Abstract
Biomarkers have been postulated as essential variables to measure the effects of exercise on the human body. To investigate the relationship between physical fitness (PF) and blood biomarkers that are associated with disease risk in Spanish older adults, four hundred and twenty-nine adults (57% females) aged older than 55 years from a cross-sectional study were included. A battery of PF test was performed, and participants were divided into 3 groups: low, medium and high fitness. Blood samples were collected, and subjects were also grouped based on a particular biomarker being within its reference range. Furthermore, drug intake and dietary intake were considered for each participant. Higher concentrations out of the reference range were observed for vitamin 25(OH)D (67.9%) and total cholesterol (TC) (58.6%). Participants from the low PF group presented lower significant concentrations out of the reference range for vitamin B12 and triglycerides; however, participants in the low PF group showed higher significant concentrations out of the reference range for total homocysteine, creatinine, TC, HDL-cholesterol and LDL-cholesterol (LDL-c) than those in the high PF group (all p<0.05). Considering drugs related to blood lipid modifications, subjects who regularly consumed lipid reducers presented higher significant concentrations out of the reference range for TC and LDL-c than participants who did not take these drugs (p<0.01). Participants from the high PF group presented better blood marker profiles, namely, lower blood markers related to disease risk out of the reference range. These blood markers could be used as a routine method for considering PF groups in older adults.
Introduction
There is an increasing need to evaluate health-related aspects (i.e., biomarkers) that can be modified by regular physical exercise [1] to provide new evidence and strategies to achieve healthy aging [2]. Aging phenomena are linked with an increased risk of cognitive dysfunction [3] and chronic diseases [4], among other issues. Both factors contribute to changes in motor function and impairments in physical performance in the older adult population. These changes could affect the ability to perform daily activities in this type of population [5].
The pro-healthy effects of regular physical activity (PA) are well-documented [6,7], and previous studies have revealed that physical fitness (PF) is a crucial independent predictor of mortality [8,9]. Attributable risk estimates for all-cause mortality show that low PF, principally low cardiorespiratory fitness and low muscular strength, is a significant risk factor in both sexes [9,10]. PF mixes most of the body functions that are involved in the performance of daily PA [11]. Decreases in PF are related to several negative health effects [12], and biomarkers have been proposed as essential health markers to evaluate the effects of physical exercise on the human body [1,13].
Vitamin deficiencies can cause disorders in behavior, cognitive and emotional status, and personality [14]. Concretely, epidemiologic data indicates that the prevalence of vitamin B12 deficiency is from 6% to 40% [15]. Additionally, the worldwide prevalence of pernicious anemia has been estimated to be 24% in older adults (aged over 65 years) [16]. B vitamins and high total homocysteine (tHcy) concentrations have been connected with cardiovascular disease (especially stroke) [17,18], cognitive decline and dementia [14,19,20], fractures [16,21] and mortality [22,23]. Likewise, low vitamin D status may stimulate adipogenesis [24], resulting in further increases in adiposity. Low vitamin D concentrations are also linked with muscle pain, muscle weakness [25,26] and poor PF in older adults [27].
High concentration of low lipoprotein density cholesterol (LDL-c) and low concentration of high lipoprotein density cholesterol (HDL-c) can increase the progression of coronary heart disease [28]. With increasing age, some changes in lipid and lipoprotein concentrations are overall unfavorable [29]. Furthermore, high cardiorespiratory fitness is related to a better blood lipid profile [29].
Through the analysis of different biomarkers, it could be possible to evaluate the reactions of the human body at several levels of PF [1]. However, research regarding the relationship between biomarkers and PF in the older adult population is still ongoing. Hence, the main purpose of this study was to investigate the relationship between blood biomarkers and PF groups associated with disease risk (more blood marker concentrations out of the reference range) in Spanish older adults.
Methods and materials
Study design, sample and ethics
The PHYSMED project is a multicenter, cross-sectional study aiming to identify cardiovascular risk factors in sedentary and active older adult subjects. Data were collected from April 2013 to May 2014, and field work took place in 2 Spanish regions: Madrid and Balearic Island. Participants were recruited through health centers, sport federations, sport facilities and municipal clubs located in Madrid and Balearic Island following a snowball system. The study population included 429 adults (57% females) aged 55–88 years. The individual exclusion criteria were as follows: individuals younger than 55 years, those who were institutionalized, and those suffering from a physical or mental illness that would have limited their participation in the physical fitness tests or their ability to respond to the questionnaires or drug intake for clinical research. Each participant signed a written informed consent prior to his/her participation.
