Abstract
Hypoxia induces myocardial injury through the activation of inflammatory and oxidative processes. The pivotal role of the renin angiotensin system (RAS) in the pathogenesis of cardiovascular diseases has been firmly established in clinical trials and practice; in fact many experimental and clinical data have highlighted that its inhibition has a cardioprotective role. Activated RAS also stimulates inflammation directly inducing proinflammatory and oxidative gene expression. This study aimed to investigate the protective role of a pre-treatment (10 and 100 μM) with irbesartan on injury induced by 24 h of hypoxia in HL-1 cardiomyocytes; in particular, we have analyzed the natriuretic peptide (BNP) expression, a biomarker able to modulate inflammatory reaction to cardiac injury and some markers involved in oxidative stress and inflammation. Our results demonstrated that a pre-treatment with 100 μM irbesartan significantly increased SOD activity and catalase expression of 15 and 25%, respectively, compared to hypoxic cells (P<0.05). On the other hand, it was able to reduce the release of peroxynitrite and iNOS protein expression of 20 and 50% respectively (P<0.05). In addition irbesartan exerts an anti-inflammatory activity reducing Toll-like receptors (TLRs)-2 and -4 mRNA expression, TNF-alpha expression and activity (20%) and increasing the expression of the cytokine IL-17 (40%) (P<0.05 vs hypoxia). Our findings also showed that BNP induced by ischemia was significantly and in a concentration-dependent manner reduced by irbesartan. The findings of our study demonstrated that the AT1 receptor antagonist irbesartan exerts a protective role in an in vitro hypoxic condition reducing oxidative stress and inflammation.
1. Introduction
Myocardial infarction (MI) is one of major cause of death and disability worldwide [1]. It occurs when coronary blood supply does not meet myocardial demand and leads to sudden necrosis of a large number of cardiomyocytes which trigger an intense inflammatory reaction. The reactive oxygen species (ROS) released during the acute phase of the ischemic damage induced detrimental effects with peculiar changes in cellular proteins and lipids, leading to cell dysfunction or death. ROS also directly induces pro-inflammatory cascades and strongly contributes to the pathogenesis of MI [2,3]. However, ROS stimulate tissue inflammation up-regulating inflammatory cytokines, e.g., tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 in the ischemic region and surrounding myocardium [4]. In a previous study Baban B. et al., showed in cardiomyocytes of ischemic-reperfused hearts that the pressure overload reduced interleukin-10 but increased interleukin-17 [5]. Additionally, the excessive intracellular ROS generation may activate the Toll-like receptor (TLR) -4 signaling pathway [6]. Previous studies have shown an involvement of TLR-4 in experimental models of ischemic injury [7,8]. It is well documented that the renin-angiotensin system (RAS) is strongly involved in the acute phase of MI and contributes to its pathophysiologic sequel [9,10]. It is well known that myocardial ischemia increases angiotensin II levels. A chronic treatment with ACE inhibitors or angiotensin II receptor antagonists has been shown to reduce ischemia-reperfusion injury [11]. Irbesartan is a potent and selective antagonist of AT1 receptors localized on vascular smooth muscle cells and in the adrenal cortex and it is usually used to treat patients with mild-to-moderate hypertension and for lower blood pressure also in drug combination [12,13]. Clinical data have demonstrated in patients with high-risk of hypertension that irbesartan reduced inflammation, oxidative stress and exerted beneficial effects on metabolic syndrome [14].The inflammatory response plays an important role in patients with cardiovascular disease and may be useful in the diagnosis of apparently healthy subjects without known coronary artery disease and without conventional risk factors. Interleukin-1, -6, -17, and TNF-α are the main investigated cytokines among those which predict cardiovascular events involved in atherosclerosis [15,16]. A large number of endoplasmatic reticulum stress-associated proteins have been shown to be involved in the development of several types of cardiomyopathies. In particular, our previous study demonstrated that an altered oxido-reductive state in the diabetic heart leads to loss of cardioprotection [17]. Thus, in the present study we evaluated the anti-inflammatory and antioxidant activity of irbesartan in a murine cellular model, HL-1 cardiomyocytes, exposed to hypoxic stress. For this purpose we investigated the beneficial effects of the AT-1 receptor antagonist irbesartan on B-type natriuretic peptide (BNP), a plasmatic marker increased in patients with myocardial ischemia, on TLRs pathway and on oxidative balance.
