Abstract
Background:
Physical rehabilitation can improve walking capacity in persons with multiple sclerosis (MS). However, changes in spatiotemporal gait parameters after rehabilitation are not frequently evaluated, and it is unknown to what extent potential effects depend on baseline disability level. The objective was to investigate the effectiveness of rehabilitation programs on gait parameters at usual and fastest speeds in persons with MS categorized according to walking speed.
Methods:
This nonrandomized multinational study in “real-world” settings evaluated participants before and after conventional rehabilitation. Outcome measurements included spatiotemporal gait parameters assessed by an electronic walkway (at usual and fastest speeds), walking capacity tests (Timed 25-Foot Walk test, 2-Minute Walk Test, 6-Minute Walk Test), and the patient-reported 12-item Multiple Sclerosis Walking Scale. Patients were allocated into three subgroups based on walking speed (<0.82 m/s and >1.14 m/s) and MS center. Results were calculated for the total group and subgroups.
Results:
Forty-two persons with MS (26 women; mean ± SD age, 44.6 ± 11.0 years; mean ± SD Expanded Disability Status Scale score, 3.5 ± 1.5) receiving rehabilitation treatment were enrolled. After rehabilitation treatment, the group demonstrated a significant decrease in double support time and an increase in stride length and step length (left leg) at usual and fastest speeds. Velocity and step length (right leg) increased only at usual speed. Subgroup analysis revealed greatest and clinically meaningful improvements in more disabled persons with MS.
Conclusions:
Physical rehabilitation induced changes in spatiotemporal gait parameters in persons with MS. The magnitude of improvement was greater in participants with more walking impairment.
Multiple sclerosis (MS) is the most common disabling disease in young persons.1 After generally 10 to 15 years of disease, up to 85% of persons with MS experience ambulation dysfunctions.2 Walking impairment is usually related to muscle weakness, spasticity, ataxia, and balance disorders and can be detected at the early stages of the disease,3 and increasing over the disease course.4 Well-established walking measures are used in MS, such as the Timed 25-Foot Walk (T25FW) test,5 the 2-Minute Walk Test (2MWT), the 6-Minute Walk Test (6MWT),6 and the 12-item Multiple Sclerosis Walking Scale (MSWS-12).7 They are commonly used for monitoring clinical disease activity and assessing efficacy of symptomatic and rehabilitation therapies. However, a disadvantage is that they detect only a deviation from normal gait performance (eg, decreased walking speed or walking distance) without giving information about the underlying gait pattern. The missing details are particularly relevant in rehabilitation, where walking treatment strategies are determined based on specific impairments.
It is well recognized that motor rehabilitation can be effective in MS.8 Exercises can improve cardiovascular fitness,9 muscle strength,9–11 and overall physical activity,8,11 as well as health perception and quality of life.11 Snook and Motl12 reviewed the effects of exercises on walking capacity in persons with MS and reported that moderate-to-severe disabled persons with MS (Expanded Disability Status Scale [EDSS] score ≥5) benefit less from treatment. But this statement was based solely on two studies,13,14 given that most studies included a mixture of persons with MS with various disability levels without reporting on subgroups. It remains unclear which persons with MS are responders to treatment and whether the potential for improvement depends on the baseline level of ambulatory function.
