Abstract
Background
Quantifying the association between adherence and the growth response to growth hormone (GH) treatment is hampered by suboptimal methods of measuring adherence, confounders associated with the growth response, and restriction of the outcome parameters to yearly growth velocities.
Aim
To investigate the effect of adherence on the two-year growth response to GH treatment in prepubertal children with idiopathic isolated growth hormone deficiency (GHD) participating in the easypod connect observational study (ECOS), a 5-year, Phase IV open-label study to continuously assess real-world adherence via the easypod electronic drug-delivery device.
Patients and methods
Outcome measures were change in height standard deviation score (ΔHSDS), index of responsiveness (IoR), and parameters of two catch-up growth (CUG) curve functions (monomolecular growth curve and second degree polynomial) with adj-HSDS (HSDS minus Target height (TH) SDS) as dependent variable. Inclusion criteria were GHD, naïve to GH treatment, known TH, age <10y in girls and <12y in boys, ≥3 measurements, HSDS <-2 at start, complete data on growth and adherence in the first and second year. Linear regression analyses were performed to test the association between adherence (continuous and high vs. low) and the outcome measures, also adjusted for potential clinical confounders (age at start, adj-HSDS at start, birth weight SDS, gestational age (<37 weeks vs ≥37 weeks), GH dose, GH max (n = 58)). The formula of IoR already adjusts for confounders.
Results
In total, 95 patients complied with the inclusion criteria. The strongest associations were found between high adherence in the second year (≥91% as cut-off value) and IoR 2y (+0.62), and average adherence and high adherence (≥78%) in the first two years and ΔHSDS 0-2y (+0.11 SD per 1 injection/week, and +0.34 SD for high vs. low adherence).
Conclusion
Suboptimal adherence negatively affected the growth response in the first two years of GH treatment.
Introduction
Growth hormone (GH) treatment for children with GH deficiency (GHD) is efficacious in generating catch-up growth (CUG) in the first years of treatment, usually followed by a maintenance phase and finally leading to a height close to the target height (TH) [1,2]. However, the growth response has been shown to be quite variable. Several groups have investigated the clinical features associated with the growth response [3,4,5,6], and in general approximately 50% of the variance could be explained by features like the severity of GHD (as assessed by the GH peak in provocation tests), age, bone age delay, birth weight, etc [7].
None of these prediction models could include adherence into their models, because there were no tools to assess adherence accurately. However, several studies have shown that adherence has an important effect on growth on GH treatment [8,9,10,11,12], although they could only be conducted over short time periods, and using a methodology based on self-reporting or number of issued and renewed prescriptions.
There are several challenges in studying the association between adherence to growth hormone (GH) treatment versus the growth response to GH. The first challenge is to make a decision on which growth parameter is the most suitable outcome parameter. So far, most studies investigating predictive factors have used yearly growth velocity data, particularly first year’s height velocity [3,6]. Others have used the change in height Standard Deviation Score (HSDS) (delta HSD, ΔHSDS) over 1 or 2 years [4,5]. All these parameters depend on the age and height SDS at start of treatment, and do not fully represent the actual shape of the catch-up growth (CUG) in the first years of GH treatment in children with GHD. The second challenge is that, besides adherence, there are many other clinical parameters that influence the growth response to GH, as illustrated by the various prediction models [7]. So, in order to obtain an unbiased effect of adherence, it is necessary to adjust for important clinical predictors that are confounders in an analysis of the effect of adherence on the growth response to GH. The third challenge is to find a reliable method for measuring adherence.
In this study we accounted for these challenges. First, we used a novel way of expressing CUG on GH treatment, using mathematical models of HSDS (adjusted for TH]) over two years of GH treatment. In an earlier study, we showed that mathematical modelling of CUG with the monomolecular growth curve is suitable for celiac disease [13]. A second degree polynomial was also used, because for most children the shape of the HSDS in the first two years is a catch-up in growth followed by stabilization. Second, we entered important potential confounders into the models. Third, we used a novel electronic tool to assess adherence accurately (easypod) [14,15,16]. For this purpose we used data from children naïve to GH treatment participating in the ECOS study, using automated continuous assessment of adherence through easypod. The easypod Connect observational study (ECOS) is a 5-year, Phase IV open label study that started in 2010 in 24 countries to assess ‘real-world’ adherence via the easypod electronic drug delivery device.[17]
Methods
Data
Adherence data were derived from the easypod device combined with physician data entry of outcome measures. Data were collected retrospectively and prospectively. Collected data were analyzed in a multinational pooled analysis.
Inclusion criteria for the ECOS study were: GH administered via the easypod electromechanical device; under 18 years of age, or over 18 without fusion of growth plates; parent’s or guardian’s written informed consent, given before entering data into the registry/observational study (if the child was old enough to read and write, a separate assent form was given as defined in the appropriate jurisdiction of each country). Exclusion criteria for the ECOS study were: Subjects taking GH in whom growth plates have fused (i.e. taking GH for its metabolic effects); contra-indications to Saizen GH; Use of an investigational drug or participation in another interventional clinical study. The study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice (ICH-GCP E6) guidelines and applicable national legal and regulatory requirements.