2. Materials and methods
2.1. Cell culture
HL-1 cells, a cardiac muscle cell line derived from the AT-1 mouse atrial myocyte tumor lineage, were a gift from William C. Claycomb, and maintained according to described protocols [18]. The cells were grown in Ex-Cell 320 medium (JRH Biosciences, Lenexa, KS) with 10% heat-inactivated fetal bovine serum (BioWhittaker), 10 mg/ml insulin (Life Technologies, Grand Island, NY), 50 mg/ml endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY), 1 mM retinoic acid (Sigma), 10 mM norepinephrine (Sigma), 100 units/ml penicillin, 100 mg/ml streptomycin (Life Technologies), and an additional 13 nonessential amino acids (Life Technologies). The cells were grown at 37°C in an atmosphere of 5% CO2 and 95% air at a relative humidity of approximately 95%.
2.2 Cell viability assay
Cell viability assay was performed after all experimental protocols. In particular, we have tested a pre- and post- treatment with three different concentrations of irbesartan (10, 50 and 100 μM, dissolved in 250 μL ethanol). 3x103 cells were seeded in 96-well plates and treated with irbesartan at 37°C for 16 h, and then placed in an hypoxia chamber for 24 h. The medium was changed before bringing the cells. To perform the post-treatment, 3x103 cells were exposed for 24 h to the hypoxic stress and later stimulated 16 h with 10, 50 and 100 μM irbesartan. The cells viability was measured by incubation of 3x103 seeded cell/well with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) vitality stain reagent formulated in fresh culture medium. MTT solution at 10% was added to each well and incubated for about 2 h. Then, excess medium was removed and 100 μL of a solution of 1N hydrochloric acid at 10% in isopropanol was added to dissolve the formazan crystals. The mixture was shaken for about 20 min and the optical density in each well was measured using a microplate spectrophotometer (Microplate Reader Model 550, BIO-RAD, California, USA) at 570 nm. Triplicate experiments were performed for each concentration. The cell viability percentage (%) was calculated by comparison with a sample’s corresponding control.
2.3 Hypoxic stress
To induce an hypoxic stress condition, 3x103 HL-1 cells were put in a sealed humidified chamber (Billups-Rothenburg, Del Mar, California) supplied with 5% carbon monoxide and 95% nitrogen for 24 h. Finally, a MTT assay was performed to test cell viability and the hypoxic threshold in cardiomyocytes.
2.4. Superoxide dismutase (SOD) assay
SOD activity was analyzed using a SOD Activity Assay Kit (BioVision, Inc., Milpitas, California) according to manufacturer’s instructions [19]. The assay is a colorimetric method to measure the rate of reduction of WST-1 reagent in a water-soluble formazan dye with superoxide anion and this rate of reduction is inhibited by SOD. Briefly 104 cells were seeded in 6-well plates after treatment with fibronectin and after 24 h incubation at 37°C they were washed once and treated with irbesartan (10 and 100 μM) at 37°C for 16 h and then were put in a hypoxic chamber. Finally, SOD Kit reagents were added to cell lysates and transferred in wells of a 96-well plate and incubated for 20 min and then the absorbance was read at 450 nm. The results were used to calculate the inhibition rate and to extract the SOD activity (U/mL).
2.5. Griess test
104 cells were seeded in 6-well plates after treatment with fibronectin and after 24 hours incubation at 37°C were washed once and stimulated with irbesartan (10 and 100 μM) at 37°C for 16 h and then were put in a hypoxic chamber. After 24 h of the hypoxic stress, the supernatants of each point were used to measure nitrite levels by the Griess reaction according to literature [20]. Briefly, 100 μL of supernatant was mixed with an equal volume of Griess reagent (0.5% sulfanilamide, 2.5% H3PO4, and 0.05% naphthylethylene diamine in H2O) and incubated for 10 min at room temperature. Absorbance was measured at 550 nm and compared with a standard curve using sodium nitrite.
2.6. Real-time PCR (RT-qPCR)
HL-1 cells were harvested and then used for RT-qPCR analysis. The total RNA was extracted from 104 cells seeded in 6-well plates according to the instructions from the supplier of the Trizol reagent (Invitrogen, Milan, Italy), then quantified using NanoDrop-1000 (Thermo Scientific, USA) [21] Using cDNA obtained by reverse transcriptase of RNA extracted from cells (Bio-Rad Laboratories, Milan, Italy), the levels of BNP, TLR-2, TLR-4 and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs were quantified by RT-qPCR using SYBR Green (Bio-Rad Laboratories, Milan, Italy). The oligonucleotide sequences are listed below; mRNA concentrations were expressed as ratio over GAPDH which was amplified as housekeeping gene.