The study followed the Declaration of Helsinki 1964 and further amendments. Additionally, the protocol study was approved by the Ethical Committee of the Universidad Politécnica de Madrid.
Specimen collection and biochemical analyses
Fasted (12 h) blood samples were collected from each participant, and lipid profiles and basic biochemical analyses were performed at the biochemical laboratories of the High Sports Council (Madrid) and Hospital Son Espases & University of Balearic Islands (Palma de Mallorca). The extraction was performed by standard venipuncture using vacuum Vacutainer tubes with separation gel. The tubes were immediately placed on ice, and after coagulum formation, the sample was centrifuged at 3,000 rpm for 10 minutes. Serum total cholesterol (TC), HDL-c, LDL-c, triglycerides (TG), glucose, urea, uric acid, total protein, albumin, glutamic oxalacetic transaminase, glutamic-pyruvic transaminase, gamma glutamyl transpeptidase, iron (FE) and ferritin (FER) were analyzed using a Beckman AU400 analyzer (Beckman AU400, Beckman Instruments, Ltd., Bucks, UK) by photometric methods. Creatinine was analyzed by a colorimetric method (Beckman AU400, Beckman Instruments, Ltd., Bucks, UK). Hematocrit and hemoglobin analyses were performed within the first hour after extraction in EDTA tubes using an automated hematology analyzer (ADVIA 120 Siemens Health Care Diagnostics, SA). These determinations were carried out in each site.
Vitamin B12, serum folate (sfolate), red blood cell folate (RBC folate, from EDTA tubes), vitamin D 25[OH]D and tHcy analyses were centralized at the High Sports Council Laboratory in Madrid. Therefore, serum samples from Palma de Mallorca were dry-ice shipped to Madrid and analyzed together with serum samples stored in Madrid at -80 °C, by an electrochemiluminescence method (Elecsys 2010, Roche Diagnostics, IN, USA), except vitamin D 25[OH]D, which was determined using an E411 analyzer (Roche Diagnostics, Switzerland).
Physical fitness tests
Each participant completed a multicomponent battery of PF tests that have been validated in the older population [30] and in Spanish older adults proposed by Pedrero-Chamizo et al. [31]. Lower body strength was measured by the chair stand test, agility/dynamic balance was performed by the 8-foot up-and-go test, aerobic endurance was assessed by the 6-min walk test, and handgrip strength was measured with a handgrip dynamometer (Takei TKK 5401, Tokyo, Japan, range = 5–100 kg, precision = 0.1 kg) [32]. The handgrip strength was assessed for both hands in a standing position. All tests were performed twice, except the 6-min walk test and the chair stand test, and the best score was retained.
The results of each PF test were stratified by sex and five age groups [divided by five-year periods, except the last group] following the criteria established by Pedrero-Chamizo et al. [31]. The score for each test ranged from 0 (worst) to 3 (best) points. Thus, the maximum score was 12 points. The scores of the PF tests were added together to create a cluster. After that, to classify our population, the PF cluster was divided into 3 different groups: low, medium and high.
Anthropometric measurements
Weight, body mass index (BMI), total body water (TBW) and fat free mass (FFM) were measured using bioimpedance analysis (TANITA Corp, BC-418MA, Tokyo, Japan) in standardized conditions. Likewise, BMI was calculated as the weight (kg) divided by the square of the height (m). A trained anthropometrist according to the International Society measured waist and hip circumference for the Advancement of Kinanthropometry. Height was assessed to the nearest millimeter using a mobile stadiometer (SECA 213, Germany), with the participant’s head in the Frankfurt plane.
Dietary assessment
Dietary intake was obtained by means of two 24-h dietary recalls collected on two nonconsecutive days within a period of 2–3 weeks by well-trained dieticians. A computer program was used to convert food into nutrients (ALIMENTA; NUCOX, Palma, Spain) based on Spanish [33,34] and European [35] food composition tables and complemented with food composition data available for Majorcan food items [36].
Socioeconomic and lifestyle questionnaire
A general questionnaire was used including smoking habits, and participants were grouped in categories as follows: (i) educational level: primary school, secondary school and college-level education; (ii) current income: <600 €/month, 600–900€/month and ≥900 €/month; and (iii) smoker (≥1 cigarette/day) and nonsmoker.
Medication intake
Participants were asked by means of the EXERNET questionnaire the following question: Do you regularly take drugs? (yes/no). If they answered yes, they were asked about type of medication, manufacturer, frequency of consumption and dose of each drug. Each drug was coded following the Spanish Agency of Medicines and Sanitary Products (https://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=detalleForm). Four groups of drug intake were considered: antidiabetics, renin-angiotensin system drugs, beta blockers and lipid-lowering drugs; all variables were grouped if participants took (coded as 1) or did not take each of the drugs (coded as 0).