To investigate the effect of adherence on the two-year growth response to GH treatment in prepubertal children with idiopathic isolated growth hormone deficiency (GHD) in the ECOS study, the following selection criteria were applied:
Naïve to GH treatment
Patients diagnosed with Idiopathic Isolated GHD by their physicians
Known parental heights, so that TH could be calculated
Age at end of 2 year study period <10 years in girls and <12 years in boys (i.e. before onset of puberty)
At least three measurements of height available from start treatment
HSDS <-2.0 at start treatment
At least two data measurements on growth and adherence in the first and second year after start of treatment
The age limit was used for two reasons. First, we wished to limit the analysis to prepubertal children, because insufficient data were available in the database about pubertal status during GH treatment. Second, beyond these age limits the SD of the population’s growth reference charts shows a non-linear pattern of an increase followed by a decrease, due to the varying ages at pubertal onset in the general population. Because HSDS is the main outcome parameter in our analysis, a higher cut-off for age would generate noise and bias.
Statistical analyses
Adherence was calculated as the number of injections received divided by the number of planned injections during the considered period, expressed as a percentage. All adherence rate analyses were based on periods of complete weeks. Data are presented over the first two years.
Outcome measures for the growth response to GH treatment in the first two years were:
Change in HSDS in the first (ΔHSDS 0–1 y), second (ΔHSDS 1–2 y) and first two years (ΔHSDS 0–2 y)
Index of responsiveness (IoR) in the first and second year [3], where IoR first year = (height velocity first year-(12.41–0.36*Age at start GH+0.47* Birthweight SDS+1.54*(log(3*GH dose at start GH (mg/kg/wk)))-0.6*(HSDS 1y-TH SDS)+0.28*weight SDS 1y))/1.72, and IoR second year = (height velocity second year-(5.69–0.09*Age at start GH+0.63*(log(3*GH dose at start GH (mg/kg/wk)))+0.24*weight SDS 2y+0.31* height velocity first year))/1.19
Parameters of the monomolecular growth curve for catch-up growth [13]: Monomolecular growth curve: adj-HSDS = A * (1—B * exp(-k * x))– 5, with x age after start GH
Parameters of a second degree polynomial: second degree polynomial: adj-HSDS = c + D*x + E*x2, with x age after start GH
Mixed-effect models were used to fit the monomolecular growth curve and the second degree polynomial on adj-HSDS (HSDS minus TH-SDS). Each curve summarizes the first phase of CUG in three parameters. For the monomolecular growth curve, these parameters are A-5 = attained adj-HSDS after two years, A*(1-B)-5 = adj-HSDS at start, k = growth rate. For the second degree polynomial, these are c = intercept (adj-HSDS at start), D = slope (CUG), E = deceleration (deceleration part after CUG). A higher value of D implies a steeper curve between 0–2 years and a higher value of E implies a stronger CUG after approximately 1 year. If E >0, then there is a stronger CUG in the second year, and if E<0 then CUG is stronger in the first year.
Linear regression analyses were performed to test the association between adherence in the first, second, and the first two years and the outcome measures. Adherence is highly skewed with peak values near or at 100%. We therefore analyzed this independent variable both as a continuous variable and categorized into a high and low level. Recursive partitioning [18] in software R (package rpart) was used to find the cut-off point (the first split) for high and low adherence that maximizes the correlation between adherence and height gain.
The parameter B from the monomolecular growth curve and c from the second degree polynomial were not used in the analyses as outcome measures, because these are related to the adj-HSDS at start and only of interest as a potential confounder. We adjusted the associations for potential clinical confounders that were significantly related to adherence in the first, second or first two years. The potential clinical confounders were age at start, adj-HSDS at start (observed data, not derived from the monomolecular growth curve model), birth weight SDS, gestational age (<37 weeks vs. ≥37 weeks), GH dose, and GH max. The formula of IoR already adjusts for confounders. We, therefore, did not adjust the IoR for the potential clinical confounders. HSDS at exactly 1.0 and 2.0y were obtained by linear interpolation using the nearest measurements around these ages. WHO growth references were used to calculate the SDS values. These references were obtained from the WHO Multicentre Growth Reference Study (MGRS) (ages 0–5 years) and the WHO 2007 reference (5–19 years for height) [19]. TH-SDS was calculated as 0.72 x (father’s HSDS + mother’s HSDS)/2 [20]. This definition corrects for assortative mating and parent-offspring correlations. We also used the first and second year Index of Responsiveness (IoR) for the KIGS prediction model as outcome measures [3], but using the WHO references for weight SDS at birth.