Sense primer:
GAPDH 5’-GCATCCTGCACCACCAACTG-3’
BNP 5’-CTGAAGGTGCTGTCCCAGAT-3’
TLR-4 5’-CCCTTATTCAACCAAGAAC-3’
TLR-2 5’-CAGAGGACTCAGGAGCAGC-3’
Antisense primer:
GAPDH 3’-CACAGTCTTCTGAGTGGCAG-5’
BNP 3’-CCTTGGTCCTTCAAGAGCTG-5’
TLR-4 3’-CTGGATAAATCCAGCCACTG-5’
TLR-2 3’-GCCTTCCCTTGAGAGGCC-5’
2.7. Western blot analysis
104 HL-1 cells were lysed by incubation on ice for 30 min with RIPA lysis buffer and 10 μL/mL leupeptin, 5 μL/mL aprotinin, 1 μmol/L pepstatin, and 10 mmol/L dithiothreitol (DTT) were added before use. After centrifugation, the supernatant was collected, and the protein concentration was measured using a commercial kit (Bio-Rad Laboratories, Milan, Italy) according to the manufacturer’s instructions. Eighty μg of protein samples were run on 10% SDS–PAGE gels and then transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Milan, Italy). Membranes were blocked for 1 h at room temperature with 5% milk in T-TBS (Tris buffer saline with 0.1% Tween 20), followed by incubation at 4°C overnight with primary antibodies against IL-17, catalase, iNOS, TNF-α, BNP (Santa Cruz Biotechnology, Milan, Italy). Membranes were then washed three times with 0.1% T-TBS solution, and incubated for 1 h at room temperature with a secondary antibody goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP, according to the primary antibodies data sheet (Santa Cruz Biotechnology, Milan, Italy). GAPDH antibody (Santa Cruz Biotechnology, Milan, Italy) was used as an internal standard. The immunoreactive bands were visualized using an enhanced chemiluminescence system (SuperSignal West Femto Maximum Sensitivity Substrate, Pierce, Rockford, USA). The protein bands were scanned and quantitated with Gel Doc-2000 (Bio-Rad, Milan, Italy).
Enzyme-linked immunosorbent assay (ELISA)
TNF-alpha and IL-17 levels were measured in the supernatants from cell cultures with a commercial ELISA kit (Elabscience, Naples, Italy). The experiments were carried out according to the manufacturer’s instructions. The values are reported as pg/mg of protein. The results derived from 3 independent experiments.
2.8. Statistical analysis
All data are expressed as the mean with standard deviation. Student's t-test was used to determine statistical significance of the results and p-values of less than 0.05 were considered statistically significant. In addition, One-way ANOVA followed by Dunnett’s test was used in order to assess the variance among the groups. The alpha critical value was set as less than 0.05 to be considered significant.
3. Results
3.1. Pre-treatment with irbesartan improves viability of HL-1 hypoxic cells
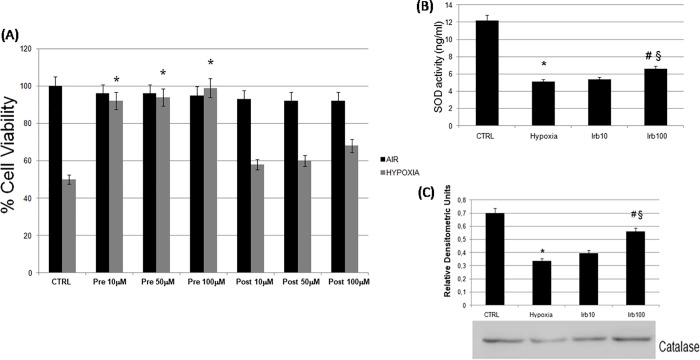
We have used an in vitro model of simulated ischemia exposing HL-1 cardiomyocytes to hypoxic stress in a suitable chamber. The exposure of cells to hypoxia for 24 h markedly reduced cell viability to 50% compared with cells grown in standard conditions (Fig 1A). When the HL-1 cells were pre-treated with irbesartan (10, 50, 100 μM) for 16 h and then incubated in hypoxic chamber for 24 h, their viability, measured by MTT test, significantly improved by about 40% compared with untreated hypoxic cells without significant difference between the concentrations. Conversely, a post-treatment with irbesartan (10, 50, 100 μM) for 16 h causes a slight recovery of cell viability (Fig 1A). Based on these results we have chosen to stimulate the cells with irbesartan in pre-treatment, in addition, from the obtained three concentrations comparable data we decided to use only minimum and maximum irbesartan concentration (10 and 100 μM) (Fig 1A).
Fig 1. Effect of irbesartan on cell viability and on oxidative stress in HL-1 hypoxic cells.
