indicate prior exposure to anaesthesia and surgery in a 20 yr period before enrolment (yes or no). The other exposure variable was a time-varying covariate indicating exposure to anaesthesia and surgery after enrolment in those with no prior exposure (20 yr before enrolment). The domain-specific components of cognitive function were analysed as secondary outcomes. Sensitivity analyses were performed that used time periods of 5 and 10 yr when defining patients with and without prior exposure. Follow-up cognitive scores were not available after a subject discontinued participation in the study or after the subject met the criteria for dementia. Those with missing data may differ from those who continued participating in the study. To assess the potential impact of informative censoring on study results, an a priori defined sensitivity analysis was performed for the primary outcome (global z-score for cognitive function), which included only assessments obtained during the first 4 yr of follow-up for each subject.
Longitudinal cognitive z-score data were analysed using linear mixed models. Baseline and all follow-up cognitive scores were included. The model estimated separate terms for no exposure, any prior exposure, and any post-enrolment exposure in those previously unexposed. In brief, the association for any prior exposure was a fixed variable defined at enrolment that allowed for a different intercept and slope over time compared with those without exposure. The effect of any post-enrolment exposure in those previously unexposed was fitted with time-dependent variables to reflect a longitudinal change in slope. All models were adjusted for covariates and their interactions with time; these covariates were chosen based on prior studies as potentially affecting cognitive scores.30, 33 Adjustment variables included sex, baseline age, education, APOE ε4 status, midlife diabetes mellitus, midlife hypertension, midlife dyslipidaemia, atrial fibrillation, Charlson Co-morbidity Index (updated at each MCSA visit), history of congestive heart failure, stroke, coronary artery disease, marital status, smoking status, diagnosed alcohol problem, and baseline MCI. Models included subject-specific random intercepts and random slopes; random terms were assessed with likelihood ratio tests, which suggested that the model including both fits the data better than a reduced model. Results are summarised by presenting slope estimates and the changes in slope associated with exposure. The significance level was set at P<0.05, with the two-tailed test. Analyses were performed with SAS statistical software (version 9.3; SAS Institute Inc, Cary, NC, USA).
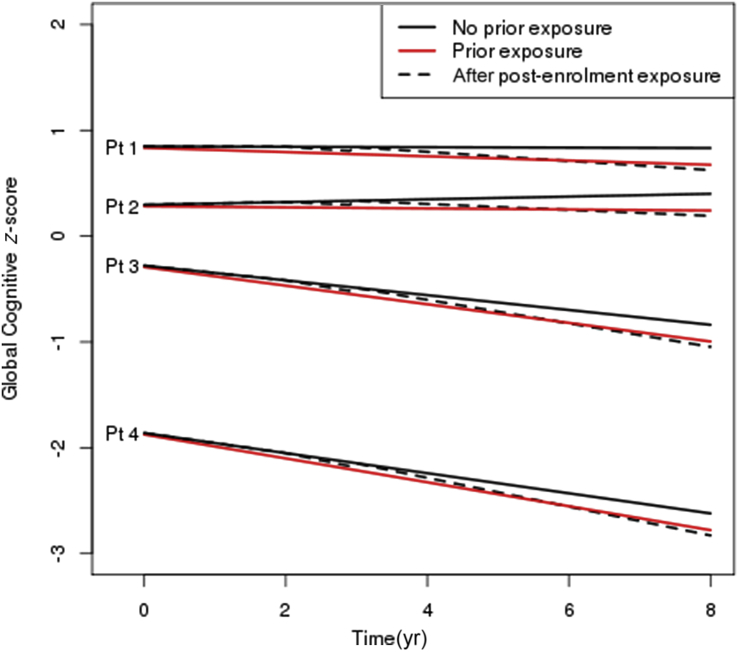
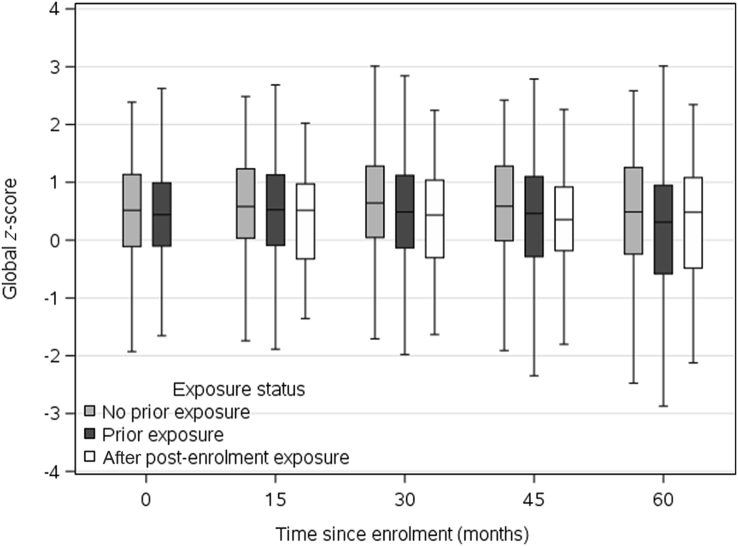
We graphically present associations in two ways. First, box plots describe cognitive z-scores over time. Study visits were grouped into intervals (enrolment, and 8–22, 23–37, 38–52, and 53–67 months) based on the expected time between visits in the MCSA. Subjects were grouped according to their exposure status at the given visit. Second, we simulated trajectories for four hypothetical patients to describe variation in cognitive z-scores at enrolment and trajectories over time. The four hypothetical patients have covariates selected to represent a range of healthy to unhealthy status (see Fig. 2 footnote for more details). Trajectories were simulated using linear combinations of the linear mixed model results with random terms set to zero to reflect an average subject with the given covariate values. Exposure status to anaesthesia and surgery was manipulated to simulate paths for (i) no exposure; (ii) prior exposure; and (iii) no prior exposure, but new exposure to anaesthesia and surgery at 2 yr after enrolment.
