Abstract
Background:
To present the current knowledge on the characteristics, assessment, and treatment of upper limb intention tremor to inform and improve future intervention studies in patients with multiple sclerosis (MS), we conducted a literature review for articles on upper limb intention tremor in patients with MS.
Methods:
Two reviewers conducted searches in PubMed, Web of Science, and MEDLINE (Ovid). Relevant articles, sorted on inclusion criteria, were examined for descriptions and assessments of upper limb intention tremor, and intervention studies were evaluated based on treatment type.
Results:
Eight descriptive studies were found reporting on the incidence and severity of tremor, impairments, and lesion load. Ten studies focused on measurement of tremor using various assessments. Intervention studies included eight articles using a diverse set of noninvasive techniques mainly showing transient reduction in tremor amplitude and temporary increase in function. Eighteen studies on pharmacologic interventions were found, with most displaying positive outcomes and mediation of tremor; others showed little to no benefit. Surgical interventions included 17 studies on thalamotomy and 20 on deep brain stimulation. Most studies showed tremor improvement after surgery; however, most sample sizes were small, and interventions were highly invasive, with potential adverse effects resulting from surgery.
Conclusions:
The literature on upper limb intention tremor in MS is relatively sparse. More studies are required to determine mechanism of action and to provide more suitable and sustainable interventions to decrease upper limb intention tremor and improve quality of life of individuals with MS.
Multiple sclerosis (MS) is an inflammatory and degenerative disease of the central nervous system that causes demyelination and axonal loss and results in increasing physical impairment over time.1 Herein, we focus on upper limb intention tremor in the context of MS. Owing to the complex interactions of multiple tremor types that can be observed in MS, it is important to have a precise definition when defining this particular phenotype. A 1998 consensus statement by the Movement Disorder Society on tremor defines action tremor as “any tremor that is produced by voluntary contraction of muscle, including postural, isometric, and kinetic tremor.” The consensus report also states that intention tremor is considered a form of kinetic tremor related to action tremor (as opposed to a rest tremor).2 Intention tremor is categorized as “target-directed movements” and is described as “[the tremor] amplitude increases during visually guided movements towards a target at the termination of the movement where the possibility of position-specific tremor or postural tremor produced at the beginning or end of a movement is excluded.”2(p4)
Previous studies have offered variable reports on the prevalence of tremor in MS, suggesting that approximately 25% to 60% of patients may display some form of tremor depending on the population studied.3–5 The different forms of MS-associated tremor can be very complex to decipher because some components of action tremors can occasionally blend together.6 For example, a particular study may describe action tremor without determining the specific components of action tremor, namely, kinetic and intention.2 Furthermore, because MS is a multifocal disease with lesions appearing in various brain regions, it can compound the difficulties in locating the sources of tremor.7–9 Further compounding the difficulty of tremor assessment in MS is overlap with other involuntary movement conditions, such as ataxia. In a recent study examining patients with MS with or without visible tremor on clinical examination, it was observed that patients with visible tremor showed more ataxia and dysfunction of the cerebellum than patients with MS without visible tremor.10 Ataxia is thought to be observed separately from tremor by the unnecessary contraction of muscle groups not associated with the goal-directed movement.6 Tremor is also associated with other debilitating symptoms, such as anxiety and depression,11 which are known comorbidities in patients with MS.