(A) Cardiomyocytes viability was determined by MTT assay in normoxia (CTRL), hypoxia alone (Hypoxia) and in combination with a pre-treatment or post-treatment with 16 h of irbesartan (Irb 10, 50, 100 μM). (B) SOD activity was determined by an ELISA assay. (C) Catalase protein expression was measured by western blot. All data are presented as mean ± S.E.M. of three independent experiments (*P<0.05 versus CTRL; #P<0.05 versus hypoxia, § P<0.05 versus irbesartan 10 μM).
3.2. Antioxidant activity of irbesartan on hypoxic cardiomyocytes
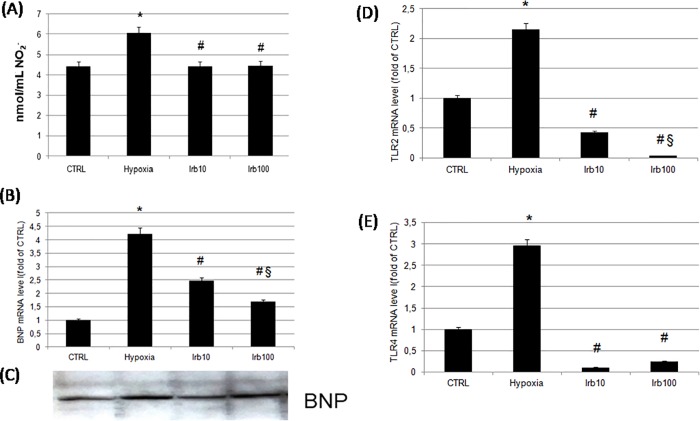
To investigate the role of the AT1 antagonist irbesartan on oxidative stress in hypoxic HL-1 cells we tested the effects of a pre-treatment with the two concentrations of irbesartan on SOD activity. Our data showed a significant decrease in the enzyme activity during hypoxia compared with control cells. Sixteen hours pre-treatment with irbesartan (100 μM) partially restored the enzyme activity (Fig 1B). The antioxidant effect of irbesartan was confirmed by measuring protein levels of catalase. A pre-treatment with 100 μM irbesartan significantly restore catalase expression reduced by hypoxia (Fig 1C). We also evaluated NO2- concentrations in cardiomyocytes by using the culture medium. In hypoxic conditions as expected we observed increased NO2- levels compared to non-stressed samples. On the other hand, a pre-treatment with irbesartan reduced significantly NO levels than in hypoxic cells (Fig 2A).
Fig 2. Effect of irbesartan pre-treatment on oxidative stress, on cardiac marker BNP and on TLRs mRNA expression.
(A) The release of peroxynitrite was measured by Griess assay. (B) mRNA levels BNP was evaluated by RT-qPCR. (C) Western blot analysis of BNP protein, blot is representative from three independent experiments. (D) TLR2 and (E) TLR4 mRNA expressions were measured by RT-qPCR. TLR2 an TLR4 mRNA levels were normalized relative to GAPDH mRNA levels. Data represent the mean ± S.E.M. of three independent experiments (*P<0.05 versus CTRL; #P<0.05 versus hypoxia, § P<0.05 versus irbesartan 10 μM).
3.3. Effects of a pre-treatment with irbesartan on cardiomyocytes BNP expression
BNP is a diagnostic and prognostic marker in the management of patients with cardiovascular diseases and it is secreted by cardiomyocytes in response to myocardial ischemia [22]. An hypoxic condition mimics a myocardial damage induced by the lack of oxygen supply and in our experimental model 24 h of hypoxia induced in HL-1 cells a significant increase of BNP mRNA compared to control cells. A pre-treatment with 10 and 100 μM of irbesartan significantly and concentration-dependent reduced mRNA levels of this peptide (Fig 2B). To confirm this result we have performed a western blot and we have measured the protein expression of BNP active peptide. Our data showed the same profile of expression obtained by Real-time PCR (Fig 2C).