Fig 2.
Simulated trajectories for four hypothetical patients under three scenarios: no prior surgery and anaesthesia, and none during follow-up; prior surgery and anaesthesia; and post-enrolment surgery and anaesthesia at 2 years after enrolment without prior surgery and anaesthesia. Follow-up is described from enrolment through 8 years. Exposure refers to exposure to surgery and anaesthesia. The four hypothetical patients were chosen to represent varying degrees of health at enrolment. Patient (Pt) 1 is an 80-year-old female, never a smoker, married, with ≥16 years of education, with no co-morbidities, APOE ε4 negative, and cognitively normal at enrolment. Pt 2 is an 80-year-old male, never a smoker, married, 13–15 years of education, with prior history of coronary artery disease and a Charlson Co-morbidity Index score of 2, APOE ε4 negative, and cognitively normal at enrolment. Pt 3 is an 80-year-old female, current smoker, single-partner status, 12 years of education, with prior history of stroke and a Charlson Co-morbidity Index score of 2, APOE ε4 positive, and cognitively normal at enrolment. Pt 4 is an 80-year-old male, former smoker, single-partner status, <12 years of education, prior history of coronary artery disease and a Charlson Co-morbidity Index score of 4, with midlife dyslipidaemia, APOE ε4 positive, and mild cognitive impairment at enrolment. The plot demonstrates that changes over time attributable to surgery and anaesthesia before enrolment or post-enrolment represent a subtle, although statistically significant, change in the average trajectory of cognitive z-scores relative to the variability in z-scores inherent in the population.
Results
Of 2563 MCSA participants who were enrolled during the study period, 744 were excluded (Supplementary Fig. S1); the present study included a total of 1819 MCSA participants (1564 with normal cognitive function and 255 with MCI at enrolment) who had a cognitive z-score calculated on at least two study visits. Amongst these participants, the median follow-up time after the index cognitive assessment was 5.1 (2.7–7.6) yrs and included a median of 4 (3–6) cognitive assessments.
Of the 1819 participants, 1218 (67%) were exposed to at least one general anaesthetic and surgery or procedure in the 20 yr period before their index cognitive assessment, 907 (50%) had at least one exposure in the last 10 yr, and 599 (33%) in the last 5 yr. Of 1218 participants with prior exposure, 431 (35%) had exposure after the first z-score and before their last z-score: of those 264, 92, 38, 18, 10, and 9 had one, two, three, four, five, and greater than or equal to six exposures, respectively. Those with prior exposure were more likely to be male and to have a higher co-morbidity burden (Table 1). Of the 601 participants who were not exposed in the 20 yr period before their index cognitive assessment, 156 were exposed during follow-up: of these, 104, 34, 8, 8, and 2 had one, two, three, four, and five procedures, respectively. Amongst all 1374 participants with an exposure before or after enrolment, there were a total of 3825 exposures (Supplementary Table S1).
Table 1.