Recently, spatiotemporal gait parameters have been increasingly used to define the characteristics of pathologic gait in persons with MS. According to previous reports, persons with MS walk at a slower speed, with a longer double support time and a wider base of support compared with healthy individuals.3,14 Furthermore, spatiotemporal gait parameters relate with the level of neurologic impairment. Consequently, clinical practitioners are advised to collect definite gait parameters to improve the assessment of disease progression and examine the efficacy of various intervention methods, providing detailed quantitative information on gait pattern compared with the standard speed variable and distance-based walking tests.15
Only a few studies reported the effects of rehabilitation programs (aerobic training, resistance training, Bobath treatment, and comprehensive in-patient rehabilitation) on spatiotemporal gait parameters.16–19 Favorable changes on these variables were reported after rehabilitation treatment, but none documented whether improvements were equally present in persons with mild compared with moderate-to-severe ambulatory dysfunctions. Moreover, evaluations were collected only at usual speed, ignoring the potential impact of rehabilitation on the fastest speed.6 Fast walking and acceleration are important in daily life activities (eg, crossing the street within the time window of the traffic lights) and are reported to decline with increased disability level.6,20
The primary aim of this study in “real-world” clinical settings was to investigate the effects of conventional physical rehabilitation programs on spatiotemporal gait parameters at usual and fastest speeds. Participants were categorized according to their baseline walking speed according to criteria applied previously in stroke survivors and persons with MS.20,21 It was hypothesized that training effects would be present at both usual and fastest speeds, with the largest effects expected in persons with low baseline walking speed.
Methods
Participants
A convenience sample of 42 people with a diagnosis of MS according to the McDonald criteria22 was recruited from four MS rehabilitation and research centers, all members of the European Rehabilitation in MS (RIMS) network: Belgium—REVAL Rehabilitation Research Center, Hasselt (n = 22); Israel—Sheba Medical Center MS, Tel-Hashomer (n = 10); and Norway—Haukeland University Hospital, Bergen (n = 2) and Kongsgaarden Physiotherapy AS, Nordland Hospital Trust, Bodø (n = 8). Each center obtained approval from the local ethical committee. This study was part of a larger study investigating the responsiveness of clinical walking-related outcome measures to rehabilitation.23 All the patients had to be aged 18 to 60 years and have an EDSS score of 6.5 or less on the date of admission. Individuals were excluded if they had any other medical conditions interfering with walking. All the participants provided written informed consent.
Study Design and Clinical Outcomes
A noncontrolled multicenter study design was applied. Age, sex, EDSS score, type of MS, and months since diagnosis were recorded at baseline. The content of physical rehabilitation (setting, volume, goal, and treatment approaches) was documented. Walking measures were assessed before and after the conventional rehabilitation programs in each setting.
Spatiotemporal gait parameters were recorded using the GAITRite system, version 4.0.3 (CIR Systems Inc, Franklin, NJ), which consisted of a 4.6-m-long electronic walkway containing 2304 compression-sensitive sensors arranged in a grid pattern.4 The following parameters were documented: gait velocity (calculated by dividing the distance walked by the ambulation time), cadence (number of steps per minute), step length (anterior-posterior distance from the heel of one footprint to the heel of the opposite footprint), and stride length (anterior-posterior distance between the heels of two consecutive footprints of the same foot, such as left to left or right to right). The heel-to-heel base of support (lateral distance from heel center of one footprint to the line of progression formed by two consecutive footprints of the opposite foot) is reported in centimeters, while swing time (time elapsed between the last contact of the current footfall to the initial contact of the next footfall of the same foot), stance time (time elapsed between the initial contact and the last contact of a single footfall), single support time (time elapsed between the last contact of the opposite footfall to the initial contact of the next footfall of the same foot), and double support time (the sum of the time elapsed during two periods of double support in the gait cycle) are reported as percentage according to gait cycle that is the normalized value to stride time (%GC). The GAITRite system has been proved to be valid and reliable in various patient populations.24
The T25FW test is a short-distance measure of walking speed.5 During the 2MWT and 6MWT, participants were instructed to walk as far as possible in 2 and 6 minutes, respectively,6 back and forth along a 30-m hallway. The MSWS-12 is a 12-item patient-rated questionnaire (on a scale from 1 to 5) about limitations in walking due to MS during the past 2 weeks.7 A total score from 12 to 60 was generated and transformed to a 0 to 100 scale. Walking improvements are indicated by negative change scores on the MSWS-12.
Procedures
The T25FW test at usual speed was the first test administered. After 1 minute of rest, the 2MWT or 6MWT was randomly performed. Between them, 15 minutes of rest was provided, during which participants completed the MSWS-12. Afterward, participants walked on the GAITRite mat for two trials at usual speed followed by two trials at fastest but safe speed. The persons with MS were allowed to use assistive devices, such as foot orthoses or canes.