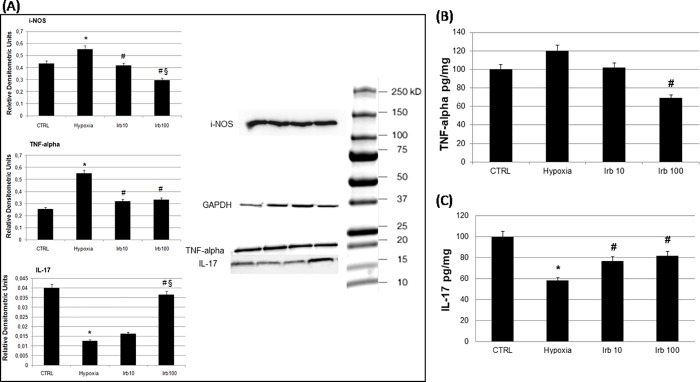
3.4. The AT1 receptor antagonist irbesartan reduces the inflammation induced by hypoxia negatively modulating the TLRs pathway
To determine the effects of irbesartan on inflammation induced by hypoxia we have chosen to measure mRNA levels of two TLRs, TLR-2 and -4, which activation by an ischemic injury aggravate tissue damage [23,24]. In particular, TLR-4 and its downstream targets such as TNF-α and i-NOS are found to facilitate the inflammatory reaction in clinical ischemic condition, i.e. myocardial infarction [25]. Our findings showed an increased mRNA of the two receptors after hypoxia. An irbesartan pre-treatment significantly reduced TLRs mRNA levels (Fig 2D and 2E). The activation of this pathway leads to an intracellular cascade of events involving inflammatory and oxidative mediators. In particular, 24 h of hypoxia induced a significant increase of inflammatory mediators such as i-NOS, TNF-α and a reduction of the antinfiammatory cytokine IL-17 (Fig 3A). An irbesartan pre-treatment (10 and 100 μM) counteracted the inflammation inducing a significant and concentration-dependent reduction of i-NOS, a reduction of TNF-α and an increase of IL-17 only at 100 μM (Fig 3A). To demonstrate and confirm that also the activity of TNF-α and IL-17 are modified by our experimental condition we performed an ELISA measurement (Fig 3B and 3C).
Fig 3. Role of irbesartan pre-treatment on inflammatory mediators and on cytokines activity.
(A) iNOS TNF-alpha and IL-17 proteins expressions were measured by western blot. GAPDH was used as internal standard. Representative blot from three independent experiments. Data are expressed as relative densitometric units and represent the mean ± S.E.M. of three independent experiments (*P<0.05 versus CTRL; #P<0.05 versus hypoxia, § P<0.05 versus irbesartan 10 μM). (B) TNF-alpha and (C) IL-17 activity was measured by ELISA. Final concentration of each cytokine was expressed as pg/mg and represent the mean ± S.E.M. of three independent experiments (*P<0.05 versus CTRL; #P<0.05 versus hypoxia).
5. Discussion
Hypoxia is a major contributor to cardiac pathophysiology; a hypoxic environment induces a disproportion between oxygen supply and demand in cardiac tissue [26]. Accumulating evidences has highlighted the importance of inflammatory response and oxidative imbalance in acute myocardial infarction pathogenesis [27]. ROS overproduction induces endothelial injury and modulates the signalling of several pathways, which are known to play a crucial role in cardiovascular diseases, i.e. ischemic heart disease, stroke, kidney disease [28–30]. Previous studies have demonstrated that AT1 blockers reduced ischemic factors and the consequences of excessive ROS production; these effects were particularly investigated in experimental models of atherosclerosis and in clinical trials [31,32]. In our experimental model a pre-treatment with irbesartan, an angiotensin II receptor type AT1 antagonist, significantly reduces oxidative stress induced by hypoxia. In particular, only the highest concentration of irbesartan (100 μM) significantly improved SOD activity reduced by hypoxia; on the other hand, in a concentration-dependent manner, the pre-treatment restored the expression of another anti-oxidant enzyme, catalase. To investigate a protective role of irbesartan in cardiac hypoxia we measure BNP expression. It is well known that the inhibition of the renin-angiotensin-aldosterone system reduced plasmatic levels of BNP in cardiovascular diseases [33,34]. Plasma levels of BNP are considered one of the markers for the diagnosis of different cardiovascular diseases associated with neuroendocrine and haemodynamic changes [35]. Many clinical trials have described elevated circulating levels of BNP in patients with myocardial ischemia [36,37]. Our results showed that twenty-four hours of hypoxia markedly increased BNP levels in cardiomyocytes; a pre-treatment with irbesartan concentration-dependently restored these levels almost to the baseline. Among multiple mechanisms involved in cardiomyocyte dysfunction during ischemia, the activation of inflammatory response plays a critical role [38]. Toll-like Receptors are one of the pattern recognition receptors that initiate a defensive response to microbial invasion. Moreover, the role of TLRs in the pathophysiology of cardiovascular diseases has been widely highlighted by different authors [39]. Among these receptors TLR-2 and TLR-4 are activated following ischemic injury in cardiomyocytes [40]. The cardioprotective role of TLR4 in cardiac injury was also confirmed in TLR-4-mutated mice where the deficiency of this receptor