Results
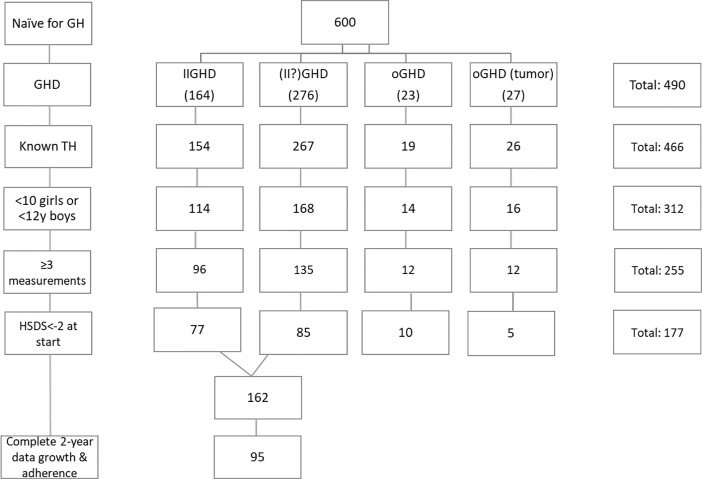
Fig 1 shows the sample size of this study after applying the inclusion criteria. In total 490 naïve patients with GHD were available within the ECOS study. The sample size of patients with an Organic GH deficiency (OGHD) due to congenital/anatomical or tumor origin was low and therefore excluded from the analysis in order to analyse a homogeneous group. In total, 95 patients with Idiopathic Isolated GH deficiency (IIGHD) were available for analysis. Table 1 shows the descriptive statistics of the background characteristics and the clinical parameters of these 95 patients. Although one patient exceeded the (arbitrary) cut-off of 10 ug/L at the GH peak after stimulation (GH peak-1 = 9.97 and GH peak -2 = 12.5 ug/L), we included this patient in our study because the treating physician interpreted this patient as GHD.
Fig 1. Selection criteria within the ECOS study.
Table 1. Background characteristics and clinical parameters of the sample (n = 95).
| Characteristics | Mean (SD) | Median (min, max) | N (%) |
|---|---|---|---|
| Sex (male) | 72 (76%) | ||
| Gestational age (<37 wks) | 12 (13%) | ||
| Birth weight SDS | -1.0 (1.5) | -0.9 (-6.4, 1.2) | |
| Age at start | 6.3 (2.1) | 6.2 (1.3, 10.0) | |
| HSDS at start | -2.8 (0.7) | -2.6 (-5.6, -2.0) | |
| HSDS 1y after start GH | -2.1 (0.7) | -2.0 (-4.4, -1.0) | |
| HSDS 2y after start GH | -1.7 (0.7) | -1.6 (-4.2, -0.3) | |
| Adj-HSDS at start | -2.1 (0.9) | -2.1 (-4.7, -0.1) | |
| Adj-HSDS 1y after start GH | -1.4 (0.8) | -1.4 (-4.0, 0.2) | |
| Adj-HSDS 2y after start GH | -1.1 (0.8) | -1.1 (-3.6, 0.5) | |
| Weight SDS at start | -1.8 (1.1) | -1.9 (-5.2, 1.8) | |
| GH dose (mg/kg/wk) | 0.21 (0.10) | 0.21 (0.04, 0.83) | |
| GH max* | 4.9 (3.0) | 4.3 (0.47, 12.5) | |
| IoR first year | -0.4 (1.1) | -0.5 (-3.4, 4.1) | |
| IoR second year | -0.1 (1.1) | -0.1 (-3.5, 3.1) | |
| Adherence 0-1y | 80.8 (31.1) | 95.1 (0, 100) | |
| Adherence 1-2y | 81.5 (23.0) | 92.9 (0, 100) | |
| Adherence 0-2y | 81.1 (22.2) | 90.6 (7.4, 99.9) |
*Data available for n = 58
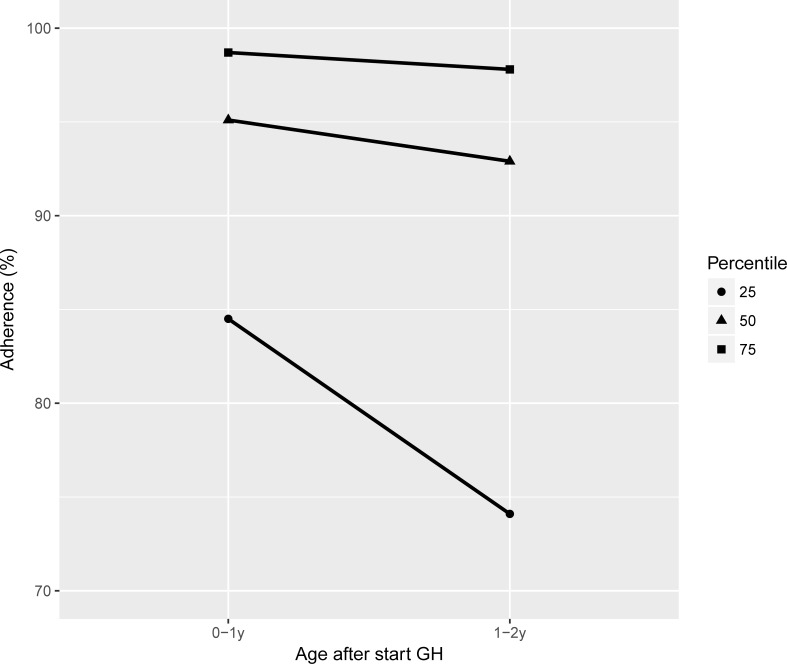
Fig 2 shows the median and P25-75 adherence % in the first two years of GH treatment. In the first year, median adherence was high with relatively little variation. In the second year, median adherence decreased, while the variation increased. Recursive partitioning for adherence resulted in a split at ≥98% in the first year, ≥91%, in the second year, and ≥78% in the first two years. With these splits, 32 children (34%) had a high adherence (≥98% as cut-off value) and 63 children (66%) a low adherence (<98% as cut-off value) in the first year. For the second year, these figures were 50 (53%) for a high adherence (≥91% as cut-off value) and 45 (47%) for a low adherence (<91% as cut-off value). For the first two years, these figures were 68 (72%) for a high adherence (≥78% as cut-off value) and 27 (28%) children (<78% as cut-off value).