Rehabilitative Intervention Protocols
Each center administered rehabilitative treatment according to its own standard protocol. In Belgium, the protocol consisted of a 24-week combined training program of five sessions per week. Each session started with cycling and treadmill walking or running. Session duration and intensity increased as the program proceeded, starting from 1 × 6 minutes per session to 3 × 10 minutes per session. The second part consisted of resistance training (leg press/curl/extension, vertical traction, arm curl, chest press).25 Repetition sets gradually increased during the intervention, from 1 × 10 to 4 × 15 repetitions. All the exercises were performed at a mild-to-moderate workload. In Israel, a 3-week comprehensive physical rehabilitation program included 1) goal-directed physical therapy (45-minute sessions, five per week) aimed at decreasing spasticity and improving muscle strength, balance, gait, and functional daily living abilities according to the Bobath concept26; 2) moderately intense aerobic exercise training on a bicycle ergometer (45-minute sessions, three per week); and 3) aquatic therapy (45-minute sessions, two per week) chiefly oriented to body structures appropriate to movement.27 Therapy domains focused on trunk mobility, postural stability, transferring oneself, and changing body positions. The Bodø, Norway, center uses a 5-week individualized group–based (three persons per group, 15 groups per session, three session per week, each 60 minutes long) outpatient physiotherapy program consisting of exercises emphasizing core stability, muscle strengthening of the lower limbs, sensory stimulation of the feet, and balance training in sitting and standing. The protocol in Bergen, Norway, was 3 weeks of daily physiotherapy (four individual sessions, each 45 minutes long, and one 45-minute group session), aiming at maintaining balance and walking. The main therapeutic approach applied was the Bobath concept, including core stability, passive mobilization, stretching, resistance training, and gait training.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp, Armonk, NY). Significance was accepted as α ≤ 0.05 for a 1-tailed test given the directional hypothesis of improvement. Nonparametric analyses were applied because data were not normally distributed. Descriptive statistics were calculated in the total sample and in the subgroups based on walking speed and MS center. The χ2 analysis and the Friedman test, followed by the post hoc Mann-Whitney U test, were used to test the differences in baseline patient characteristics between MS centers. The Wilcoxon signed rank test was used to investigate the effects of the rehabilitative treatments in the total sample and in subgroups based on usual walking speed. An analysis based on MS center subgroups was executed. The two Norway centers were grouped due to their cultural similarity, comparable therapeutic approaches, and relatively small sample sizes. The classification of persons with MS to subgroups with different baseline walking speeds was based on the protocol developed by Perry et al,21 originally developed for persons with stroke. Participants were classified as community walkers (CWs), with walking speed greater than 1.14 m/s; limited community walkers (LCWs), with walking speed greater than 0.82 m/s; and most limited community walkers (MLCWs), with walking speed less than 0.82 m/s. We report for the total group to allow comparisons with previous studies and for subgroups to answer the research question on the effect of walking impairment level on gait changes after rehabilitation.
Results
Participant Characteristics
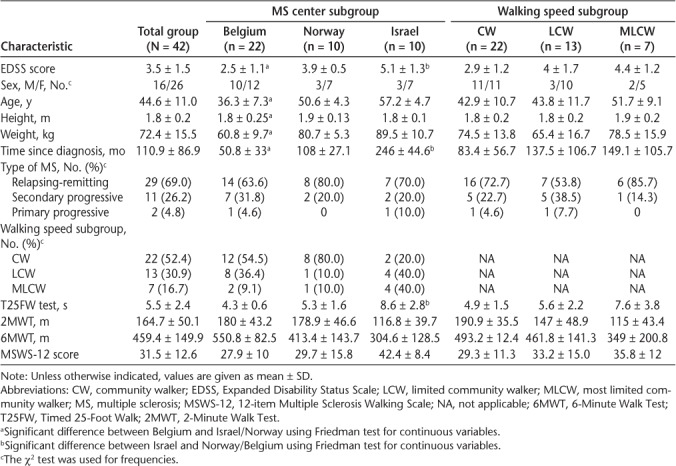
Table 1 shows the demographic and clinical characteristics for the MSWS-12, T25FW test, 2MWT, and 6MWT for the total sample and subgroups based on MS center and baseline walking speed. The frequency distribution of the participants of the different MS centers in the walking speed−based subgroups is also reported in this table.