Patient and clinical characteristics at enrolment
| Characteristic | Prior anaesthesia and surgery exposure∗† |
||
|---|---|---|---|
| No exposure (n=601) | ≥1 Exposure (n=1218) | P-value | |
| Age, mean (sd) (yr) | 78.9 (5.3) | 78.8 (5.0) | 0.68 |
| Sex | 0.003 | ||
| Male | 282 (47) | 662 (54) | |
| Female | 319 (53) | 556 (46) | |
| Education (yr) | 0.34 | ||
| <12 (did not complete high school) | 47 (8) | 120 (10) | |
| 12 (high school graduate) | 200 (33) | 411 (34) | |
| 13–15 (some college or technical school) | 146 (24) | 306 (25) | |
| ≥16 (4 yr college degree or graduate studies) | 208 (35) | 381 (31) | |
| Smoking status | 0.34 | ||
| Never | 331 (55) | 627 (51) | |
| Former | 249 (41) | 548 (45) | |
| Current | 21 (3) | 43 (4) | |
| Marital status | 0.10 | ||
| Single | 68 (11) | 107 (9) | |
| Married | 368 (61) | 801 (66) | |
| Widowed | 165 (27) | 310 (25) | |
| APOE ε4 allele (n=1816) | 154 (26) | 330 (27) | 0.54 |
| Ever had a diagnosis of an alcohol problem (n=1816) | 15 (2) | 54 (4) | 0.043 |
| Charlson Co-morbidity Index | <0.001 | ||
| 0 | 98 (16) | 71 (6) | |
| 1 | 151 (25) | 213 (17) | |
| ≥2 | 352 (59) | 934 (77) | |
| Midlife diabetes mellitus | 34 (6) | 61 (5) | 0.56 |
| Midlife hypertension | 176 (29) | 470 (39) | <0.001 |
| Midlife dyslipidaemia | 229 (38) | 572 (47) | <0.001 |
| Atrial fibrillation | 60 (10) | 234 (19) | <0.001 |
| Congestive heart failure | 41 (7) | 139 (11) | 0.002 |
| Stroke | 29 (5) | 67 (6) | 0.54 |
| Coronary artery disease | 174 (29) | 537 (44) | <0.001 |
| Cognitive status | 0.64 | ||
| Normal cognition | 520 (87) | 1044 (86) | |
| Mild cognitive impairment | 81 (13) | 174 (14) | |
APOE ε4, apolipoprotein ε4; sd, standard deviation.
∗Exposure in the 20 years before the first available z-score.
†Categorical data are reported as number of patients (percentage); continuous data are reported as mean (sd).
Results for global cognitive z-scores
In adjusted linear mixed models, any exposure to anaesthesia and surgery in the previous 20 yr was associated with a more negative slope for global cognitive function in comparison to those unexposed (slope: –0.077 vs –0.059; difference: –0.018; 95% confidence interval [CI]: –0.032, –0.003; P=0.015) (Table 2). Any post-enrolment exposure in those previously unexposed was associated with a greater decline in slope after exposure (post-exposure slope: –0.100 vs –0.059 for pre-exposure, respectively; difference: –0.041; 95% CI: –0.074, –0.008; P=0.016) (Table 2). The results of the full model for the primary analysis are presented in Supplementary Table S2.
Table 2.
Analysis of global cognitive z-score and domain scores according to the pattern of exposures to anaesthesia and surgery in the 20 year period before enrolment
| Cognitive scores | Slope estimate | Difference in slope estimate (95% CI) | P-value |
|---|---|---|---|
| Global z-score | |||
| No prior exposure | –0.059 | Reference | |
| Prior exposure | –0.077 | –0.018 (–0.032, –0.003) | 0.015 |
| After post-enrolment exposure∗ | –0.100 | –0.041 (–0.074, –0.008) | 0.016 |
| Domain scores | |||
| Attention/executive | |||
| No prior exposure | –0.079 | Reference | |
| Prior exposure | –0.096 | –0.016 (–0.031, –0.001) | 0.033 |
| After post-enrolment exposure∗ | –0.132 | –0.053 (–0.094, –0.012) | 0.012 |
| Memory | |||
| No prior exposure | –0.005 | Reference | |
| Prior exposure | –0.022 | –0.018 (–0.034, –0.001) | 0.035 |
| After post-enrolment exposure∗ | –0.072 | –0.068 (–0.103, –0.033) | <0.001 |
| Language | |||
| No prior exposure | –0.064 | Reference | |
| Prior exposure | –0.078 | –0.014 (–0.029, 0.001) | 0.076 |
| After post-enrolment exposure∗ | –0.096 | –0.032 (–0.065, 0.001) | 0.060 |
| Visuospatial | |||
| No prior exposure | –0.031 | Reference | |
| Prior exposure | –0.038 | –0.008 (–0.020, 0.005) | 0.249 |
| After post-enrolment exposure∗ | –0.027 | 0.004 (–0.026, 0.034) | 0.785 |
CI, confidence interval.