Fig 2. Adherence in the first two years after starting growth hormone therapy.
Table 2 shows the results of the effect of adherence on ΔHSDS, the parameters of the growth curves and IoR. Ten out of eighteen associations from the final model were statistically significant. The strongest associations were found between high versus low adherence in the second year and IoR 2y, between adherence (continuous, in %) and ΔHSDS 0-2y, and between high versus low adherence in the first two years and ΔHSDS 0-2y. Mean SD) IoR at 2y was 0.19 (0.99) in the high adherence group and -0.44 (1.04) in the low adherence group. Without adjustment, mean (SD) ΔHSDS 0-2y was +1.16 (0.52) in the high adherence group and +0.88 (0.41) in the low adherence group. After adjustment for the clinical confounders, the difference in mean height between the high and low adherence group increased from +0.28 SD to +0.34 SD. The size of the effect of adherence (continuous) in the first two years on HSDS 0-2y can be calculated by multiplying the model estimate by the percentage gain of adherence. For example, a 14% lower adherence (missed 1 injection/week) in the first two years is associated with 14 x 0.00792 = 0.11 SD less height gain, and a 28% lower adherence (missed 2 injections/week) with 0.22 SDS less height gain.
Table 2. Results of linear regression analyses with ΔHSDS and the parameters of the growth curves as outcome and adherence as dependent variable with and without adjustment for potential clinical confounders (n = 95).
| Outcome | Dependent | Model 1a B (SE) |
Model 2a Adj. B (SE) |
Model 3a Adj. B (SE) |
Model 4a Adj. B (SE) |
|---|---|---|---|---|---|
| ΔHSDS 0-1y | Adherence 0-1y (high vs lowb) | 0.19508 (0.07277)** | 0.15268 (0.06674)* | 0.15281 (0.06741)* | 0.156532 (0.086071) |
| Adherence 0-1y (in %) | 0.00114 (0.00115) | 0.00101 (0.00101) | 0.00111 (0.00111) | 0.00193 (0.00143) | |
| ΔHSDS 1-2y | Adherence 1-2y (high vs lowb) | 0.13908 (0.05231)** | 0.15606 (0.05261)** | 0.15972 (0.05250)** | 0.15416 (0.05783)* |
| Adherence 1-2y (in %) | 0.00254 (0.00115)* | 0.00282 (0.00115)* | 0.00294 (0.00115)* | 0.00306 (0.00113)** | |
| ΔHSDS 0-2y | Adherence 0-2y (high vs lowb)^ | 0.27744 (0.11175)* | 0.29110 (0.09754)** | 0.33681 (0.10161)** | 0.34356 (0.12583)** |
| Adherence 0-2y (in %) | 0.00465 (0.00230)* | 0.00500 (0.00205)* | 0.00596 (0.00216)** | 0.00792 (0.00253)** | |
| Ac | Adherence 0-2y (high vs lowb)^ | 0.2199 (0.2356) | 0.36290 (0.20320) | 0.42619 (0.21318)* | 0.38919 (0.26092) |
| Adherence 0-2y (in %) | 0.00406 (0.00481) | 0.00887 (0.00418)* | 0.01053 (0.00441)* | 0.01253 (0.00518) * | |
| kc | Adherence 0-2y (high vs lowb)^ | 0.004739 (0.071399) | -0.02255 (0.06929) | -0.04837(0.07252) | -0.06807 (0.08505) |
| Adherence 0-2y (in %) | -0.00086 (0.00145) | -0.00181 (0.00142) | -0.00256 (0.00149) | -0.00375 (0.00168)* | |
| Dd | Adherence 0-2y (high vs lowb)^ | 0.22788 (0.07813)** | 0.23054 (0.06822)** | 0.24612 (0.07175)** | 0.224221 (0.091026)* |
| Adherence 0-2y (in %) | 0.00318 (0.00163) | 0.00324 (0.00146)* | 0.00345 (0.00155)* | 0.00408 (0.00189)* | |
| Ed | Adherence 0-2y (high vs lowb)^ | 0.05306 (0.02061)* | 0.05134 (0.01975)* | 0.04698 (0.02077)* | 0.03313 (0.02403) |
| Adherence 0-2y (in %) | -0.00061 (0.00043) | -0.00056 (0.00042) | -0.00041 (0.00044) | -0.00030 (0.00050) | |
| IoRe 1y | Adherence 0-1y (high vs lowb) | 0.5456 (0.2442)* | |||
| Adherence 0-1y (in %) | -0.00299 (0.00381) | ||||
| IoRe 2y | Adherence 1-2y (high vs lowb) | 0.6237 (0.2078)** | |||
| Adherence 1-2y (in %) | 0.00882 (0.00466) |
aModel 1: Adherence
Model 2: Model 1 + age at start + adj-HSDS at start + birth weight SDS + gestational age (<37 weeks vs ≥37 weeks)
Model 3: Model 2 + GH dose
Model 4: Model 3 + GH max (n = 58)
bhigh ≥98%, low<98% adherence 0-1y, high ≥91%, low<91% adherence 1-2y, high ≥78%, low<78% adherence 0-2y
^Both adherence in the first and second year in the model
cParameters of the monomolecular growth curve: A-5 = attained adj-HSDS after two years-5, A*(1-B)-5 = adj-HSDS at start, k = growth rate.