Upper limb intention tremor is a common cause of disability in MS, affecting up to 25% of patients.12 Patients with MS with intention tremor have increased difficulty with a variety of daily activities,3,13,14 such as eating, writing, dressing, and bathing. The purpose of this review was to provide a report on the current literature surrounding upper limb intention tremor in patients with MS. We examine pharmacologic and nonpharmacologic treatments as reported in the literature, provide descriptions of severity, and present the methods that have been used to evaluate upper limb intention tremor in past studies. We aimed to address whether there is a consensus on treatment paradigms or methods of evaluating upper limb intention tremor in the literature. We also critically examined the literature to inform and improve future studies on the assessment and treatment of upper limb intention tremor in patients with MS.
Methods
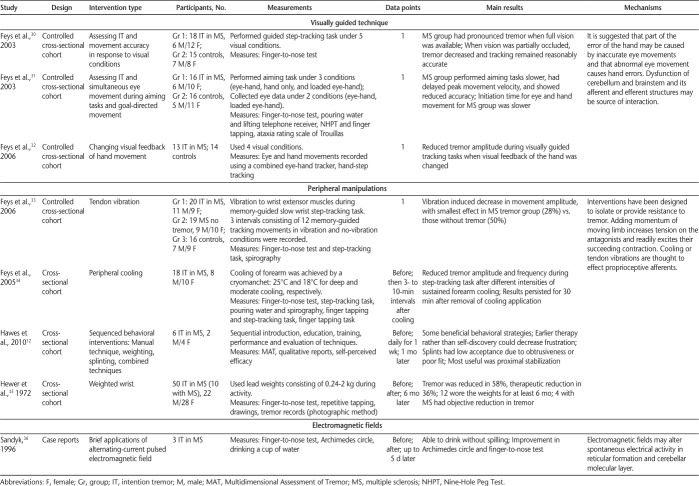
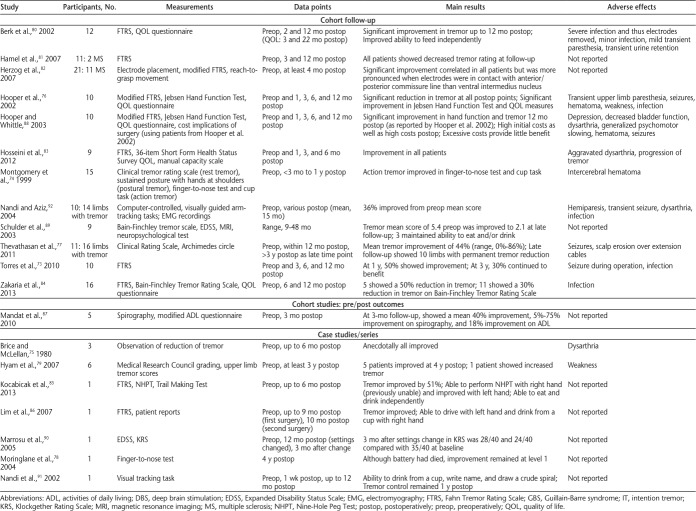
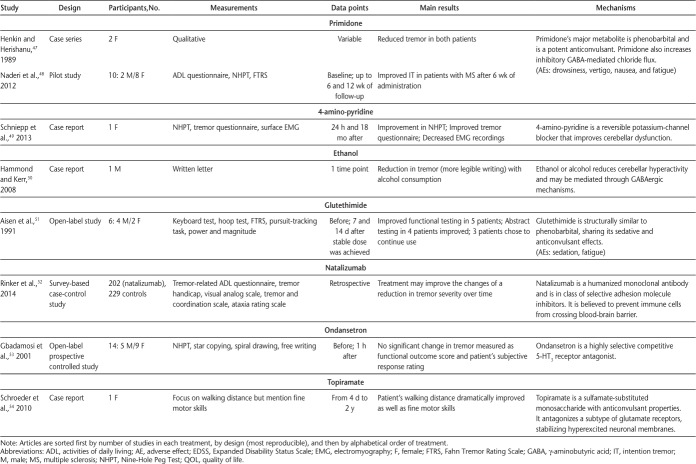
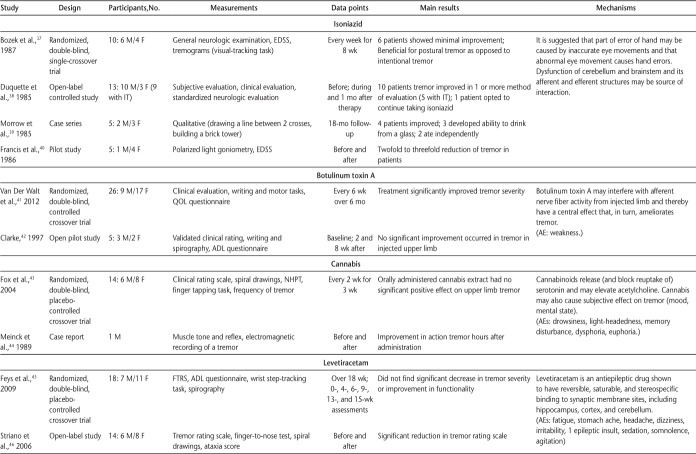
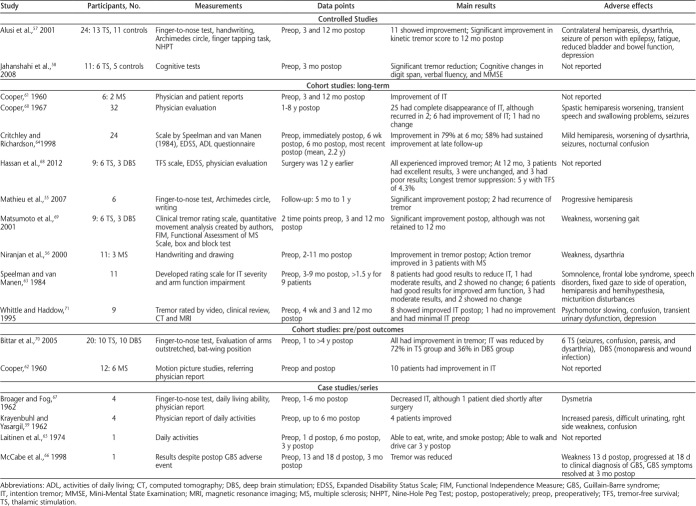
In October 2014, we searched Web of Science, PubMed, MEDLINE (Ovid), and MS-related journals that were not included in the databases. For the initial search, a qualified librarian helped provide the most effective search strategy. The search terms were tremor, intention tremor, action tremor, and multiple sclerosis. Two reviewers (J.K.M. and J.A.R.) independently performed the search using the same search strategy. The independent search results were then merged, and duplicates were removed. The inclusion criteria were English-language articles, non–conference proceedings, research articles, human studies, and articles that included MS-related intention or action tremor. The two reviewers independently applied the inclusion criteria to article titles and then to abstracts, and any disagreements or questionable articles were resolved and decided on by discussion. Standardized data extraction tables created a priori by the senior author (S.J.F.) were completed by both reviewers. Four tables were created summarizing literature related to noninvasive interventions, pharmacology, thalamotomy, and deep brain stimulation (DBS). Data collected on interventions included study design, intervention type, sample size, measurements, main results, and mechanisms of action, as well as adverse effects for pharmacologic and surgical interventions (Tables 1–4).
Table 1.
Noninvasive interventions used to investigate intention tremor in MS
Table 4.
Surgical intervention DBS used to treat intention tremor in MS
Table 2.
Pharmacologic interventions used to investigate intention tremor in MS (page 2 of 2)
Results
Search Results
The initial search in each database yielded the following results: Web of Science (n = 369), PubMed (n = 426), MEDLINE (Ovid) (n = 279), and additional MS-related journals (n = 4). After removing duplicates, the initial search yielded 623 articles. After applying the inclusion criteria, a final capture of 81 articles8,9,12,14–71,73–92 was used for data extraction (Figure 1).
Figure 1.
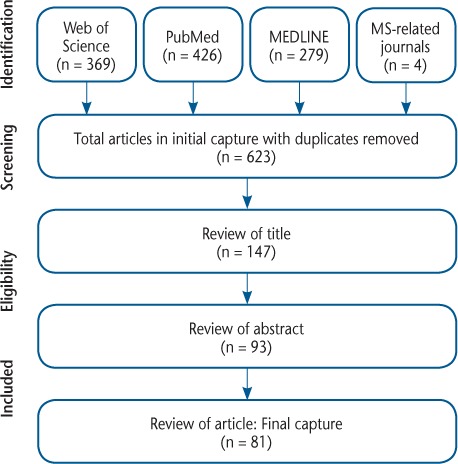
Search strategy
The initial capture included 623 articles. After excluding duplicates, conference proceedings, and non–English-language articles, 147 articles remained. After excluding articles based on relevance of title, 93 articles remained. Articles were then reviewed based on their abstracts, and 81 articles remained, which was the final capture. MS, multiple sclerosis.