∗Amongst participants with no exposure in 20 year period before enrolment, but with exposure after enrolment. Data were analysed using mixed-effect linear models with random intercepts and slopes for time and time after new exposure in those previously unexposed. Time was modelled as years after first available z-score. Adjustment covariates not shown, but included in the model were smoking status, midlife diagnoses of diabetes mellitus, hypertension, dyslipidaemia, history of atrial fibrillation, congestive heart failure, stroke, coronary artery disease, alcohol problems, marital status, sex, APOE ε4 allele, education, Charlson Co-morbidity Index, and cognitive status at enrolment. Separate analyses were performed for global score and for each cognitive domain. For each exposure category, a model-based estimate of the slope was calculated using covariate information for the entire cohort. Point estimates and 95% CIs are presented for the estimated difference in slope for those with prior exposure compared with no prior exposure, and for the change in slope after exposure in those previously unexposed. Six patients were excluded from the analysis because of missing covariate information.
In a post hoc analysis for the primary endpoint of global cognitive function, we further modelled the association of post-enrolment exposure amongst those with a prior exposure in addition to the comparisons estimated previously. Amongst patients with a prior exposure, a post-enrolment exposure was associated with a non-significant trend towards greater decline (difference in slope after post-enrolment exposure: –0.026; 95% CI: –0.053, 0.001; P=0.060).
Fig. 1 shows box plots for z-scores according to approximate visit month (0, 15, 30, 45, and 60 months) and exposure status for subjects who had a cognitive assessment at the 60 month visit. At the enrolment visit, the median (25th, 75th percentiles) z-score was 0.20 (–0.52, 0.82). This illustrates that the variability between subjects is substantial at all time points and differences in scores associated with exposure are small relative to this variability. This z-score variability is affected by subject characteristics, age at study entry (70–91 yr), and other characteristics that affect cognitive scores. Fig. 2 shows simulated paths of four hypothetical patients with escalating risks for cognitive decline (see legend). The graph illustrates that the differences in cognitive slope over time attributable to prior exposure, or post-enrolment exposure in those previously unexposed, are relatively small when compared with the wide variation in z-scores attributable to patient characteristics.
Fig 1.
Box plots for z-scores presented according to visit month (0, 15, 30, 45, and 60 months) and exposure to surgery and anaesthesia during the 20 years before enrolment. To ensure that the same individuals are represented at each time point, only individuals who had a cognitive assessment at the 60 month visit are included. Each time point represents a variety of individuals with variable global cognitive z-scores.
From sensitivity analyses defining any prior exposure at 10 or 5 yr before enrolment, there was still evidence that post-enrolment exposure in those previously unexposed was associated with an accelerated decline in global cognitive function (prior exposure defined as 10 yr: slope difference, –0.030; 95% CI, –0.057, –0.004; P=0.025; and prior exposure defined as 5 yr: slope difference, –0.027; 95% CI, –0.050, –0.005; P=0.017) (Supplementary Table S3). In a sensitivity analysis limited to 4 yr follow-up per participant, the estimated differences in slopes associated with prior or new exposure to anaesthesia and surgery compared with no exposure were numerically similar in direction and magnitude, but no longer significant (–0.008; 95% CI: –0.027, 0.009; P=0.329 and –0.060; 95% CI: –0.130, 0.010; P=0.096, respectively, for prior and new exposure vs no exposure).
Results for domain-specific z-scores
For attention/executive function, the slope difference for those with any exposure in the 20 yr before enrolment vs unexposed was –0.016 (95% CI: –0.031, –0.001; P=0.033); for those with no prior exposure, the change in slope after any post-enrolment exposure was –0.053 (95% CI: –0.094, –0.012; P=0.012) (Table 2). For memory domain, the slope difference for those with any exposure in the 20 yr before enrolment vs no exposure was –0.018 (95% CI: –0.034, –0.001; P=0.035); for those with no prior exposure, the change in slope after any post-enrolment exposure was –0.068 (95% CI: –0.103, –0.003; P<0.001). There was not enough evidence to suggest different slopes for the language and visuospatial domains over time.