dParameters of the second degree polynomial: D = slope (CUG), E = deceleration (deceleration after CUG)
eIndex of Responsiveness
*p<0.05
**p<0.01
Discussion
Our study is the first to show the effect of suboptimal adherence in a large group of patients with GHD using a reliable method to automatically assess adherence through an electronic device over the first two years of GH treatment (Easypod). The effect size of suboptimal adherence is dependent on the percentage of missed injections; if a cut-off of 78% is used, the loss of height gain was 0.34 SD, approximately 2–2.5 cm after two years of GH treatment.
It is noteworthy that average adherence in the first year was not associated with first year growth response, in contrast to the second year and both years combined. We believe that there are three explanations for this observation. The first is that average adherence in the first year is very high with little variation, which limits the power to detect statistically significant associations. Second, adherence has a skewed distribution, which may reduce the associations. Third, the effect of GH on growth is most prominent in the first year of treatment, with a relatively strong effect on growth velocity with relatively little effect of GH dose [3].
Our data show that in order to obtain a good insight into the effect of suboptimal adherence, two conditions have to be met. First, an outcome parameter has to be used that represents the natural shape of CUG. In the first years of treatment, CUG has the shape of a sharp increase of HSDS in the first year, followed by gradually decreasing height velocities until HSDS adjusted for TH-SDS is close to 0. HSDS remains stable until the onset of puberty, when the pubertal growth spurt begins. This implies that the choice for the outcome parameter is dependent on the number of years after start of GH treatment. In the first years, growth should be compared with a model for CUG, thereafter to a stable HSDS, and from pubertal onset the effect of adherence becomes impossible to analyze because of interference by the pubertal growth spurt. Our data show that over the first two years the change in HSDS gives similar or higher effects than the growth rate parameters of the two mathematical models. Although the cut-offs for adherence were based on the difference in HSDS, a similar cut-off for 0–2 y was found for the slope parameter D. We propose that this outcome measure may be superior to the various indicators of “poor response” reported in the literature [21].
The second condition that has to be met is that sufficient adjustment is made for clinical parameters that influence the growth response to GH treatment. In our study, adherence in the first two years strongly correlated with height gain 0–2 y, even unadjusted for clinical predictors. However, the effect of adherence became considerably stronger when adjustment was made to well established predictors.
While automatic recording of adherence is helpful to give insight into the frequency of suboptimal adherence and its effect on the growth response to GH treatment, for clinical care its most important benefit could be that it gives the clinician a signal to intervene and try to improve adherence. In theory, supportive accountability, including human support, motivational interviewing and communication “bandwidth” could be successful, and potentially successful approaches have been suggested [22,23,24]. However, controlled trials on the efficacy of such programs are lacking.
The limitations of this study include its non-interventional nature, which is associated with a high level of missing data, high inter-patient variability, and the absence of detailed recording of actions performed by health-care providers and carers when poor adherence and/or poor response to treatment was recorded. However, these limitations occur in all surveillance studies [25], whereas the observational nature means that it reflects normal clinical practice. Another limitation is that the cut-off values for defining a high and low adherence were constructed in the same dataset as the correlation analysis. This may have overestimated the correlations. However, similar conclusions could be drawn based on the adjusted correlations between ΔHSDS in the first, second and first two years and adherence in % (linear) and adherence in two categories (high versus low according to the cut-off values that we developed). Also, when we apply the Cutfield cut-off values [9] of high (≥86%) versus low adherence (<86%) in the first, second and first two years, similar significance levels were found with the exception of ΔHSDS in the first two years (Adj. B (SE) = 0.23156 (0.12338), p = 0.066). The cut-off value of ≥86% for a high adherence was chosen by Cutfield et al. [9], because it corresponds to no more than one missed dose a week on average. However, it is to be preferred to take the cut-off value that maximizes the correlation between adherence and height gain, because it provides more insight into the doses that are needed per several days, week or several weeks to have an optimal height gain. Further research is needed to investigate if the performance of the constructed cut-off values in our study provides similar correlations between high/low adherence and height gain in new datasets compared to our study. Another limitation of our study is that we did not include IGF-I measurements to assess GH status and predict growth response and adherence during GH therapy in our patients. IGF-I measurements were not available for the majority of patients, because ECOS was a surveillance study [26]. For this study, we only selected adherence from the easypod electronic drug-delivery device, growth outcomes and several clinical and background parameters as potential confounders.
Strengths include that it is the first study that has used a device with an eHealth platform to report adherence data directly from patients to health-care providers. A number of individual cases from ECOS have been reported [27,28,29,30]; these indicate that direct access to adherence monitoring can make the difference in a patient’s management and motivation. For example, a 14% lower adherence (missed 1 injection/week) in the first two years is associated with 14 x 0.00792 = 0.11 SD less height gain, and a 28% lower adherence (missed 2 injections/week) with 0.22 SDS less height gain.