Descriptive Literature
Overall, there were eight articles that analyzed descriptive characteristics of intention tremor in MS.8,9,14–19 These articles used a combination of behavioral measurements, neuroimaging, and questionnaires as measures to determine associated descriptors of intention tremor. Across all studies there were 180 patients with MS with upper limb intention tremor and 96 controls. The types of studies included cohort cross-sectional controlled (n = 3), cohort cross-sectional (n = 3), and case studies (n = 2). Characteristics of intention tremor reported on by the studies related to the incidence and severity of tremor, abnormal eye movements, impairment in the extremities of patients, and lesion load. One article studied 100 patients with MS to determine the prevalence of intention tremor and found that 58% of the patients had tremor, and six patients had intention tremor.15 Moreover, Feys and colleagues14 investigated the level of disability in patients with MS and found that 94% had a moderate-to-severe cerebellar impairment and 81% experienced tremor while writing. Descriptive characteristics of abnormal eye movements as they relate to upper limb intention tremor included abnormal saccadic eye coupling16 and increased size of fixational eye movements.17 These studies suggested that imprecise eye movements may contribute to the abnormal hand movements in intention tremor. Measures of impairment in the extremities included delayed tendon reflexes18 and the influence of central oscillations on voluntary movements.19 These studies indicated that the action of intention tremor is influenced by multiple mechanisms, including pathologic control at a supraspinal level, possibly due to lesions in the cerebellar system, or slowed nerve conduction velocity.18,19 Two studies investigated lesion load, with one study finding that lesion load was highest in the pons and cerebellum, with a significant correlation between tremor amplitude and lesion load in the contralateral pons,8 suggesting that the lesions in the pons may be associated with tremor. Although the previous study examined the infratentorial regions of the brain, a series of case studies looking at upper limb intention tremor and ataxia in five patients observed that these patients did not show cerebellar-associated lesions but rather lesion load in the percentral sulcus region in the five patients examined.9 Other areas of investigation by the two studies included the midbrain and the medulla oblongata, and the results suggest that intention tremor is primarily caused by lesions in the afferent and/or efferent pathways of the cerebellum.8,9
Assessment Literature
A multitude of assessments are used in the measurement of tremor in MS, including, but not limited to, performance-based assessments (including clinician-rated tests [ie, tremor rating scales] and objective tests [ie, the Nine-Hole Peg Test]) and patient-reported outcomes, such as quality-of-life questionnaires (for a review on assessments, see the study by Panicker and Pal20). The search found ten articles that were specifically focused on assessments, which were applied to and verified for use in upper limb intention tremor. Of the ten articles, four demonstrated the application of existing assessments to tremor, including the visually guided tracking task21–23 and the Stewart-Holmes maneuver.24 Five studies examined the validity and reliability of assessments. One study examined the comparative reliability and validity of rating upper limb intention tremor using the finger-to-nose task, spiral drawing, and handwriting by correlating them to finger tapping, the Nine-Hole Peg Test, and the activities of daily living questionnaire.25 The study used three blinded examiners and found that all three ways of rating tremor in patients with MS were proved to be valid.25 Another study confirmed the validity and reliability of digitized circle and square drawing for quantifying intention tremor severity and related disability in patients with MS.26 The reliability of the Fahn Tremor Rating Scale (FTRS) was also examined in patients with MS.27 Overall, the results of this study demonstrated good intrarater and interrater reliability.27 A single-item questionnaire, the Tremor and Coordination Scale (TACS), was also assessed for criterion and construct validity.28 The TACS was assessed using correlations with multiple measures, including the Expanded Disability Status Scale, the Nine-Hole Peg Test, age, and body mass index. The findings from this study supported the criterion and construct validity of the TACS.28 Last, one study examined the reliability, validity, and use of a tool called the Multidimensional Assessment of Tremor (MAT).29 The MAT characterizes many dimensions of tremor in MS, including subjective descriptions on how tremor affects daily life and a person's psychosocial life, as well as an objective and clinician-rated measure of tremor severity.29 The reliability, criterion validity, and clinical utility of the MAT were supported.29 In general, all the assessments were proved to be reliable and valid for use in MS tremor.
Noninvasive Interventions
Eight articles were categorized as noninvasive interventions (Table 1). Noninvasive interventions are generally administered to reduce tremor and allow for compensation to improve functionality. The main results were a transient reduction in tremor amplitude and a temporary increase in function using noninvasive techniques. Diverse interventions included visually guided techniques,30–32 tendon vibration,33 peripheral cooling,34 weighting extremities,35 proximal positioning, manual techniques,12 positioning techniques, and the administration of electromagnetic fields.36 One clear benefit of noninvasive interventions was the lack of adverse effects, where only minor inconveniences were mentioned, such as one intervention in which patients with MS reported that the given splints were obtrusive or fit poorly,12 or a peripheral cooling intervention that was reported by some to be uncomfortable.34
Pharmacologic Interventions
Eighteen articles were categorized as pharmacologic interventions (Table 2). Eleven pharmacologic drugs were investigated, including isoniazid,37–40 botulinum toxin A,41,42 cannabis,43,44 levetiracetam,45,46 primidone,47,48 4-amino-pyridine,49 ethanol,50 glutethimide,51 natalizumab,52 ondansetron,53 and topiramate.54 Pharmacologic interventions were used because of their ability to increase inhibitory action on the central nervous system and afferent nerve activity in the peripheral nervous system. Overall, isoniazid was the most studied pharmacologic drug and was successful in approximately 60% to 80% of patients. Ondansetron was the only pharmacologic drug that was found to have no effect on tremor severity. Several of the pharmacologic drugs investigated revealed conflicting outcomes, with most studies finding some positive results and approximately one-quarter of the studies showing null findings, sometimes in the same study. The most relevant adverse effects across all pharmacologic interventions were fatigue and weakness. An overall methodological limitation in the pharmacologic studies included a lack of controlled trials, and, therefore, blinded studies.
Table 2.
Pharmacologic interventions used to investigate intention tremor in MS (page 1 of 2)
Surgical Interventions
Overview
Surgical interventions for upper limb intention tremor were composed of two categories: thalamotomy and DBS. In both cases, the surgical targets are the thalamus; although specific targets in the thalamus vary by patient, they commonly include the ventrolateral nucleus or the ventral intermediate nucleus.
Thalamotomy
Thalamotomy is a process by which an area of the thalamus is ablated by various methods. There were 17 articles that fell under the category of thalamotomy as their main intervention (Table 3). Two of the studies55,56 used a radiofrequency method, which limits the invasiveness of the surgery. Thalamotomy was generally effective in most groups, although some patients experienced a relapse of tremor often 1 year or more after surgery. Two articles in the search included control groups,57,58 although one of the articles was retrospective and included information from patients in the first study. Some studies were older and included only physicians' descriptions as outcomes.59–62 Speelman and van Manen63 proposed a tremor rating scale for severity of arm intention tremor, which was used in a later study by another group.64 Of the remaining studies, three were case studies,65–67 three compared thalamotomy and DBS,68–70 and one included the use of magnetic resonance imaging (MRI)– and computed tomography–guided thalamotomy.71 In general, studies showed tremor improvement after surgery; however, outcome measures were varied because there was no standard evaluation method. Sample sizes in studies tended to be small. Although thalamotomy is overall effective in treating upper limb intention tremor in patients with MS, it is a highly invasive procedure and has a risk of damaging areas outside of the therapeutic target, leading to serious postoperative complications. Some potential adverse effects resulting from surgery can include weakness and fatigue, seizures, or increased paresis.