Table 1.
Demographic and clinical characteristics of total group and by MS center and baseline usual walking speed
Gait Changes After Rehabilitation
Total Sample
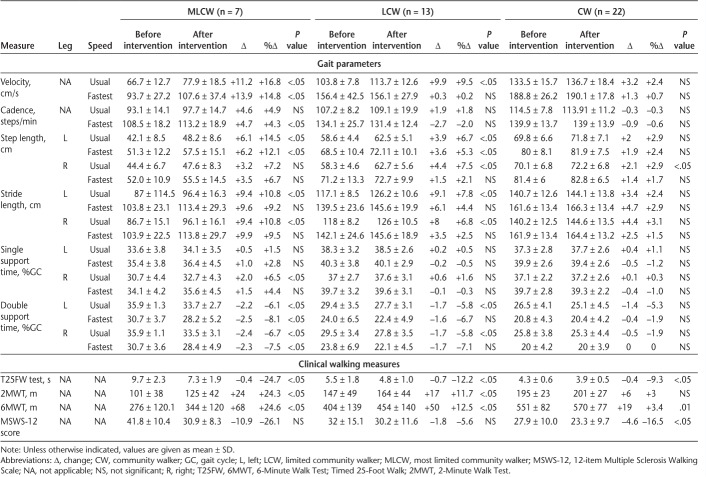
The results for the total sample are shown in Table 2. A significant increase was found for step length of the left leg at usual speed (+5.2%, P < .001) and fastest speed (+4.3%, P < .001); for stride length of both legs at usual speed (+5.1%, P < .001) and fastest speed (right: +2.8%, left: +4.1%, P < .05); and for velocity (+5.8%, P < .05), step length (+5.0%, P < .001), and single support time (%GC) (+1.9%, P < .05) of the right leg only at usual speed. Double support time (%GC) of both legs significantly decreased at usual speed (right: −3.6%, P < .05; left: −4.6%, P < .001) and fastest speed (right: −4.1%, P < .05; left: −4.5%, P < .05).
Table 2.
Spatiotemporal parameters at usual and fastest speeds and clinical walking measures before and after rehabilitation intervention

To judge the validity of the changes in gait speed, we verified whether there were participants who changed subgroups. Of 13 persons with MS in the LCW subgroup (mean walking speed >0.82 m/s), eight moved into the CW subgroup (mean walking speed >1.14 m/s); two of seven persons with MS moved from the MLCW subgroup (mean walking speed <0.82 m/s) into the LCW subgroup (mean walking speed >0.82 m/s).
Regarding the standard walking tests, it was found that the T25FW test, 2MWT, 6MWT (P < .001), and MSWS-12 (P < .05) results significantly improved after treatment.
MS Centers
Differences in baseline characteristics among centers were verified: whereas the proportion of men and women (χ2 = 1.06, P = .58) and the MS phenotype (χ2 = 1.9, P = .76) were not statistically different, the mean age, height, weight, EDSS score, and time to diagnosis were significantly different between Belgium and the other centers. The mean EDSS score and mean time to diagnosis were also significantly different between Israel and Norway. Overall, similar changes across the MS centers were found after treatment (Table 3).
Table 3.