Discussion
Older adults with any exposure to surgery with anaesthesia in the 20 yr before enrolment in the MCSA experienced a greater decline in cognitive z-scores compared with those not exposed. A more prominent decline was also found in previously unexposed participants who had any surgery with anaesthesia after enrolment in the study. Amongst those with pre-enrolment exposure, we did not have enough evidence to conclude that a post-enrolment surgery with anaesthesia was associated with a change in the trajectory of cognitive decline. Cognitive decline was primarily attributable to impairment of memory and attention executive domains.
There are numerous challenges in assessing the association between exposure to surgery and anaesthesia and cognitive decline. First, ageing is per se associated with measurable cognitive decline,34 and at the same time increases the likelihood of undergoing surgery: for individuals 65 yr of age or older, the incidence of undergoing a surgical procedure is substantially higher than for younger individuals.35 Second, a daunting challenge remains, separating the effects of anaesthesia and surgery from those of co-morbidities that are independently associated with cognitive deterioration, and also likely more frequent in the surgical population.3 Third, it is impossible to isolate the effects of anaesthesia from a systemic inflammatory response or other responses to surgery.36 Fourth, quantifying exposure to anaesthesia is not straightforward because exposures occur at variable times before and during longitudinal follow-up, which requires the use of complex statistical modelling when assessing this association. Given these interrelating complexities, it is not surprising that the debate concerning the association between anaesthesia and cognitive decline continues.3, 5, 6, 8, 10, 11, 12, 16
Several studies have examined the association between anaesthesia and longitudinal assessments of cognition. Using a linear mixed effects model, Avidan and colleagues7 did not find an association between surgery or illness and the rate of change in longitudinal cognitive trajectories assessed from comprehensive in-person assessment that included neurological evaluation and neuropsychological testing.7 They studied 575 patients, of whom 180 were exposed to non-cardiac surgery, although the timing of exposure was not precisely described.7 Hughes and colleagues11 conducted a multicentre prospective cohort study, and reported that surgery with anaesthesia was not a risk for cognitive impairment when tested at 12 months after major non-cardiac surgery or critical illness.11 Their definition of exposure to surgery and anaesthesia was restricted to exposure during current hospital admission or immediately preceding the admission, and cognitive assessments were performed 3 and 12 months after hospital discharge; therefore, this study did not assess change in cognition from before surgery.11 Another report from a large Danish registry of middle-aged and elderly twins reported a negligible decrease in a sensitive composite cognitive score for twins who underwent at least one major surgery, and no difference when a same-sex intra-pair sub-analysis was performed, suggesting the absence of an association between surgery with anaesthesia and long-term cognitive decline.8 Schenning and colleagues5 performed a longitudinal cohort study of cognitive ageing and reported faster decline in memory in those exposed to surgery. In their study, surgical procedures before enrolment were not considered, and patients were assigned to surgical or non-surgical groups according to post-enrolment exposure. Patel and colleagues16 recorded accelerated cognitive decline after surgery in older adults already diagnosed with cognitive impairment, but not in cognitively normal patients. In contrast, Bratzke and colleagues17 reported that surgery and anaesthesia affected cognition in middle-aged individuals without pre-existing cognitive dysfunction.