Table 3.
Surgical intervention thalamotomy used to treat intention tremor in MS
Deep Brain Stimulation
Deep brain stimulation is a more recently developed surgical technique for treating tremor and involves the implantation of electrodes in the brain.72 These electrodes are implanted and battery operated to stimulate the target area. Twenty studies met the criteria for DBS and upper limb intention tremor in MS (Table 4). None of the studies contained a control group. Multiple studies reported improvement of tremor after surgery as a result of a thalamotomy-like effect before initiations of stimulation.73–76 Also, several studies reported continued benefit in patients after the electrodes were turned off.77–79 As in thalamotomy, there were no standard outcomes across studies, making it difficult to compare results, although multiple studies used the FTRS.73,80–87 Others used the modified FTRS,76,88 a clinical tremor rating scale,74,77 the Bain-Finchley Tremor Rating Scale,89 the Expanded Disability Status Scale,90 the finger-to-nose test,78 and a novel visual tracking task.91,92 Similar to the studies that included the FTRS, most of these studies also included other outcome measures. Also similar to thalamotomy articles, the n values tended to be small in these studies, likely due to the invasiveness of the surgery. Although DBS has the advantage of being able to stimulate a brain region instead of simply ablating it, it is still a complex neurosurgery, which imposes increased risks to the patient's health after surgery. Potential adverse effects resulting from surgery can include postoperative infection, seizures, hematoma, or dysarthria.
Discussion
Although there have been previous reviews on MS-related tremor,5,12 overall this review provides the most comprehensive overview of upper limb intention tremor in MS related to the descriptive literature, the assessment literature, and the intervention literature, including noninvasive, pharmacologic, and surgical interventions. The literature on noninvasive interventions included a diverse set of strategies to investigate tremor, with studies mostly achieving a transient reduction in tremor amplitude and a temporary increase in function. The suggested mechanism being investigated by interventions using visually guided techniques for upper limb intention tremor was that of inaccurate and abnormal eye movements.30–32 The source of this interaction may be dysfunction in the cerebellum and brainstem and its afferent and efferent structures. Interventions using peripheral manipulations have been designed to isolate or provide resistance to tremor.33,35 The suggested mechanism of these manipulations is based on adding momentum of the moving limb to increase the tension on the antagonists, which readily excites their succeeding contraction.
Regarding the pharmacologic literature, many studies had small samples of patients. Most pharmacologic interventions had outcomes with some participants displaying positive results and mediation of tremor and others having little to no benefit from the intervention. Overall, isoniazid was the most common pharmacologic agent investigated and was successful in approximately 60% to 80% of patients. Isoniazid was investigated due to its inhibition of γ-aminobutyric acid aminotransferase that leads to an increase in γ-aminobutyric acid.38 However, all of these studies were conducted before the 1990s; therefore, a follow-up study, even if it is retrospective, may be beneficial. On the other hand, ondansetron was the only pharmacologic agent that was found to have no effect on tremor severity. Ondansetron is a highly selective and competitive 5-HT3 receptor antagonist, and it has previously been suggested that the heterogenicity of patients with MS makes this type of pharmacologic intervention inappropriate.53,56 Along with the limitation of the pharmacologic literature having an uncharacteristically small number of participants, there was also a lack of controlled trials. A previous review of tremor in MS similarly found that the published studies on medical treatment of MS-related tremor consisted mostly of case reports and uncontrolled open-label studies, or a scarce number of small randomized controlled trials.5 Follow-up studies on some pharmacologic agents that showed potential, such as glutethimide and 4-amino-pyridine, would be warranted. Larger controlled studies, even if retrospective, may benefit this area of research.
One issue with studies conducted in upper limb intention tremor has been inconsistent outcome measures. More than 40 outcome measurements were used across all the literature reviewed. We found that the most commonly used measure of assessment was the FTRS (21 references), or individual functional assessments from within this scale (ie, finger-to-nose test, spiral drawing, pouring water, bat-wing; 37 references). The most frequent individual assessment from the FTRS was the finger-to-nose test (13 references). Subsequently, the most frequent measurements outside the FTRS were handwriting and drawing (ten references), the Nine-Hole Peg Test (seven references), and finger tapping (five references). For a useful review of these measurements, see the article by Lamers et al.93 Note that subjective assessments, including qualitative assessments, physician reports, and self-perceived evaluations, were present in 18 articles. In a past review of surgical treatments for MS-related tremor, most studies provided basic, minimal information on the effect on functional status and tremor-associated disability causing great caution in making any comparisons across studies.5 Similarly, in the present review, a comparison of decreased tremor amplitude due to DBS is impossible to compare with a study of thalamotomy that was evaluated by Archimedes spiral. Furthermore, in studies using similar outcome measures, discrepancies can be observed based on instructions given to participants and variations of certain tests. For example, although the finger-to-nose test is commonly used, there are variations on how the test is administered, leading to potentially altered outcomes. For example, Feys et al.94 examined the finger-to-nose test following four separate protocols for its administration and found that clinically observable tremor severity could be altered depending on whether an individual is required to touch his or her nose or hold a stable position. Nevertheless, given the wide use of the FTRS (or one of its individual functional tests) in past studies, with further studies of its psychometric properties and improved standardization techniques, it seems to be the most promising comprehensive measure of assessment for upper limb intention tremor. It is clear from the findings of this review that a consistent scoring system should be used across future studies in upper limb intention tremor. This, however, becomes more complicated depending on the research study questions being addressed. Advantages to a standardized testing protocol would be the ability for comparisons between studies where interventions have been used. An ideal test battery would assess multiple functions of both coordination and functional daily activity outcomes using a scale with the least amount of interexaminer variation and standardized quantification methods.
The mechanism by which tremor occurs is still not fully documented in upper limb intention tremor. The treatment of upper limb intention tremor in MS may be dramatically improved if there were clearer data on its mechanism. In surgical interventions, the area of the thalamus to be stimulated or lesioned is generally evaluated individually in patients. One emerging technology in future surgical interventions is focused ultrasound. Although two of the studies reviewed used gamma-knife radiosurgery,55,56 MRI-focused ultrasound has improved the accuracy of the surgical target definition and may be useful in tremor.95 This technology is already showing promise in the treatment of essential tremor when used for a thalamotomy procedure.96
In terms of strengths, this review provided a summary of evidence from studies conducted on MS-related upper limb intention tremor. Unfortunately, we were unable to perform a meta-analysis owing to high heterogeneity across study protocols and high variation in the measures of assessment. The review also highlighted some limitations in the literature that should be acknowledged. First, an overall limitation included a lack of randomized trials and a lack of controlled, blinded studies. Second, there was a lack of objective evaluation methods due to a variety of assessment tools used (with no universal scoring system used and, therefore, no consistent measurement of recovery). Moreover, there were a variety of physician-reported outcomes that led to contradictory information. Last, the studies were generally limited to a small sample population.