The success of therapy depends mainly on the ability of the patients and their parents to carefully adhere to the recommended treatment regimen. Complex schedules should be avoided. This study shows that each missed injection/week in the first two years resulted in 0.11 SD less height gain, which implies that a daily routine of taking an injection, especially in the second year, is recommended. Although accurate and up-to-date adherence data can be obtained from the easypoddevice, at the moment this data is not directly available for the physician. So complete non-adherence to GH therapy is relatively easy to detect on the basis of growth failure, but suboptimal and/or intermittent adherence are more difficult to assess. Linking the adherence data from the easypod device to the physician can be helpful in the future. At the moment, regularly interviewing the patients and their parents are efficacious means of detecting the degree of adherence [31]. Different strategies can be incorporated to enhance adherence to GH therapy, i.e. providing early patient and parent education and support, medication reminder systems and longer duration of GH prescriptions [32]. A personalized approach seems to be promising, because a previous study on the ECOS data showed that several clinical parameters and background characteristics of the patients are important determinants to predict the level of adherence. Early age of self-administration, weight at start of treatment, and teenage years are associated with a lower adherence to GH treatment [33]. Patient adherence support programs that take into account these factors may improve adherence and subsequent clinical outcomes. Further studies are required to design personalized patient support programs to attain and maintain good adherence to GH treatment.
In conclusion, electronically monitoring adherence enables obtaining reliable information on adherence in a “real-life” situation, and shows a statistically significant effect on the growth response in the first two years of treatment in children with GHD. Each missed injection per week in the first two years, resulted in 0.11 SDS less height gain. The next step should be to develop interventional tools to explore the hypothesis that patient adherence support programs improve adherence and subsequent clinical outcomes.
Supporting information
(DOCX)
Acknowledgments
We are grateful to the patients and their families who participated in the study and to the members of the steering committee of the ECOS study: Alicia Belgorovski from Argentina, Martin Borkenstein from Austria, Peter Davies from Australia, John VanderMeulen from Canada, Milian Du from China, Jan Lebl from Czech Republic, Marc Nicolino from France, Evangelia Charmandari from Greece, Andrea Luczay from Hungary, Sandro Loche from Italy, Ho-Seong Kim from Korea, Raul Calzada Leon from Mexico, Ludmila Kostalova from Slovakia, Dolores Rodriguez Arnao from Spain, Svante Norgren from Sweden, and Jeremy Kirk from the United Kingdom.
Data Availability
The analysis was performed from data from ECOS study, which is Merck KgaA sponsored phase IV clinical trial, data sharing should be done in accordance with the European Federation of Pharmaceutical Industries and Associations (EFPIA) and the Pharmaceutical Research and Manufacturers of America’s (PhRMA) Principles for Responsible Clinical Trial Data Sharing. Merck KGaA, Darmstadt, Germany, believes that as a biopharmaceutical company, the sharing of information related to company sponsored clinical trials is central to our mission. The sharing of clinical trial Information enables the medical and scientific community to further develop the medical and scientific knowledge base and permits the public to make informed healthcare decisions. However, because information from company sponsored clinical trials may include confidential personal information and proprietary company information, Merck must ensure that information is provided only in response to legitimate scientific and medical requests and that information disseminated outside of the company properly protects all confidential. All clinical trial information must further be provided only in accordance with applicable laws and codes. Merck will share anonymized patient level, and study level data and redacted clinical study reports from clinical trials in patients with qualified scientific and medical researchers, upon researcher request via the Merck website portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html), as necessary for conducting legitimate research. More information for researchers can be found on this website. Evaluation of the Research Proposal, as well as the qualifications and experience of the Lead Researcher and Research Team, will be conducted by appropriate and qualified person or board. The researcher must enter into a Data Sharing Agreement (“DSA”) with Merck. The standard DSA which must be entered into is posted on Merck’s website. Merck uploads the data to Cloud-Based Data Sharing Analytical Solution where researchers can conduct the approved analysis. A request form is available on the Merck website portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). A point of contact for information regarding data requests is Sascha-Marc Seidl (Sascha-Marc.Seidl@merckgroup.com).
Funding Statement
This study was sponsored by Merck KGaA, Darmstadt, Germany. Medical writing assistance was provided by David Candlish, inScience Communications, Tattenhall, UK, and sponsored by Merck KGaA, Darmstadt, Germany. The data collection was performed via the easypodTM electronic drug-delivery device, which is a product of Merck KGaA. Also, physician data entry of outcome measures were retrospectively and prospectively collected by Merck KGaA. This data collection was part of the ECOS study, which was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice (ICH-GCP E6) guidelines and applicable national legal and regulatory requirements. The ECOS study was published in Endocrine Connections (Koledova E, Stoyanov G, Ovbude L, Davies PSW (2018) Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect. 7: 914-923). Post hoc analysis on the ECOS study was performed by PvD from TNO. TNO is an independent RTO from the Netherlands founded by law. TNO’s professionals put their knowledge and experience to work in creating smart solutions to complex issues. These innovations help to sustainably strengthen social wellbeing and industrial competitiveness. The core values of TNO are integrity, independence, professionalism, and engagement with society. TNO’s Code of Conduct, published on the website of TNO (https://www.tno.nl/en/about-tno/mission-and-strategy/tno-code), contains a chapter on scientific integrity. Research is conducted without any undue influence from commercial or other interests. Merck KGaA provided support in the form of salaries for author PvD, but did not have any additional role in performing the post hoc analysis. Merck has provided the ECOS data to TNO for this post hoc analysis. Manuscript was reviewed according to Merck publication procedures. The specific roles of all authors are articulated in the ‘author contributions’ section.