In the present study, we found an association between an accelerated decline in global cognitive function and exposure to anaesthesia and surgery, both in individuals with exposure within 20 years before MCSA enrolment and in individuals who had exposure only after enrolment. However, these changes over time were small relative to the overall population variation in z-scores. For an average individual with no prior exposure who is subsequently exposed after enrolment, the decline in cognitive function over a 5 yr period after the exposure would be 0.2 standard deviations more than expected (i.e. a decline of 0.50 standard deviations compared with an expected decline of 0.30 standard deviations below normative means). Although such a decline may be relevant to patients and providers, it represents only 5% of the roughly –2.18 to +1.99 range of z-scores present at enrolment in the sample. This represents a small effect size of uncertain clinical importance that likely could be meaningful for individuals with already low baseline cognition, as depicted in Fig. 2. This figure simulates trajectories for four hypothetical 80-yr-old patients with different risk factors for cognitive decline, and illustrates that even a small contribution from exposure to surgery and anaesthesia might, in some individuals, critically affect cognition by crossing a threshold for diagnosis of pre-dementia or even dementia. The observed small effect on cognitive decline in our study could account for our inability to detect associations between exposure to anaesthesia with surgery and higher rates of prevalent MCI10 or Alzheimer's dementia12 in prior analyses that also utilised the MCSA data set. However, it may be consistent with our analysis of incident MCI in this population (i.e. analyses restricted to individuals who were cognitively normal at the time of MCSA enrolment). Although cumulative exposure after age 40 yr was not associated with the development of incident MCI, in planned sensitivity analyses, we could not exclude the possibility that exposure later in life is associated with the subsequent development of MCI.6
Common cognitive complaints associated with normal ageing include impairment of memory37 and executive function.38 We found that, compared with unexposed participants, individuals exposed to anaesthesia and surgery have subtle, but statistically significant accelerated deterioration of cognitive scores for these two domains, which are potential drivers of future progression to dementia.13 The visuospatial domain represents a group of cognitive functions that involve the ability to understand space in two and three dimensions (e.g. the ability to recognise familiar objects or faces, spatial perception, etc.).37 The visuospatial domain is relatively resistant to normal ageing,39 and exposure was not associated with changes in the rate of change of this domain. Language is a complex cognitive domain that remains relatively intact with ageing,37, 38, 40 and likewise, we did not detect a significant deterioration in exposed individuals. Age-related cognitive decline affects specific domains differentially,41 and this non-uniformity appears to remain after exposure to anaesthesia.13 It remains to be determined whether the differential pattern of domain effects represents a form of accelerated normal ageing, or rather reflects a specific neuropathology induced by factors associated with exposure to surgery or anaesthesia.
Limitations
The most important limitation of our study is the possibility of unmeasured confounding. As exposure to anaesthesia is nearly always part of a surgical episode, we cannot separate the potential effects of anaesthesia and the surgery itself. In addition, exposure to anaesthesia and surgery could serve simply as a marker for other factors, including the underlying conditions necessitating surgery, which might be associated with accelerated cognitive decline. Available data do not include indications of other life events, such as recent extensive travel or death of family members that can be associated with accelerated cognitive decline. An additional limitation is that we included only exposure to surgery under general but not regional anaesthesia. Finding a similar effect on cognitive decline for both regional and general anaesthesia would argue against the direct contributory neurodegenerative effects of general anaesthetic agents, and would implicate surgical exposure as the primary culprit for cognitive deterioration.
The MCSA is a prospective study with information of cognitive scores collected in planned 15 month time intervals; however, given that it was not designed for the current investigation, the timing of the MCSA visits did not correspond to any consistent time interval surrounding exposures, which were retrospectively ascertained from the medical records. In addition, our cohort includes participants with varying degrees of cognitive function from normal to MCI, such that the average z-scores represent a mixture of cognitive function level, and may be less sensitive to subtle changes in individuals as a result of anaesthesia and surgery. Finally, cognition was not assessed after diagnosis of dementia, which might lead to informative censoring of patient data. However, numerically consistent findings were observed in the sensitivity analysis, which restricted follow-up to the first 4 yr after the index cognition score.
In conclusion, in this series of older adults, undergoing surgical or interventional procedures with general anaesthesia was associated with accelerated cognitive decline beyond that expected for normal ageing. The accelerated cognitive decline was predominantly related to impairments in memory and attention/executive function. Whilst the magnitude of the observed cognitive deterioration appears to be small, the clinical effect could be meaningful in individuals who undergo surgery with already lower baseline cognition.
Authors' contributions
Study conception and design: J.S.
Study supervision: J.S.
Data acquisition: J.S., R.C.P., D.S.K.
Data analysis: P.J.S., J.S., A.C.H., D.R.S.
Data interpretation: J.S., R.O.R., T.N.W., D.P.M., R.C.P., D.S.K., D.O.W.
Statistical modelling: P.J.S.
Drafting of manuscript: P.J.S., J.S.
Critical revisions for important intellectual content: all authors.
Final approval of manuscript: P.J.S., J.S., R.O.R., T.N.W., D.P.M., R.C.P., D.S.K., D.O.W., D.R.S.
J.S. and D.R.S. have full access to all the study data, and take responsibility for the integrity of the data and the accuracy of the data analysis. They also have final responsibility for the decision to submit for publication.
Acknowledgements
The authors thank J. Aakre, statistician for the Mayo Clinic Study of Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Handling editor: H.C. Hemmings Jr
Editorial decision: May 28, 2018
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2018.05.060.