Effects of rehabilitation treatment on spatiotemporal parameters at usual and fastest speeds and clinical walking measures for each multiple sclerosis center
A significant decrease in left double support time (%GC) was found in all the centers at usual speed (Belgium: −4.2%, Norway: −4.5%, Israel: −5.5%, all P < .05) and in the Israel center at fastest speed (−3.3%, P < .05), and left single support time (%GC) significantly increased in the Belgium center at usual speed (+2.0%, P < .05). A significant increase was found in terms of right and left step lengths at usual speed and left step length at fastest speed in all the centers (except the Norway centers) and in stride length at usual speed in all the centers and at fastest speed in the Belgium center. The 2MWT and MSWS-12 results did not improve, respectively, in the Norway and Belgium centers.
Walking Speed–Based Subgroups
Table 4 shows the results for walking speed–based subgroups. The MLCW subgroup showed a significant increase in velocity (P < .05) at usual speed (+16.8%) and fastest speed (+14.8%) and in cadence at fastest speed (+4.3%, P < .05). In addition, an increase was observed in left leg step length at usual and fastest speeds (+14.5% and +12.1%, P < .05) and in stride length at usual speed (+10.8%, P < .05). There was a significant decrease in double support time at usual speed (right: −6.7%, left: −6.1%, P < .05) and fastest speed (right: −7.5%, left: −8.1%, P < .05).
Table 4.
Effects of rehabilitation intervention on spatiotemporal gait parameters at usual and fastest speeds and clinical walking measures by walking speed subgroup
At usual speed, the LCW subgroup showed a significant increase in velocity (+9.5%, P < .05), right leg step length (+7.5%, P < .05), and stride length (right: +6.8%, left: +7.8%, P < .05). In addition, left step length increased at usual and fastest speeds (+6.7% and +5.3%, P < .05). There was a significant decrease in double support time at usual speed (−5.8%, P < .05).
The CW subgroup demonstrated a significant increase only in right step length at usual speed (+2.9%, P < .05).
The results of the T25FW test (MLCWs −24.7%, LCWs −12.2%, and CWs −9.3%, P < .05) and the 6MWT (MLCWs +24.6%, LCWs +12.5%, and CWs +3.4%, P < .05) improved in all the subgroups. The results of the 2MWT increased in the MLCW (+24.3%, P < .05) and LCW (+11.7%, P < .05) subgroups only, whereas the MSWS-12 score decreased only in the CW subgroup (−16.5%, P < .05) (Figure 1).
Figure 1.

Mean spatiotemporal gait parameter values at usual (A–C) and fastest (D–F) speeds before and after rehabilitation treatment in persons with multiple sclerosis, stratified according to their baseline walking speed
CW, community walker; GC, gait cycle; LCW, limited community walker; MLCW, most limited community walker; POST, after treatment; PRE, before treatment. (*) refers to significant differences (P < .05) before and after rehabilitation treatment.
Discussion
This study investigated the effects of conventional rehabilitation programs on the gait pattern during usual and fastest speeds in persons with MS classified according to usual walking speed. We observed that regardless of the rehabilitative treatment content and immediately after the 3-week programs, there were positive changes on several spatiotemporal gait parameters associated with concurrent improvement in the results of standard short and longer walking tests as well as the MSWS-12. Interestingly, changes were clearly most pronounced and consistently meaningful in the more disabled group (MLCWs).
To date, limited research has been conducted to investigate the effects of rehabilitative training on spatiotemporal gait parameters in MS.16–19 Gutierrez et al16 found that persons with MS increased their swing-phase duration and decreased time in the stance and double support phases after an 8-week program of lower-limb resistance exercises. Newman et al17 reported a decrease in the stance phase of the weaker leg and a longer stride length of the stronger leg after 12 sessions of treadmill aerobic training. Sacco et al18 reported faster walking speed, longer stride length, and less stride length variability after a 3-week multidisciplinary inpatient rehabilitation program. Motl et al19 found that persons with MS with moderate disability improved their walking speed, stride length, and single support and swing-phase periods after an 8-week combined training program.