In this review, we were unable to find articles reviewing pathology in patients with MS with or without intention tremor. These types of studies may be beneficial in determination of partial causes of upper limb intention tremor because, for example, lesion location may be examined and correlated with upper limb intention tremor in patients with MS. Currently, it is unknown whether lesion location is directly responsible for the development of this condition. Besides postmortem studies, lesions in patients developing upper limb intention tremor may be evaluated and correlated using MRI. This would allow evaluation of patients with MS with and without upper limb intention tremor to perform a properly controlled MRI study looking at lesion sizes and locations.
Furthermore, the shortcomings of the study protocols and the use of small sample sizes could be improved by future larger-scale multicenter studies. To address these limitations, future studies should be more proactive in their research design, including controlled studies and more objective methods of assessment. To improve the potential for a future meta-analysis of findings, future studies would benefit from more consistency in outcome measures because it is currently difficult to compare outcomes across studies.
In conclusion, after reviewing articles on upper limb intention tremor in patients with MS, a variety of studies were found; however, owing to design differences, lack of controls, and inconsistent outcome measures, it is currently not possible to compare many of the studies. This leaves physicians and health care professionals with a lack of specific guidelines on how to approach the treatment of upper limb intention tremor in patients with MS. What is clear from this literature is the importance of and the need for a multidisciplinary approach (including physicians, specialists, and physical and occupational therapists) to investigate and treat upper limb intention tremor. With more consistent outcome measures and larger multicenter studies, as well as improvements in technology and mechanisms of upper limb intention tremor in MS, we may be able to develop clear treatment paradigms for patients and improve their quality of life in the future.
PRACTICE POINTS
One of the debilitating physical manifestations in MS can be intention tremor, which in general consists of rhythmic, oscillatory movement of a body part produced involuntarily. This article offers a literature review of intervention studies focused on upper limb intention tremor specifically in patients with MS.
The current literature on upper limb intention tremor in MS is relatively sparse, but a variety of studies were found in the following categories: surgical interventions (thalamotomy, deep brain stimulation), pharmacologic interventions, and noninvasive interventions.
Owing to the heterogeneity in existing studies, it is currently not possible to compare and combine findings, which confirms the importance of and need for consistent outcome measures and larger multicenter studies to investigate and treat upper limb intention tremor. With a multidisciplinary approach (including physicians, specialists, and physical and occupational therapists), it may be possible to develop clear treatment paradigms for patients and improve their quality of life in the future.
Financial Disclosures
The authors declare no conflicts of interest.
Funding/Support
This work was completed as part of an endMS Scholar Program for Researchers in Training (SPRINT) interdisciplinary learning project while Drs. McCreary and Rogers were enrolled in SPRINT and Dr. Forwell acted as mentor. SPRINT is part of the endMS National Training Program funded by the MS Society of Canada through its related MS Scientific Research Foundation. Dr. McCreary receives student funding from the Natural Sciences and Engineering Research Council of Canada Collaborative Research and Training Experience Program (grant 371155). Dr. Rogers receives student funding from an Alberta Innovates Health Solutions studentship.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee Consensus statement of the Movement Disorder Society on tremor. Mov Disord. 1998;13(suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 3.Alusi S, Worthington J, Glickman S et al. A study of tremor in multiple sclerosis. Brain. 2001;124(pt 4):720–730. doi: 10.1093/brain/124.4.720. [DOI] [PubMed] [Google Scholar]
- 4.Pittock S, McClelland R, Mayr W et al. Prevalence of tremor in multiple sclerosis and associated disability in the Olmsted County population. Mov Disord. 2004;19:1482–1485. doi: 10.1002/mds.20227. [DOI] [PubMed] [Google Scholar]
- 5.Koch M, Mostert J, Heersema D et al. Tremor in multiple sclerosis. J Neurol. 2007;254:133–145. doi: 10.1007/s00415-006-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alusi S, Glickman S, Aziz T et al. Tremor in multiple sclerosis. J Neurol. 1999;66:131–134. doi: 10.1136/jnnp.66.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickman S, Brierley C, Silver N et al. Infratentorial hypointense lesion volume on T1-weighted magnetic resonance imaging correlates with disability in patients with chronic cerebellar ataxia due to multiple sclerosis. J Neurol Sci. 2001;187:35–39. doi: 10.1016/s0022-510x(01)00519-6. [DOI] [PubMed] [Google Scholar]
- 8.Feys P, Maes F, Nuttin B et al. Relationship between multiple sclerosis intention tremor severity and lesion load in the brainstem. Neuroreport. 2005;16:1379–1382. doi: 10.1097/01.wnr.0000176521.26971.58. [DOI] [PubMed] [Google Scholar]
- 9.Karmon Y, Morrow S, Weinstock A et al. Limb ataxia originating from pericentral sulcus demyelinating lesion in multiple sclerosis. J Neurol Sci. 2012;320:136–140. doi: 10.1016/j.jns.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Ayache S, Chalah M, Al-Ani T et al. Tremor in multiple sclerosis: the intriguing role of the cerebellum. J Neurol Sci. 2015;358:351–356. doi: 10.1016/j.jns.2015.09.360. [DOI] [PubMed] [Google Scholar]
- 11.Louis E, Barnes L, Albert S et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16:914–920. doi: 10.1002/mds.1184. [DOI] [PubMed] [Google Scholar]