Declaration of interest
The authors declare no competing interests. D.S.K. previously served as deputy editor for Neurology, and he serves on a Data Safety Monitoring Board for Lundbeck and for the Dominantly Inherited Alzheimer Network Trial Unit. He is an investigator in clinical trials sponsored by Biogen, Eli Lilly and Company, and TauRx Therapeutics Ltd., and receives research support from the National Institutes of Health (NIH). R.C.P. is the chair of Data Monitoring Committees for Pfizer, Inc. and Janssen Alzheimer Immunotherapy, LLC, and has served as a consultant for F. Hoffmann–La Roche Ltd.; Merck and Co, Inc.; and Genentech, Inc. He receives royalties from sales of the book Mild Cognitive Impairment (Oxford University Press). R.O.R. receives research support from the NIH and from F. Hoffmann–La Roche Ltd. J.S., T.N.W., P.J.S., A.C.H., D.P.M., D.R.S., and D.O.W. have nothing to disclose.
Funding
US National Institutes of Health (P50 AG016574 and U01 AG006786) to R.C.P.; Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program; Rochester Epidemiology Project (R01 AG034676); Mayo Clinic Center for Translational Sciences Activities (UL1 TR000135 from the US National Center for Advancing Translational Sciences); Department of Anesthesiology, Mayo Clinic.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig S1.
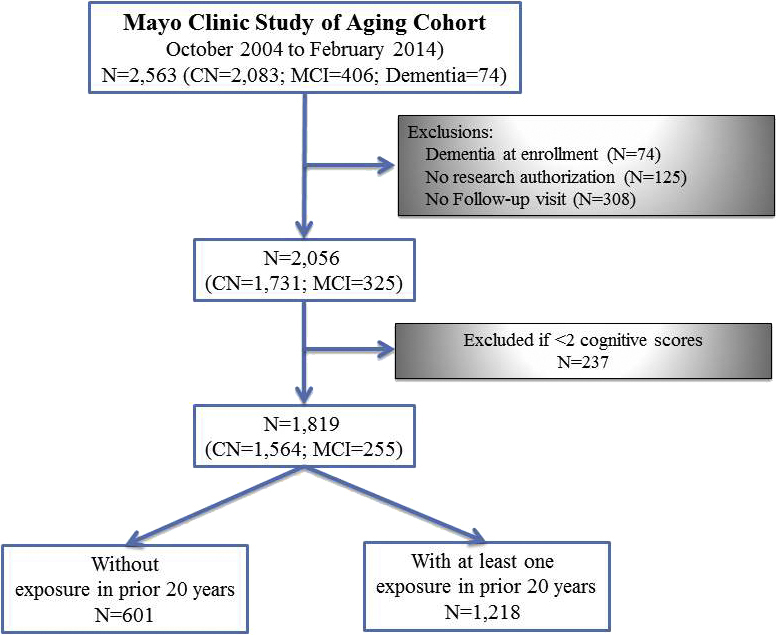
Flow diagram of the Mayo Clinic Study of Aging. Participants in the present study. CN, cognitively normal; MCI, mild cognitive impairment.
Surgeries and procedures performed under general anaesthesia.
Full model from analysis of global cognitive z-scores with prior exposure to general anaesthesia and surgery defined from the 20 yr before enrolment*.
Sensitivity analyses of global cognitive z-score based on timing of prior general anaesthesia and surgery exposure∗ (10, 5, and 20 yr limited to 4 yr follow-up).