References
- 1.Kristrom B, Wikland KA (2002) Growth prediction models, concept and use. Horm Res 57 Suppl 2: 66–70. [DOI] [PubMed] [Google Scholar]
- 2.Westphal O, Lindberg A (2008) Final height in Swedish children with idiopathic growth hormone deficiency enrolled in KIGS treated optimally with growth hormone. Acta Paediatrica 97: 1698–1706. 10.1111/j.1651-2227.2008.01053.x [DOI] [PubMed] [Google Scholar]
- 3.Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, et al. (1999) Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab 84: 1174–1183. 10.1210/jcem.84.4.5634 [DOI] [PubMed] [Google Scholar]
- 4.Kristrom B, Jansson C, Rosberg S, Albertsson-Wikland K (1997) Growth response to growth hormone (GH) treatment relates to serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in short children with various GH secretion capacities. Swedish Study Group for Growth Hormone Treatment. J Clin Endocrinol Metab 82: 2889–2898. 10.1210/jcem.82.9.4234 [DOI] [PubMed] [Google Scholar]
- 5.Wikland KA, Kristrom B, Rosberg S, Svensson B, Nierop AF (2000) Validated multivariate models predicting the growth response to GH treatment in individual short children with a broad range in GH secretion capacities. Pediatr Res 48: 475–484. 10.1203/00006450-200010000-00010 [DOI] [PubMed] [Google Scholar]
- 6.Schonau E, Westermann F, Rauch F, Stabrey A, Wassmer G, Keller E, et al. (2001) A new and accurate prediction model for growth response to growth hormone treatment in children with growth hormone deficiency. Eur J Endocrinol 144: 13–20. [DOI] [PubMed] [Google Scholar]
- 7.Wit JM, Ranke MB, Albertsson-Wikland K, Carrascosa A, Rosenfeld RG, Van Buuren S, et al. (2013) Personalized approach to growth hormone treatment: clinical use of growth prediction models. Horm Res Paediatr 79: 257–270. 10.1159/000351025 [DOI] [PubMed] [Google Scholar]
- 8.Kapoor RR, Burke SA, Sparrow SE, Hughes IA, Dunger DB, Ong KK, et al. (2008) Monitoring of concordance in growth hormone therapy. Archives of Disease in Childhood 93: 147–148. 10.1136/adc.2006.114249 [DOI] [PubMed] [Google Scholar]
- 9.Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, et al. (2011) Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One 6: e16223 10.1371/journal.pone.0016223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher BG, Acerini CL (2013) Understanding the growth hormone therapy adherence paradigm: a systematic review. Hormone Research in Paediatrics 79: 189–196. 10.1159/000350251 [DOI] [PubMed] [Google Scholar]
- 11.Aydin BK, Aycan Z, Siklar Z, Berberoglu M, Ocal G, Cetinkaya S, et al. (2014) Adherence to growth hormone therapy: results of a multicenter study. Endocr Pract 20: 46–51. 10.4158/EP13194.OR [DOI] [PubMed] [Google Scholar]
- 12.De Pedro S, Murillo M, Salinas I, Granada ML, Martinez M, Puig-Domingo M, et al. (2016) Variability in adherence to rhGH treatment: Socioeconomic causes and effect on children's growth. Growth Horm IGF Res 26: 32–35. 10.1016/j.ghir.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Boersma B, Wynne HJ, Wit JM (1994) A mathematical model describing catch-up growth in celiac disease. Acta Paediatr 83: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 14.Dahlgren J, Veimo D, Johansson L, Bech I (2007) Patient acceptance of a novel electronic auto-injector device to administer recombinant human growth hormone: results from an open-label, user survey of everyday use. Current Medical Research and Opinion 23: 1649–1655. 10.1185/030079907X210589 [DOI] [PubMed] [Google Scholar]
- 15.Tauber M, Payen C, Cartault A, Jouret B, Edouard T, Roger D. (2008) User trial of Easypod, an electronic autoinjector for growth hormone. Annales d'endocrinologie 69: 511–516. 10.1016/j.ando.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Lion F (2010) Electronic recording of growth hormone dosing history: the easypod auto-injector. Current Drug Therapy 5: 271–276. [Google Scholar]
- 17.Koledova E, Stoyanov G, Ovbude L, Davies P (2018) Adherence and long-term growth: the easypod connect observational study (ECOS) in children with growth disorders. Endocrine Connections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breiman L, Friedman JH, Olshen RA, Stone CJ (1984) Classification and Regression Trees Boca Raton, Florida, USA: Chapman and Hall/CRC. [Google Scholar]
- 19.(2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 450: 76–85. [DOI] [PubMed] [Google Scholar]
- 20.Hermanussen M, Cole J (2003) The calculation of target height reconsidered. Horm Res 59: 180–183. 10.1159/000069321 [DOI] [PubMed] [Google Scholar]
- 21.Bang P, Bjerknes R, Dahlgren J, Dunkel L, Gustafsson J, Juul A, et al. (2011) A comparison of different definitions of growth response in short prepubertal children treated with growth hormone. Hormone Research in Paediatrics 75: 335–345. 10.1159/000322878 [DOI] [PubMed] [Google Scholar]