exacerbated the cardiac dysfunction during ischemia [41]. The activation of TLR signaling through nuclear factor-κB pathway increases the expression of pro-inflammatory cytokines such as TNF-α [42,43]. For the above mentioned reasons we chose to measure the expressions of TLR- 2 and -4 after hypoxia; their expression significantly increase after 24 hours of hypoxia. A pre-treatment with irbesartan at both concentrations reported the values to baseline. To confirm the anti-inflammatory effects of irbesartan we measured the expression of two inflammatory mediators, i-NOS and TNF-α, and a specific cytokine, IL-17. As expected, hypoxia increased both inflammatory molecules but a pre-treatment with irbesartan significantly counteracted this effect. Conversely, the AT-1R blocker restored to control levels the IL-17 amount. Previous studies have shown that the biological actions of IL-17 are pro-inflammatory but there are several other studies suggesting that its role is controversial and is not yet fully understood. In fact Maione et al. show that IL-17 play a key role in sustaining chronic inflammation in autoimmune diseases. In particular, IL-17 does not initiate an inflammatory response but it is able to amplify biochemical and cellular events of this reaction [44]. Furthermore, Ke Y et al. show that IL-17 has anti-inflammatory activity and that this cytokine can suppress the development of autoimmune disease [45]. Other authors sustain that innate-derived IL-17 constitutes a major element in the altered immune response against self antigens or the perpetuation of inflammation, particularly at mucosal sites [46]. In our proposed model IL-17 could play a protective role in the presence of irbesartan in cardiomyocytes. In this perspective further studies will be needed to better understand the causative and crucial role of IL-17. Our results aimed to better understand the molecular changes of cardiomyocytes under hypoxia; any cause of hypoxia, in fact, may have a negative impact on cardiac function, and the majority of heart diseases are associated with a cardiac hypoxic stress. However, a limitation of this study is not to completely investigate the TLRs involvement; i.e. TLRs protein expression. The findings of this study add new information about the role of renin angiotensin system in the control of oxidative and inflammatory response to an experimental hypoxic condition highlighting the protective effect of the AT1 receptor antagonist irbesartan. Thus, therapeutic approaches that target components of the inflammatory and oxidative response have been investigated as potential and useful treatments for ischemic myocardial injury. Certainly a number of questions remain unsolved and further study will be needed on the hypoxia signaling to understand its pathological processes in more detail.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:447–454. 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 2.Fineschi V, Measuring myocyte oxidative stress and targeting cytokines to evaluate inflammatory response and cardiac repair after myocardial infarction. Curr. Vasc. Pharmacol. 2015;13:3–5. [DOI] [PubMed] [Google Scholar]
- 3.Hashem SI, Perry CN, Bauer M, Han S, Clegg SD, Ouyang K, et al. Brief Report: Oxidative Stress Mediates Cardiomyocyte Apoptosis in a Human Model of Danon Disease and Heart Failure. Stem Cells 2015;33:2343–2350. 10.1002/stem.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Wang D, Yu B, Wang Y, Ren H, Zhang B, et al. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid Med Cell Longev. 2014:134862 10.1155/2014/134862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baban B, Liu JY, Mozaffari MS. Pressure overload regulates expression of cytokines, γH2AX, and growth arrest- and DNA-damage inducible protein 153 via glycogen synthase kinase-3β in ischemic-reperfused hearts. Hypertension 2013;61:95–104. 10.1161/HYPERTENSIONAHA.111.00028 [DOI] [PubMed] [Google Scholar]
- 6.Deftereos S, Angelidis C, Bouras G, Raisakis K, Gerckens U, Cleman MW, et al. Innate immune inflammatory response in the acutely ischemic myocardium. Med. Chem. 2014;10: 653–662. [DOI] [PubMed] [Google Scholar]