- 12.Hawes F, Billups C, Forwell S. Interventions for upper-limb intention tremor in multiple sclerosis. Int J MS Care. 2010;12:122–132. [Google Scholar]
- 13.Gillen G. Improving activities of daily living performance in an adult with ataxia. Am J Occup Ther. 2000;54:89–96. doi: 10.5014/ajot.54.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Feys P, Romberg A, Ruutiainen J et al. Interference of upper limb tremor on daily life activities in people with multiple sclerosis. Occup Ther Health Care. 2004;17:81–95. doi: 10.1080/J003v17n03_06. [DOI] [PubMed] [Google Scholar]
- 15.Rinker J, Salter A, Walker H et al. Prevalence and characteristics of tremor in the NARCOMS multiple sclerosis registry: a cross-sectional survey. BMJ Open. 2015;5:e006714. doi: 10.1136/bmjopen-2014-006714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feys P, Helsen W, Liu X et al. Interaction between eye and hand movements in multiple sclerosis patients with intention tremor. Mov Disord. 2005;20:705–713. doi: 10.1002/mds.20382. [DOI] [PubMed] [Google Scholar]
- 17.Feys P, Helsen W, Nuttin B et al. Unsteady gaze fixation enhances the severity of MS intention tremor. Neurology. 2008;70:106–113. doi: 10.1212/01.wnl.0000287071.51180.b1. [DOI] [PubMed] [Google Scholar]
- 18.Feys P, Helsen W, Ilsbroukx S et al. Is MS intention tremor amplitude related to changed peripheral reflexes? ISRN Neurol. 2011;2011:192414. doi: 10.5402/2011/192414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong M, Miall R, Bain P et al. The onset of voluntary reactive movement is temporally influenced by the central oscillation in action tremor caused by multiple sclerosis. Neurosci Lett. 2008;445:122–125. doi: 10.1016/j.neulet.2008.08.086. [DOI] [PubMed] [Google Scholar]
- 20.Panicker J, Pal P. Clinical features, assessment and treatment of essential tremor. J Assoc Physicians India. 2003;51:276–279. [PubMed] [Google Scholar]
- 21.Liu X, Miall C, Aziz T et al. Analysis of action tremor and impaired control of movement velocity in multiple sclerosis during visually guided wrist-tracking tasks. Mov Disord. 1997;12:992–999. doi: 10.1002/mds.870120624. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Miall R, Aziz T et al. Distal versus proximal arm tremor in multiple sclerosis assessed by visually guided tracking tasks. J Neurol Neurosurg Psychiatry. 1999;66:43–47. doi: 10.1136/jnnp.66.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Aziz T, Miall R et al. Frequency analysis of involuntary movements during wrist tracking: a way to identify MS patients with tremor who benefit from thalamotomy. Stereotact Funct Neurosurg. 2000;74:53–62. doi: 10.1159/000056464. [DOI] [PubMed] [Google Scholar]
- 24.Waubant E, Tezenas du Montcel S, Jedynak C et al. Multiple sclerosis tremor and the Stewart-Holmes manoeuvre. Mov Disord. 2003;18:948–952. doi: 10.1002/mds.10454. [DOI] [PubMed] [Google Scholar]
- 25.Alusi S, Worthington J, Glickman S et al. Evaluation of three different ways of assessing tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:756–760. doi: 10.1136/jnnp.68.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feys P, Helsen W, Prinsmel A et al. Digitised spirography as an evaluation tool for intention tremor in multiple sclerosis. J Neurosci Methods. 2007;160:309–316. doi: 10.1016/j.jneumeth.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Hooper J, Taylor R, Pentland B et al. Rater reliability of Fahn's tremor rating scale in patients with multiple sclerosis. Arch Phys Med Rehabil. 1998;79:1076–1079. doi: 10.1016/s0003-9993(98)90174-5. [DOI] [PubMed] [Google Scholar]
- 28.Marrie R, Goldman M. Validation of the NARCOMS Registry: Tremor and Coordination Scale. Int J MS Care. 2011;13:114–120. doi: 10.7224/1537-2073-13.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daudrich B, Hurl D, Forwell S. Multidimensional assessment of tremor in multiple sclerosis. Int J MS Care. 2010;12:23–32. [Google Scholar]
- 30.Feys P, Helsen W, Liu X et al. Effect of visual information on step-tracking movements in patients with intention tremor due to multiple sclerosis. Mult Scler. 2003;9:492–502. doi: 10.1191/1352458503ms949oa. [DOI] [PubMed] [Google Scholar]
- 31.Feys P, Helsen W, Lavrysen A et al. Intention tremor during manual aiming: a study of eye and hand movements. Mult Scler. 2003;9:44–54. doi: 10.1191/1352458503ms863oa. [DOI] [PubMed] [Google Scholar]
- 32.Feys P, Helsen W, Buekers M et al. The effect of changed visual feedback on intention tremor in multiple sclerosis. Neurosci Lett. 2006;394:17–21. doi: 10.1016/j.neulet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Feys P, Helsen WF, Verschueren S et al. Online movement control in multiple sclerosis patients with tremor: effects of tendon vibration. Mov Disord. 2006;21:1148–1153. doi: 10.1002/mds.20938. [DOI] [PubMed] [Google Scholar]
- 34.Feys P, Helsen W, Liu X et al. Effects of peripheral cooling on intention tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:373–379. doi: 10.1136/jnnp.2004.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewer R, Cooper R, Morgan M. An investigation into the value of treating intention tremor by weighting the affected limb. Brain. 1972;95:579–590. doi: 10.1093/brain/95.3.579. [DOI] [PubMed] [Google Scholar]
- 36.Sandyk R. Treatment with electromagnetic field alters the clinical course of chronic progressive multiple sclerosis: a case report. Int J Neurosci. 1996;88:75–82. doi: 10.3109/00207459608999814. [DOI] [PubMed] [Google Scholar]
- 37.Bozek C, Kastrukoff L, Wright J et al. A controlled trial of isoniazid therapy for action tremor in multiple sclerosis. J Neurol. 1987;234:36–39. doi: 10.1007/BF00314007. [DOI] [PubMed] [Google Scholar]
- 38.Duquette P, Pleines J, du Souich P. Isoniazid for tremor in multiple sclerosis: a controlled trial. Neurology. 1985;35:1772–1775. doi: 10.1212/wnl.35.12.1772. [DOI] [PubMed] [Google Scholar]