Statistical analysis
Statistics were performed using the statistical software SPSS (IBM Corp. Released 2012. Statistics for Window, V 21.0. Armonk, NY). Descriptive characteristics were summarized by calculating means and standard deviations unless otherwise stated. Each variable was checked for normality of distribution using the Kolmogorov-Smirnov test. The urea, uric acid, total protein, albumin, TC, HDL-c, LDL-c, hemoglobin, hematocrit and vitamin 25[OH]D concentrations showed a normal distribution. The differences between descriptive characteristics and sexes and the differences between biomarkers and PF groups were performed using a one-way ANOVA (for normally distributed variables) and the Kruskal-Wallis test (for nonnormally distributed variables). Post hoc analyses were conducted with the Bonferroni adjustment. All variables of the PF tests were checked for normality of distribution by the Kolmogorov-Smirnov test, and these presented a normal distribution.
Regarding vitamin D, the season and the date of blood sample collection were considered for statistical analysis. Samples collected from October to March were classified as “autumn-winter samples”, and those collected from April to September were classified as “spring-summer samples”. The latitudes of each city are similar: Mallorca (39° 34’ N) and Madrid (40° 24’ N).
Likewise, reference ranges established by each laboratory were used to categorize each biomarker as in or out of the reference range (See S1 Table). For vitamin B12 and related biomarkers, a different cut-off was applied [15]. Each biomarker within its reference range was coded as 1, and each biomarker out of its reference range was coded as 0.
The association between the status of each biomarker and the PF groups was assessed using the general lineal model. Each biomarker coded as 0 (out of the reference range) was used as a reference in this statistical model. The PF groups were used as an independent variable in which the high PF group was considered the reference. Depending on the biomarkers, drug intake was included into the model as an independent variable in which drug intake was the reference. Moreover, several diet covariables were considered depending on the biomarkers analyzed. The model was not considered when the level of significance of the omnibus test was higher than 0.05. In turn, the lower Akaike information criterion (AIC) was chosen in the final models. Albumin was not analyzed by means of the generalized linear model because only one participant presented abnormal concentrations. Values of p<0.05 were considered statistically significant.
Results
Table 1 includes the subjects’ characteristics split by PF groups.
Table 1. Descriptive characteristics of the studied sample split by PF groups.
| Physical fitness groups | ||||
|---|---|---|---|---|
| Low (n = 131) | Medium (n = 172) | High (n = 126) | p-value | |
| Mean±SD Median (min-max) |
Mean±SD Median (min-max) |
Mean±SD Median (min-max) |
||
| Sex (female) | 76 (58.6) | 98 (56.9) | 70 (55.6) | >0.05 |
| Age (y) | 66.8±7.1 66.0 (55.0–87.8) |
66.6±6.6 66.0 (55.0–85.4) |
66.5±6.2 66.0 (55.1–80.8) |
>0.05 |
| City (Madrid) | 30 (22.6) | 83 (47.7) | 87 (69.0) | >0.05 |
| Height (cm) | 161.2±8.8 159.5 (143.0–183.0) |
162.2±9.2 160.0 (142.0–184.0) |
163.9±8.9 164.0 (145.5–185.5) |
<0.05b |
| Weight (kg) | 72.4±13.5 70.3 (44.9–116.6) |
72.9±12.9 71.5 (44.0–99.5) |
69.1±11.4 68.2 (46.1–104.6 |
<0.05c |
| BMI (kg/m2) | 27.8±3.6 27.4 (17.1–39.1) |
27.7±4.1 27.5 (18.4–41.8) |