The present study findings are in line with previous results: We found a significant improvement in walking speed and stride length after the intervention programs. However, we did not observe an overall increase in cadence (except for the MLCW subgroup at fastest speed). In other words, step length and cadence did not change proportional to changes in gait velocity, indicating that the quality of the gait pattern itself was likely beneficially altered after rehabilitation. However, previous methodological reports advise researchers to include at least three speed instruction variations per evaluation point to validate the latter conclusion with certainty.28
Regarding the impact of walking impairment level on gait, we found that slower-walking persons with MS demonstrated pronounced improvements in clinical measures and spatiotemporal gait parameters. Improvements were reflected in terms of velocity, double support time, step length, and stride length. Previous studies reported positive effects in persons with MS with mild-to-moderate disability,16–18 as in more severely impaired patients,19 suggesting that modifications in gait parameters might occur regardless of the baseline physical impairment. Nevertheless, according to a meta-analysis by Snook and Motl,12 persons with MS with higher disability have fewer tendencies to improve as a result of treatment. Yet, the systematic review included a relatively small number of studies examining the intervention programs in more disabled patients.
Interestingly, Gutierrez et al16 reported that after the physical intervention period, gait parameters changed solely in patients with a higher disability score (EDSS score > 5.5), supporting the belief that the disability status affects the improvement magnitude. However, we did not analyze patients according to EDSS score because our aim was to investigate the effect on gait changes of walking performance in terms of speed and not in terms of overall neurologic impairment.
When comparing gait performance according to walking speed (usual or fastest speed), we found that parameters that improved at usual speed also improved at fastest speed in the most disabled subgroup. These findings advocate for treating patients with moderate-to-severe walking impairments, who have a limited ability to accelerate. This restriction negatively affects7,19 daily ambulation activities, such as crossing the street or avoiding hazards. However, due to the small sample size in the latter group, we acknowledge the need for replication of the findings in a larger sample.
Overall, the results of the present study are positive, indicating statistically significant effects of conventional rehabilitation on walking measures and the gait pattern. However, interpretation of magnitude of change is needed to judge whether changes are exceeding statistically defined noise levels and are perceived by patients as clinically meaningful. Therefore, we compare the present results with those of Baert et al,23 who provided clinically meaningful changes for the clinical walking measures, and Schwartz et al.29 Looking at the present results, the whole sample exceeded reported thresholds of minimally important change for the long-distance walking tests (2MWT, 6.8 m and 6MWT, 9 m), which, however, was not the case for the MSWS-12 (11.3 and 14.9, respectively), which exceeded thresholds of smallest real change (4.6). The meaningful improvements on the walking capacity tests may be hypothesized to be mirrored by meaningful improvement on the spatiotemporal parameters. Values for 6-month longitudinal changes of spatiotemporal parameters have been provided,30 but not values for clinically meaningful change.
When interpreting the results for subgroups with different baseline walking speeds, distinguishing conclusions can be made. Persons without marked walking impairment (CWs) did not reach changes that are clinically meaningful. Persons with rather mild impairment (LCWs) reached clinically meaningful changes after rehabilitation on the walking capacity tests but not on the MSWS-12. Persons with marked walking impairment (MLCWs) showed clinically meaningful improvement on the walking capacity tests and borderline improvement on the MSWS-12 as well. These findings demonstrate rehabilitation treatment indications for persons with marked walking impairment.
Methodologically, two long-walking capacity tests, the 2MWT and the 6MWT, were included in the present study because it was hypothesized that rehabilitation could have focused more on walking capacity in the more disabled patients and walking endurance in the mildly disabled patients. However, the results of the present study indicate that rehabilitation effects are similar for both tests across all walking impairment levels and that one test could be sufficient for future studies.
This study has several limitations. First, although the classification by Perry et al that was used to compare groups based on walking speed has been applied before in MS31 to differentiate groups, its validity has not yet been tested in MS. As such, the presented thresholds were based on the original study in patients with stroke, potentially limiting the validity of the allocated group names, such as CWs. Second, despite the different content of rehabilitative treatment, similar results were observed among the MS centers, suggesting that changes were obtained independently from the specific treatment administered. The present study did not aim to compare between the different treatments strategies because differences in setting, content, duration, and volume of rehabilitation were present in this “real-world” study. As such, we cannot argue whether a specific rehabilitation approach was particularly effective. This query needs to be further investigated. We assume that the standard conventional rehabilitation approach of each center guaranteed the best treatment option that each center could provide.