- 7.Rohde D, Schön C, Boerries M, Didrihsone I, Ritterhoff J, Kubatzky KF, et al. S100A1 is released from ischemic cardiomyocytes and signals myocardial damage via Toll-like receptor 4. EMBO Mol Med. 2014;6:778–794. doi: 10.15252/emmm.201303498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao JM, Meng XW, Zhang J, Chen WR, Xia F, Peng K, et al. Dexmedetomidine protects cardiomyocytes against hypoxia/reoxygenation injury by suppressing TLR4-MyD88-NF-κB signaling. Biomed Res Int. 2017;1674613 10.1155/2017/1674613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parashar A., Agarwal S., Krishnaswamy A., Garg A., Poddar K.L., Sud K., et al. Renin-Angiotensin System Antagonists in Patients Without Left Ventricular Dysfunction After Percutaneous Intervention for ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2015;116:508–514. 10.1016/j.amjcard.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Wen ZZ, Cai MY, Mai Z, Jin DM, Chen YX, Huang H, et al. Angiotensin II receptor blocker attenuates intrarenal renin-angiotensin-system and podocyte injury in rats with myocardial infarction. PLoS One 2013;8: e67242 10.1371/journal.pone.0067242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babiker F, Al-Jarallah A, Joseph S. The Interplay between the Renin Angiotensin System and Pacing Postconditioning Induced Cardiac Protection. PLoS One 2016;11:e0165777 10.1371/journal.pone.0165777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roksnoer LC, van Veghel R, van Groningen MC, de Vries R, Garrelds IM, Bhaggoe UM, et al. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin Sci (Lond) 2016;130:1209–1220. [DOI] [PubMed] [Google Scholar]
- 13.Kusunoki H, Taniyama Y, Rakugi H, Morishita R. Cardiac and renal protective effects of irbesartan via peroxisome proliferator-activated receptorγ-hepatocyte growth factor pathway independent of angiotensin II Type 1a receptor blockade in mouse model of salt-sensitive hypertension. J Am Heart Assoc. 2013;22: e000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taguchi I, Toyoda S, Takano K, Arikawa T, Kikuchi M, Ogawa M, et al. Irbesartan, an angiotensin receptor blocker, exhibits metabolic, anti-inflammatory and antioxidative effects in patients with high-risk hypertension. Hypertens. Res. 2013;36:608–613. 10.1038/hr.2013.3 [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Zhao Y, Wei F, Ye L, Lu F, Zhang H, et al. Treatment with telmisartan/rosuvastatin combination has a beneficial synergistic effect on ameliorating Th17/Treg functional imbalance in hypertensive patients with carotid atherosclerosis. Atherosclerosis 2014;233:291–299. 10.1016/j.atherosclerosis.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Min X, Lu M, Tu S, Wang X, Zhou C, Wang S, et al. Serum Cytokine Profile in Relation to the Severity of Coronary Artery Disease. Biomed Res Int. 2017;4013685 10.1155/2017/4013685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toldo S, Boccellino M, Rinaldi B, Seropian IM, Mezzaroma E, Severino A, et al. Altered oxido-reductive state in the diabetic heart: loss of cardioprotection due to protein disulfide isomerase. Mol. Med. 2011;17:1012–1021. 10.2119/molmed.2011.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. 1998;U S A 95:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ukeda H, Maeda S, Ishii T, Sawamura M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3'—1—(phenylamino)-carbonyl—3, 4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal. Biochem. 1997;251:206–209. 10.1006/abio.1997.2273 [DOI] [PubMed] [Google Scholar]
- 20.Hernández-Díaz Y, Tovilla-Zárate CA, Juárez-Rojop I, Baños-González MA, Torres- Helaleh MI, Korenaga T. Sensitive spectrophotometric determination of nitrite in human saliva and rain water and of nitrogen dioxide in the atmosphere. J. AOAC 2001;Int. 84:53–58. [PubMed] [Google Scholar]
- 21.Rinaldi B, Donniacuo M, Esposito E, Capuano A, Sodano L, Mazzon E, et al. PPARα mediates the anti-inflammatory effect of simvastatin in an experimental model of zymosan-induced multiple organ failure. Br. J. Pharmacol. 2011;163:609–623. 10.1111/j.1476-5381.2011.01248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: focus on natriuretic peptides. Can. J. Cardiol. 2014;30:1027–1034. 10.1016/j.cjca.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 23.Mkaddem SB, Bens M, Vandewalle A. Differential activation of Toll-like receptor-mediated apoptosis induced by hypoxia. Oncotarget 2010;1:741–750. doi: 10.18632/oncotarget.101203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balistreri CR, Ruvolo G, Lio D, Madonna R. Toll-like receptor-4 signaling pathway in aorta aging and diseases: "its double nature". J Mol Cell Cardiol. 2017;110:38–53. 10.1016/j.yjmcc.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, et al. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed. Pharmacother. 2017;91:1042–1052. 10.1016/j.biopha.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 26.Farías JG, Molina VM, Carrasco RA, Zepeda AB, Figueroa E, Letelier P et al. Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 2017;9(9): pii: E966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Reséndiz S, Chinda K, Ong SB, Cabrera-Fuentes H, Zazueta C, Hausenloy D. The role of redox dysregulation in the inflammatory response to acute myocardial ischaemia-reperfusion injury—adding fuel to the fire. Curr Med Chem. 2018;25(11):1275–1293. 10.2174/0929867324666170329100619 [DOI] [PubMed] [Google Scholar]