References
- 1.Xie Z., Dong Y., Maeda U. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman S., Stygall J., Hirani S., Shaefi S., Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Chen P.L., Yang C.W., Tseng Y.K. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204:188–193. doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Pan N., Ma Y. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci. 2013;345:355–360. doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 5.Schenning K.J., Murchison C.F., Mattek N.C., Silbert L.C., Kaye J.A., Quinn J.F. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimers Dement. 2016;12:590–597. doi: 10.1016/j.jalz.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprung J., Roberts R.O., Knopman D.S. Association of mild cognitive impairment with exposure to general anesthesia for surgical and nonsurgical procedures: a population-based study. Mayo Clin Proc. 2016;91:208–217. doi: 10.1016/j.mayocp.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avidan M.S., Searleman A.C., Storandt M. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dokkedal U., Hansen T.G., Rasmussen L.S., Mengel-From J., Christensen K. Cognitive functioning after surgery in middle-aged and elderly Danish twins. Anesthesiology. 2016;124:312–321. doi: 10.1097/ALN.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprung J., Knopman D., Warner D.O. Risk of dementia after anaesthesia and surgery: study design may affect reported outcome. Br J Psychiatry. 2014;204:323. doi: 10.1192/bjp.204.4.323. [DOI] [PubMed] [Google Scholar]
- 10.Sprung J., Roberts R.O., Knopman D.S. Mild cognitive impairment and exposure to general anesthesia for surgeries and procedures: a population-based case-control study. Anesth Analg. 2017;124:1277–1290. doi: 10.1213/ANE.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes C.G., Patel M.B., Jackson J.C. Surgery and anesthesia exposure is not a risk factor for cognitive impairment after major noncardiac surgery and critical illness. Ann Surg. 2017;265:1126–1133. doi: 10.1097/SLA.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprung J., Jankowski C.J., Roberts R.O. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc. 2013;88:552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopman D.S., Beiser A., Machulda M.M. Spectrum of cognition short of dementia: framingham heart study and Mayo clinic study of aging. Neurology. 2015;85:1712–1721. doi: 10.1212/WNL.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen R.C. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 15.Roberts R.O., Knopman D.S., Mielke M.M. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82:317–325. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel D., Lunn A.D., Smith A.D., Lehmann D.J., Dorrington K.L. Cognitive decline in the elderly after surgery and anaesthesia: results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia. 2016;71:1144–1152. doi: 10.1111/anae.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratzke L.C., Koscik R.L., Schenning K.J. Cognitive decline in the middle-aged after surgery and anaesthesia: results from the Wisconsin Registry for Alzheimer's Prevention cohort. Anaesthesia. 2018;73:549–555. doi: 10.1111/anae.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts R.O., Geda Y.E., Knopman D.S. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Kokmen E., Smith G.E., Petersen R.C., Tangalos E., Ivnik R.C. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 21.Hachinski V.C., Iliff L.D., Zilhka E. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 22.Rosen W.G., Terry R.D., Fuld P.A., Katzman R., Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 23.Fahn S., Elton R.L. UPDRS committee. Unified Parkinson’s disease rating scale. In: Fahn S., Marsden C.D., Calne D.B., Goldstein M., editors. Recent developments in Parkinson's disease. MacMillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 24.Wechsler D.A. Psychological Corporation; New York: 1987. Wechsler adult intelligence scale-revised. [Google Scholar]
- 25.Reitan R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–276. [Google Scholar]
- 26.Kaplan E.F., Goodglass H., Weintraub S. 2nd ed. Lea & Febiger; Philadelphia, PA: 1982. The boston naming test. [Google Scholar]
- 27.Lucas J.A., Ivnik R.J., Smith G.E. Mayo's older americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D.A. Psychological Corporation; New York: 1987. Wechsler memory scale-revised. [Google Scholar]
- 29.Ivnik R.J., Malec J.F., Smith G.E. WAISR, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 30.Vemuri P., Lesnick T.G., Przybelski S.A. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 2014;71:1017–1024. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association . American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders. DSM-IV. [Google Scholar]
- 33.Rocca W.A., Yawn B.P., St Sauver J.L., Grossardt B.R., Melton L.J. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadelson M.R., Sanders R.D., Avidan M.S. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112:440–451. doi: 10.1093/bja/aet420. [DOI] [PubMed] [Google Scholar]
- 35.Etzioni D.A., Liu J.H., Maggard M.A., Ko C.Y. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckenhoff R.G., Laudansky K.F. Anesthesia, surgery, illness and Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:162–166. doi: 10.1016/j.pnpbp.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden K.M., Welsh-Bohmer K.A. Epidemiology of cognitive aging and Alzheimer's disease: contributions of the cache county Utah study of memory, health and aging. Curr Top Behav Neurosci. 2012;10:3–31. doi: 10.1007/7854_2011_152. [DOI] [PubMed] [Google Scholar]
- 39.Greenwood P.M., Parasuraman R., Haxby J.V. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31:471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- 40.Singh-Manoux A., Kivimaki M., Glymour M.M. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goh J.O., An Y., Resnick S.M. Differential trajectories of age-related changes in components of executive and memory processes. Psychol Aging. 2012;27:707–719. doi: 10.1037/a0026715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgeries and procedures performed under general anaesthesia.
Full model from analysis of global cognitive z-scores with prior exposure to general anaesthesia and surgery defined from the 20 yr before enrolment*.
Sensitivity analyses of global cognitive z-score based on timing of prior general anaesthesia and surgery exposure∗ (10, 5, and 20 yr limited to 4 yr follow-up).