- 39.Morrow J, McDowell H, Ritchie C et al. Isoniazid and action tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1985;48:282–283. doi: 10.1136/jnnp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis D, Grundy D, Heron J. The response to isoniazid of action tremor in multiple sclerosis and its assessment using polarized-light goniometry. J Neurol Neurosurg Psychiatry. 1986;49:87–89. doi: 10.1136/jnnp.49.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Der Walt A, Sung S, Spelman T et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012;79:92–99. doi: 10.1212/WNL.0b013e31825dcdd9. [DOI] [PubMed] [Google Scholar]
- 42.Clarke C. Botulinum toxin type A in cerebellar tremor caused by multiple sclerosis. Eur J Neurol. 1997;4:68–71. doi: 10.1111/j.1468-1331.1997.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 43.Fox P, Bain P, Glickman S et al. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology. 2004;62:1105–1109. doi: 10.1212/01.wnl.0000118203.67138.3e. [DOI] [PubMed] [Google Scholar]
- 44.Meinck H, Schonle P, Conrad B. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol. 1989;236:120–122. doi: 10.1007/BF00314410. [DOI] [PubMed] [Google Scholar]
- 45.Feys P, D'Hooghe M, Nagels G et al. The effect of levetiracetam on tremor severity and functionality in patients with multiple sclerosis. Mult Scler. 2009;15:371–378. doi: 10.1177/1352458508099142. [DOI] [PubMed] [Google Scholar]
- 46.Striano P, Coppola A, Vacca G et al. Levetiracetam for cerebellar tremor in multiple sclerosis: an open-label pilot tolerability and efficacy study. J Neurol. 2006;253:762–766. doi: 10.1007/s00415-006-0112-4. [DOI] [PubMed] [Google Scholar]
- 47.Henkin Y, Herishanu Y. Primidone as a treatment for cerebellar tremor in multiple sclerosis: two case reports. Isr J Med Sci. 1989;25:720–721. [PubMed] [Google Scholar]
- 48.Naderi F, Javadi SA, Motamedi M et al. The efficacy of primidone in reducing severe cerebellar tremors in patients with multiple sclerosis. Clin Neuropharmacol. 2012;35:224–226. doi: 10.1097/WNF.0b013e31826249bb. [DOI] [PubMed] [Google Scholar]
- 49.Schniepp R, Jakl V, Wuehr M et al. Treatment with 4-aminopyridine improves upper limb tremor of a patient with multiple sclerosis: a video case report. Mult Scler. 2013;19:506–508. doi: 10.1177/1352458512461394. [DOI] [PubMed] [Google Scholar]
- 50.Hammond E, Kerr D. Ethanol responsive tremor in a patient with multiple sclerosis. Arch Neurol. 2008;65:142–143. doi: 10.1001/archneurol.2007.13. [DOI] [PubMed] [Google Scholar]
- 51.Aisen M, Holzer M, Rosen M et al. Glutethimide treatment of disabling action tremor in patients with multiple sclerosis and traumatic brain injury. Arch Neurol. 1991;48:513–515. doi: 10.1001/archneur.1991.00530170077023. [DOI] [PubMed] [Google Scholar]
- 52.Rinker J, Salter A, Cutter G. Improvement of multiple sclerosis-associated tremor as a treatment effect of natalizumab. Mult Scler Relat Disord. 2014;3:505–512. doi: 10.1016/j.msard.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Gbadamosi J, Buhmann C, Moench A et al. Failure of ondansetron in treating cerebellar tremor in MS patients: an open-label pilot study. Acta Neurol Scand. 2001;104:308–311. doi: 10.1034/j.1600-0404.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 54.Schroeder A, Linker R, Lukas C et al. Successful treatment of cerebellar ataxia and tremor in multiple sclerosis with topiramate: a case report. Clin Neuropharmacol. 2010;33:317–318. doi: 10.1097/WNF.0b013e3181f84a39. [DOI] [PubMed] [Google Scholar]
- 55.Mathieu D, Kondziolka D, Niranjan A et al. Gamma knife thalamotomy for multiple sclerosis tremor. Surg Neurol. 2007;68:394–399. doi: 10.1016/j.surneu.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 56.Niranjan A, Kondziolka D, Baser S et al. Functional outcomes after gamma knife thalamotomy for essential tremor and MS-related tremor. Neurology. 2000;55:443–446. doi: 10.1212/wnl.55.3.443. [DOI] [PubMed] [Google Scholar]
- 57.Alusi S, Aziz T, Glickman S et al. Stereotactic lesional surgery for the treatment of tremor in multiple sclerosis: a prospective case-controlled study. Brain. 2001;124(pt 8):1576–1589. doi: 10.1093/brain/124.8.1576. [DOI] [PubMed] [Google Scholar]
- 58.Jahanshahi M, Pieter S, Alusi S et al. Effects on cognition of stereotactic lesional surgery for the treatment of tremor in multiple sclerosis. Behav Neurol. 2008;20:1–9. doi: 10.3233/BEN-2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krayenbuhl H, Yasargil M. Relief of intention tremor due to multiple sclerosis by stereotaxic thalamotomy. Confin Neurol. 1962;22:368–374. doi: 10.1159/000104388. [DOI] [PubMed] [Google Scholar]
- 60.Cooper I. Relief of intention tremor of multiple sclerosis by thalamic surgery. JAMA. 1967;199:689–694. [PubMed] [Google Scholar]
- 61.Cooper I. Neurosurgical alleviation of intention tremor of multiple sclerosis and cerebellar disease. N Engl J Med. 1960;263:441–444. doi: 10.1056/NEJM196009012630905. [DOI] [PubMed] [Google Scholar]
- 62.Cooper I. Neurosurgical relief of intention tremor due to cerebellar disease and multiple sclerosis. Arch Phys Med Rehabil. 1960;41:1–4. [PubMed] [Google Scholar]
- 63.Speelman J, van Manen J. Stereotactic thalamotomy for the relief of intention tremor of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1984;47:596–599. doi: 10.1136/jnnp.47.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Critchley G, Richardson P. Vim thalamotomy for the relief of the intention tremor of multiple sclerosis. Br J Neurosurg. 1998;12:559–562. doi: 10.1080/02688699844439. [DOI] [PubMed] [Google Scholar]
- 65.Laitinen L, Arsalo A, Hanninen A. Combination of thalamotomy and longitudinal myelotomy in the treatment of multiple sclerosis. Acta Neurochir. 1974;(suppl 21):89–91. doi: 10.1007/978-3-7091-8355-7_12. [DOI] [PubMed] [Google Scholar]
- 66.McCabe P, Blakeslee M, Tenser R. Guillain-Barre syndrome after thalamotomy for tremor in MS. Neurology. 1998;51:1229–1230. doi: 10.1212/wnl.51.4.1229. [DOI] [PubMed] [Google Scholar]
- 67.Broager B, Fog T. Thalamotomy for the relief of intention tremor in multiple sclerosis. Acta Neurol Scand Suppl. 1962;38:153–156. doi: 10.1111/j.1600-0404.1962.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 68.Hassan A, Ahlskog J, Rodriguez M et al. Surgical therapy for multiple sclerosis tremor: a 12-year follow-up study. Eur J Neurol. 2012;19:764–768. doi: 10.1111/j.1468-1331.2011.03626.x. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto J, Morrow D, Kaufman K et al. Surgical therapy for tremor in multiple sclerosis: an evaluation of outcome measures. Neurology. 2001;57:1876–1882. doi: 10.1212/wnl.57.10.1876. [DOI] [PubMed] [Google Scholar]