Results may not yet be generalized to the overall MS population given the limited sample sizes in the different walking groups as well as the differences in rehabilitation program content and in the intensity and duration of the treatments offered at the different centers. The small and different sample sizes of the contributing centers and different ambulation groups did not allow “center” to be factored into the statistical analyses. Further studies that differentiate effects of identical rehabilitation programs on gait function according to baseline walking speed are advocated. Another limitation is the lack of a control group, which hampers speculation about whether the found effects depend on the treatments or are placebo findings that relate to therapies other than physical rehabilitation. Indeed, the aim of the study was to document real-world practice effects in different walking impairment groups, assuming that centers applied the best available treatment accordingly. We agree that the availability of control data would assist in interpreting whether rehabilitation effects are superior compared with no treatment.30 However, there are an increasing number of scientific studies published that document that significant improvements can be obtained with a variety of rehabilitation interventions.
Moreover, the sample size in the different subgroups did not allow for statistical analyses of another important factor, such as the disease phenotype. Investigating whether people with progressive forms of MS show similar restorative potentials after rehabilitative treatment should be one of the future directions for MS rehabilitation research.
In conclusion, assessing spatiotemporal gait parameters revealed changes induced by physical rehabilitation treatment in persons with MS. Gait changes occurred at both usual and fastest speeds, and the magnitude of improvement was greater and clinically meaningful in more disabled persons with MS. Future research should take into account that different levels of walking impairment at usual speed are related to different margins of improvement.
PRACTICE POINTS
Double support time, stride length, and step length significantly changed after rehabilitation at usual and fastest speeds, but velocity changed significantly at usual speed only.
There were meaningful improvements after rehabilitation on walking capacity tests but not systematically on perceived walking ability.
The magnitude of improvement was greatest and most frequently clinically meaningful in patients with lower baseline walking speed.
Acknowledgments
Participating patients and therapists at each site are thanked for their cooperation (among others, Kari Jones). We acknowledge the contribution of Niels Vreysen in data processing.
Financial Disclosures
The authors declare no conflicts of interest.
Funding/Support
The coordination of this study, as part of multicenter joint data collection studies, was partially funded via an unrestricted educational grant from Novartis Pharma AG to RIMS.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Scheinberg L, Holland N, La Rocca NG. Multiple sclerosis: earning a living. N Y State J Med. 1980;80:1395–1400. [PubMed] [Google Scholar]
- 3.Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture. 2012;36:154–156. doi: 10.1016/j.gaitpost.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 5.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 6.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Kesselring J. Rehabilitation in MS is effective. Int MS J. 2001;8:68–71. [Google Scholar]
- 8.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 9.Debolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85:290–297. doi: 10.1016/j.apmr.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Patti F, Ciancio MR, Reggio E et al. The impact of outpatient rehabilitation on quality of life in multiple sclerosis. J Neurol. 2002;249:1027–1033. doi: 10.1007/s00415-002-0778-1. [DOI] [PubMed] [Google Scholar]
- 11.Latimer-Cheung AE, Pilutti LA, Hicks AL et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94:1800–1828. doi: 10.1016/j.apmr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. 2009;23:108. doi: 10.1177/1545968308320641. [DOI] [PubMed] [Google Scholar]