- 28.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. 10.1016/j.freeradbiomed.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 29.Vanacore D, Messina G, Lama S, Bitti G, Ambrosio P, Tenore GC, et al. Effect of Restriction Vegan Diet's on Muscle Mass, Oxidative Status and Myocytes Differentiation: a Pilot Study. J Cell Physiol. 2018;10 10.1002/jcp.26427 [DOI] [PubMed] [Google Scholar]
- 30.D'Angelo S, La Porta R, Napolitano M, Galletti P, Quagliuolo L, Boccellino M. Effect of Annurca apple polyphenols on human HaCaT keratinocytes proliferation. J Med Food 2012;15(11):1024–31. 10.1089/jmf.2012.0076 [DOI] [PubMed] [Google Scholar]
- 31.Hirohata A, Yamamoto K, Miyoshi T, Hatanaka K, Hirohata S, Yamawaki H, et al. Impact of olmesartan on progression of coronary atherosclerosis a serial volumetric intravascular ultrasound analysis from the OLIVUS (impact of OLmesarten on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) trial. J Am Coll Cardiol. 2010;55:976–982. 10.1016/j.jacc.2009.09.062 [DOI] [PubMed] [Google Scholar]
- 32.Shimada K, Murayama T, Yokode M, Kita T, Fujita M, Kishimoto C. Olmesartan, a novel angiotensin II type 1 receptor antagonist, reduces severity of atherosclerosis in apolipoprotein E deficient mice associated with reducing superoxide production. Nutr Metab Cardiovasc Dis 2011;21:672–678. 10.1016/j.numecd.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 33.Meno H, Inou T, Tanaka M, Tsuchiya Y, Shiga Y, Kobayashi K, et al. Antihypertensive efficacy of the losartan/hydrochlorothiazide combination and its effect on plasma B-type natriuretic peptide in hypertensive patients uncontrolled by angiotensin II type 1 receptor antagonist-based therapy: a multicentre prospective observational study. Clin Drug Investig. 2012;32:171–178. 10.2165/11597620-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 34.Helanova K, Littnerova S, Kubena P, Ganovska E, Pavlusova M, Kubkova L, et al. Prognostic impact of neutrophil gelatinase-associated lipocalin and B-type natriuretic in patients with ST-elevation myocardial infarction treated by primary PCI: a prospective observational cohort study. BMJ Open. 2015;5:e006872 10.1136/bmjopen-2014-006872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong PC, Guo J, Zhang A. The renal and cardiovascular effects of natriuretic peptides. Adv. Physiol. Educ. 2017;41:179–185. 10.1152/advan.00177.2016 [DOI] [PubMed] [Google Scholar]
- 36.Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic. Res. Cardiol. 2007;102,529–541. 10.1007/s00395-007-0672-1 [DOI] [PubMed] [Google Scholar]
- 37.Goetze JP, Christoffersen C, Perko M, Arendrup H, Rehfeld JF, Kastrup J, et al. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003;17,1105–1107. 10.1096/fj.02-0796fje [DOI] [PubMed] [Google Scholar]
- 38.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018; 9. pii: S0163-7258(18)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilahur G, Badimon L. Ischemia/reperfusion activates myocardial innate immune response: the key role of the toll-like receptor. Front Physiol. 2014;5,496 10.3389/fphys.2014.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, et al. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol. 2010;48,1236–1244. 10.1016/j.yjmcc.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 41.Zhao P, Wang J, He L, Ma H, Zhang X, Zhu X, et al. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med. 2009;13,1513–25. 10.1111/j.1582-4934.2009.00798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C, et al. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: Role of the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2015;11,1120–1126. 10.3892/mmr.2014.2955 [DOI] [PubMed] [Google Scholar]
- 43.Lu M, Tang F, Zhang J, Luan A, Mei M, Xu C, et al. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-κB signaling pathway. Phytother Res. 2015;29,599–606. 10.1002/ptr.5297 [DOI] [PubMed] [Google Scholar]
- 44.Maione F, Paschalidis N, Mascolo N, Dufton N, Perretti M, D'Acquisto F. Interleukin 17 sustains rather than induces inflammation. Biochem Pharmacol. 2009;77(5):878–87. 10.1016/j.bcp.2008.11.011 [DOI] [PubMed] [Google Scholar]
- 45.Ke Y, Liu K, Huang GQ, Cui Y, Kaplan HJ, Shao H, et al. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182(5):3183–90. 10.4049/jimmunol.0802487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. 10.1016/j.jaut.2015.04.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.