- 70.Bittar R, Hyam J, Nandi D et al. Thalamotomy versus thalamic stimulation for multiple sclerosis tremor. J Clin Neurosci. 2005;12:638–642. doi: 10.1016/j.jocn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Whittle I, Haddow L. CT guided thalamotomy for movement disorders in multiple sclerosis: problems and paradoxes. Acta Neurochir Suppl. 1995;64:13–16. doi: 10.1007/978-3-7091-9419-5_4. [DOI] [PubMed] [Google Scholar]
- 72.Tarazi F, Sahli Z, Wolny M et al. Emerging therapies for Parkinson's disease: from bench to bedside. Pharmacol Ther. 2014;144:123–133. doi: 10.1016/j.pharmthera.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Torres C, Moro E, Lopez-Rios A et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for tremor in patients with multiple sclerosis. Neurosurgery. 2010;67:646–651. doi: 10.1227/01.NEU.0000375506.18902.3E. [DOI] [PubMed] [Google Scholar]
- 74.Montgomery E, Baker K, Kinkel R et al. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625–628. doi: 10.1212/wnl.53.3.625. [DOI] [PubMed] [Google Scholar]
- 75.Brice J, McLellan L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet. 1980;1:1221–1222. doi: 10.1016/s0140-6736(80)91680-3. [DOI] [PubMed] [Google Scholar]
- 76.Hooper J, Taylor R, Pentland B et al. A prospective study of thalamic deep brain stimulation for the treatment of movement disorders in multiple sclerosis. Br J Neurosurg. 2002;16:102–109. doi: 10.1080/02688690220131769. [DOI] [PubMed] [Google Scholar]
- 77.Thevathasan W, Schweder P, Joint C et al. Permanent tremor reduction during thalamic stimulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:419–422. doi: 10.1136/jnnp.2010.213900. [DOI] [PubMed] [Google Scholar]
- 78.Moringlane J, Spiegel J, Fuss G et al. Improvement of upper limb ataxia and intention tremor allowing cessation of thalamic electrostimulation after four years. Mult Scler. 2004;10:708–710. doi: 10.1191/1352458504ms1102cr. [DOI] [PubMed] [Google Scholar]
- 79.Hyam J, Aziz T, Bain P. Post-deep brain stimulation: gradual non-stimulation dependent decrease in strength with attenuation of multiple sclerosis tremor. J Neurol. 2007;254:854–860. doi: 10.1007/s00415-006-0433-3. [DOI] [PubMed] [Google Scholar]
- 80.Berk C, Carr J, Sinden M et al. Thalamic deep brain stimulation for the treatment of tremor due to multiple sclerosis: a prospective study of tremor and quality of life. J Neurosurg. 2002;97:815–820. doi: 10.3171/jns.2002.97.4.0815. [DOI] [PubMed] [Google Scholar]
- 81.Hamel W, Herzog J, Kopper F et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir. 2007;149:749–758. doi: 10.1007/s00701-007-1230-1. [DOI] [PubMed] [Google Scholar]
- 82.Herzog J, Hamel W, Wenzelburger R et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130(pt 6):1608–1625. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- 83.Hosseini H, Mandat T, Waubant E et al. Unilateral thalamic deep brain stimulation for disabling kinetic tremor in multiple sclerosis. Neurosurgery. 2012;70:66–69. doi: 10.1227/NEU.0b013e31822da55c. [DOI] [PubMed] [Google Scholar]
- 84.Zakaria R, Vajramani G, Westmoreland L et al. Tremor reduction and quality of life after deep brain stimulation for multiple sclerosis-associated tremor. Acta Neurochir. 2013;155:2359–2364. doi: 10.1007/s00701-013-1848-0. [DOI] [PubMed] [Google Scholar]
- 85.Kocabicak E, Terzi M, Alptekin O et al. Targeting thalamic tremor cells in deep brain stimulation for multiple sclerosis-induced complex tremor. Surg Neurol Int. 2013;4:31. doi: 10.4103/2152-7806.109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim D, Khandhar S, Heath S et al. Multiple target deep brain stimulation for multiple sclerosis related and poststroke Holmes' tremor. Stereotact Funct Neurosurg. 2007;85:144–149. doi: 10.1159/000099072. [DOI] [PubMed] [Google Scholar]
- 87.Mandat T, Koziara H, Tutaj M et al. Thalamic deep brain stimulation for tremor among multiple sclerosis patients. Neurol Neurochir Pol. 2010;44:542–545. doi: 10.1016/s0028-3843(14)60150-x. [DOI] [PubMed] [Google Scholar]
- 88.Hooper J, Whittle I. Costs of thalamic deep brain stimulation for movement disorders in patients with multiple sclerosis. Br J Neurosurg. 2003;17:40–45. [PubMed] [Google Scholar]
- 89.Schulder M, Sernas T, Karimi R. Thalamic stimulation in patients with multiple sclerosis: long-term follow-up. Stereotact Funct Neurosurg. 2003;80:48–55. doi: 10.1159/000075160. [DOI] [PubMed] [Google Scholar]
- 90.Marrosu F, Maleci A, Cocco E et al. Vagal nerve stimulation effects on cerebellar tremor in multiple sclerosis. Neurology. 2005;65:490. doi: 10.1212/01.wnl.0000172343.45110.79. [DOI] [PubMed] [Google Scholar]
- 91.Nandi D, Chir M, Liu X et al. Electrophysiological confirmation of the zona incerta as a target for surgical treatment of disabling involuntary arm movements in multiple sclerosis: use of local field potentials. J Clin Neurosci. 2002;9:64–68. doi: 10.1054/jocn.2001.1012. [DOI] [PubMed] [Google Scholar]
- 92.Nandi D, Aziz T. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21:31–39. doi: 10.1097/00004691-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Lamers I, Kelchtermans S, Baert I et al. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch Phys Med Rehabil. 2014;95:1184–1200. doi: 10.1016/j.apmr.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 94.Feys P, Davies-Smith A, Jones R et al. Intention tremor rated according to different finger-to-nose test protocols: a survey. Arch Phys Med Rehabil. 2003;84:79–82. doi: 10.1053/apmr.2003.50068. [DOI] [PubMed] [Google Scholar]
- 95.Monteith S, Sheehan J, Medel R et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery. J Neurosurg. 2013;118:215–221. doi: 10.3171/2012.10.JNS12449. [DOI] [PubMed] [Google Scholar]
- 96.Lipsman N, Schwartz M, Huang Y et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]