- 22.van Dongen N, Kaptein AA (2012) Parents' views on growth hormone treatment for their children: psychosocial issues. Patient Prefer Adherence 6: 547–553. 10.2147/PPA.S33157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haverkamp F, Norgren S, Horne R, Gasteyger C (2012) The role of electronic assessment of adherence in the education and counseling of children taking growth hormone: progress and challenges. Pediatr Endocrinol Rev 10: 199–208. [PubMed] [Google Scholar]
- 24.Mohr DC, Cuijpers P, Lehman K (2011) Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. Journal of Medical Internet Research 13: e30 10.2196/jmir.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranke M, Price D, Reiter E (2007) Growth Hormone Therapy in Pediatrics 20 Years of KIGS. Basel, Switzerland: Karger. [Google Scholar]
- 26.Koledova E, Stoyanov G, Ovbude L, Davies PSW (2018) Adherence and long-term growth outcomes: results from the easypo connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect 7: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Arnao M, Sánchez A, López I, Fernández J, Bermúdez de la Vega J, Ballano V, et al. (2016) Spanish ECOS Study Analysis: Socioeconomic data, adherence and growth outcomes with case Studies. Hormone Research in Paediatrics 86: 381. [Google Scholar]
- 28.Nicolino M, Coutant R, Tauber M, López Y. Easypod Connect Observational Study (ECOS)–French case histories and growth outcomes2017; Washington DC, USA: Horm Res Paediatr; pp. 1–628. [Google Scholar]
- 29.Ayala-Estrada A, Antillon-Ferreira C, Saavedra-Castillo E, Barrientos-Pérez M, Rivero-Escalante H, Flores-Caloca O, et al. (2017) The Easypod Connect Observational Study (ECOS): Descriptive analysis of adherence to treatment of growth hormone deficient and small for gestational age naïve to easypod patients in Mexico 2012–2015. Horm Res Paediatr 86: 76. [Google Scholar]
- 30.Stoyanov G, Koledova E, Vandermeulen J, The Canadian ECOS group. Objectively measured treatment adherence in the easypod connect observational study (ECOS) Canadian interim analysis: population data and case reports 18–20 Feb 2016; Edmonton, Canada: CPEG-GCEP. [Google Scholar]
- 31.Bozzola M, Pagani S, Iughetti L, Maffeis C, Bozzola E, Meazza C (2014) Adherence to growth hormone therapy: a practical approach. Horm Res Paediatr 81: 331–335. 10.1159/000357975 [DOI] [PubMed] [Google Scholar]
- 32.Mohseni S, Heydari Z, Qorbani M, Radfar M (2018) Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab 31: 13–20. 10.1515/jpem-2017-0157 [DOI] [PubMed] [Google Scholar]
- 33.van Dommelen P, Koledova E, Wit JM (2018) Low adherence to growth hormone treatment depends on a child’s age, weight and whether they inject themselves. Endocrine Reviews 39, 2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The analysis was performed from data from ECOS study, which is Merck KgaA sponsored phase IV clinical trial, data sharing should be done in accordance with the European Federation of Pharmaceutical Industries and Associations (EFPIA) and the Pharmaceutical Research and Manufacturers of America’s (PhRMA) Principles for Responsible Clinical Trial Data Sharing. Merck KGaA, Darmstadt, Germany, believes that as a biopharmaceutical company, the sharing of information related to company sponsored clinical trials is central to our mission. The sharing of clinical trial Information enables the medical and scientific community to further develop the medical and scientific knowledge base and permits the public to make informed healthcare decisions. However, because information from company sponsored clinical trials may include confidential personal information and proprietary company information, Merck must ensure that information is provided only in response to legitimate scientific and medical requests and that information disseminated outside of the company properly protects all confidential. All clinical trial information must further be provided only in accordance with applicable laws and codes. Merck will share anonymized patient level, and study level data and redacted clinical study reports from clinical trials in patients with qualified scientific and medical researchers, upon researcher request via the Merck website portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html), as necessary for conducting legitimate research. More information for researchers can be found on this website. Evaluation of the Research Proposal, as well as the qualifications and experience of the Lead Researcher and Research Team, will be conducted by appropriate and qualified person or board. The researcher must enter into a Data Sharing Agreement (“DSA”) with Merck. The standard DSA which must be entered into is posted on Merck’s website. Merck uploads the data to Cloud-Based Data Sharing Analytical Solution where researchers can conduct the approved analysis. A request form is available on the Merck website portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). A point of contact for information regarding data requests is Sascha-Marc Seidl (Sascha-Marc.Seidl@merckgroup.com).