- 13.Patti F, Ciancio MR, Cacopardo M et al. Effects of a short outpatient rehabilitation treatment on disability of multiple sclerosis patients: a randomized controlled trial. J Neurol. 2003;250:861–866. doi: 10.1007/s00415-003-1097-x. [DOI] [PubMed] [Google Scholar]
- 14.Kalron A, Dvir Z, Frid L, Achiron A. Quantifying gait impairment using an instrumented treadmill in persons with multiple sclerosis. ISRN Neurol. 2013;2013:867575. doi: 10.1155/2013/867575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bethoux F, Bennett S. Evaluating walking in patients with multiple sclerosis: which assessment tools are useful in clinical practice? Int J MS Care. 2011;13:4–14. doi: 10.7224/1537-2073-13.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez GM, Chow JW, Tillman MD, McCoy SC, Castellano V, White LJ. Resistance training improves gait kinematics in persons with multiple sclerosis. Arch Phys Med Rehabil. 2005;86:1824–1829. doi: 10.1016/j.apmr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Newman MA, Dawes H, van den Berg M, Wade DT, Burridge J, Izadi H. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: a pilot study. Mult Scler. 2007;13:113–119. doi: 10.1177/1352458506071169. [DOI] [PubMed] [Google Scholar]
- 18.Sacco R, Bussman R, Oesch P, Kesselring J, Beer S. Assessment of gait parameters and fatigue in MS patients during inpatient rehabilitation: a pilot trial. J Neurol. 2011;258:889–894. doi: 10.1007/s00415-010-5821-z. [DOI] [PubMed] [Google Scholar]
- 19.Motl RW, Smith DC, Elliott J, Weikert M, Dlugonski D, Sosnoff JJ. Combined training improves walking mobility in persons with significant disability from multiple sclerosis: a pilot study. J Neurol Phys Ther. 2012;36:32–37. doi: 10.1097/NPT.0b013e3182477c92. [DOI] [PubMed] [Google Scholar]
- 20.Feys P, Severijns D, Vantenderloo S et al. Spatio-temporal gait parameters change differently according to speed instructions and walking history in MS patients with different ambulatory dysfunction. Mult Scler Relat Disord. 2013;2:238–246. doi: 10.1016/j.msard.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Perry J, Garrett M, Gronley JK et al. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 22.McDonald WI, Compston A, Edan G, Mulroy SJ. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 23.Baert I, Freeman J, Smedal T et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabil Neural Repair. 2014;28:621–631. doi: 10.1177/1545968314521010. [DOI] [PubMed] [Google Scholar]
- 24.van Uden CJT, Besser MP. Test-retest reliability of temporal and spatial parameters measured with an instrumented walkway system (GAITRite) BMC Musculoskelet Disord. 2004;5:27–32. doi: 10.1186/1471-2474-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broekmans T, Roelants M, Feys P et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler. 2011;17:468–477. doi: 10.1177/1352458510391339. [DOI] [PubMed] [Google Scholar]
- 26.Smedal T, Lygren H, Myhr KM et al. Balance and gait improved in patients with MS after physiotherapy based on the Bobath concept. Physiother Res Int. 2006;11:104–116. doi: 10.1002/pri.327. [DOI] [PubMed] [Google Scholar]
- 27.Tripp F, Krakow K. Effects of an aquatic therapy approach (Halliwick-Therapy) on functional mobility in subacute stroke patients: a randomized controlled trial. Clin Rehabil. 2014;28:432–439. doi: 10.1177/0269215513504942. [DOI] [PubMed] [Google Scholar]
- 28.Moe-Nilssen R, Helbostad J. Estimation of gait cycle characteristics by trunk accelerometry. J Biomech. 2004;37:121–126. doi: 10.1016/s0021-9290(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz CE, Ayandeh A, Motl RW. Investigating the minimal important difference in ambulation in multiple sclerosis: a disconnect between performance-based and patient-reported outcomes? J Neurol Sci. 2014;347:268–274. doi: 10.1016/j.jns.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Sosnoff JJ, Klaren RE, Pilutti LA, Dlugonski D, Motl RW. Reliability of gait in multiple sclerosis over 6 months. Gait Posture. 2015;41:860–862. doi: 10.1016/j.gaitpost.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Feys P, Bibby B, Romberg A et al. Within-day variability on short and long walking tests in persons with multiple sclerosis. J Neurol Sci. 2014;338:183–187. doi: 10.1016/j.jns.2014.01.001. [DOI] [PubMed] [Google Scholar]