25.6±3.1 25.5 (17.0–33.4) |
<0.001 a,b,c |
| Waist circumference (cm) | 91.6±10.9 91.4 (67.0–121.3) |
90.2±11.5 89.9 (61.3–113.7) |
86.2±11.2 85.2 (62.7–124.7) |
<0.001 b,c |
| Hip circumference (cm) | 102.6±8.1 102.0 (82.7–140.0) |
102.5±8.3 102.0 (84–136.8) |
99.2±6.7 99.1 (84.0–121.8) |
<0.001 b,c |
| Energy (kcal/day) | 1666±486 1618 (540.7–3121) |
1650±438 1603 (722–3154) |
1778 ±492 1722 (884–3515) |
<0.01b |
| Smoker | 16 (12.0) | 6 (3.5) | 12 (9.5) | <0.05a |
| Education | ||||
| Primary school | 78 (58.6) | 30 (22.6) | 25 (18.8) | <0.05 a,b,c |
| Secondary school | 68 (39.9) | 57 (34.1) | 45 (26.0) | |
| University graduate | 40 (31.8) | 41 (32.5) | 45 (35.7) | |
| Current income | ||||
| <600 €/month | 43 (32.3) | 19 (14.3) | 71 (53.4) | <0.001 b,c |
| 600–900 €/month | 41 (24.1) | 23 (13.5) | 106 (62.4) | |
| >900 €/month | 11 (8.7) | 17 (13.5) | 98 (77.8) | |
| Systolic blood pressure (mm Hg) | 141.0±18.3 138.8 (93.5–219.5) |
140.3±17.6 139.3 (100-213-5) |
139.6±17.2 139.0 (104.5–185.5) |
>0.05 |
| Diastolic blood pressure (mm Hg) | 81.0±9.8 81.0 (54.5–108.5) |
79.4±10.4 79.5 (50.0–126.0) |
77.5±8.1 76.5 (58.0–102.0) |
<0.05a |
Data are presented as the means±SD; median (minimum-maximum); n, no. of subjects and values (%). BMI, body mass index. Comparisons between physical fitness groups were analyzed by one-way ANOVA or the Kruskal-Wallis test, according to the normality of the variables. Post hoc analyses were conducted with the Bonferroni adjustment.
a: Low fitness vs. medium fitness;
b: low fitness vs. high fitness;
c: medium fitness vs. high fitness.
Level of significance p<0.05.
The descriptive data of biomarkers according to PF groups are shown in Table 2. Blood levels of creatinine (p<0.001), TC (p<0.05), HDL-c (p<0.001), LDL-c (p<0.01), sfolate (p<0.001) and RBC folate (p<0.01) were significantly higher in the high PF group than in the low and medium PF groups. Participants from the high PF group also showed lower significant blood levels than those in the low and medium PF groups for total protein and TG concentrations (both p<0.05).
Table 2. Descriptive data of biomarkers divided by PF groups.
| Biomarkers | Physical fitness groups | ||||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | |||||
| n | Mean±SD Median (min-max) |
n | Mean±SD Median (min-max) |
n | Mean±SD Median (min-max) |
p-value | |
| Glucose (mg/dL) | 129 | 99.5±15.7 96.0 (75.0–190.0) |
172 | 99.1±12.7 98.0 (75.0–144.0) |
126 | 98.1±14.8 97.0 (71.0–181.0) |
>0.05 |
| Urea (mg/dL) | 130 | 36.3±9.3 35.9 (19.0–69.0) |
171 | 37.5±8.2 37.0 (20.0–59.8) |
126 | 37.5±8.2 37.0 (17.7–62.2) |
>0.05 |
| Uric acid (mg/dL) | 130 | 5.4±1.3 5.1 (2.8–9.4) |
171 | 5.4±1.4 5.5 (2.6–9.4) |
126 | 5.3±1.3 5.2 (3.0–8.9) |
>0.05 |
| Creatinine (mg/dL) | 131 | 0.82±0.21 0.78 (0.53–2.18) |
172 | 0.86±0.17 0.84 (0.57–1.46) |
126 | 0.93±0.17 0.91 (0.64–1.41) |
<0.001 |
| Total protein (g/L) | 62 | 70.4±3.6 70.0 (60.5–81.0) |
116 | 70.3±3.9 70.0 (62.0–81.0) |
97 | 69.0±3.7 69.0 (60.0–78.0) |
<0.05 |
| Albumin (g/L) | 61 | 42.2±2.1 42.0 (37.7–46.0) |
116 | 42.1±2.1 42.0 (37.0–49.0) |
97 | 42.0±2.5 42.0 (35.0–48.0) |
>0.05 |
| TC (mg/dL) | 130 | 204.0±35.3 198.5 (112.0–293.0) |
172 | 210.8±34.6 206.0 (141.0–351.0) |
126 | 218.4±33.3 219.0 (131.0–321.0) |
<0.01 |
| HDL-c (mg/dL) | 129 | 53.0±12.6 53.0 (20.0–91.0) |
169 | 55.8±13.1 54.5 (27.7–95.2) |
124 | 59.2±13.5 57.9 (32.6–98.0) |
<0.001 |
| LDL-c (mg/dL) | 129 | 129.7±30.1 126.0 (69.4–218.8) |
169 | 135.1±29.6 134.0 (69.0–249.5) |
124 | 141.3±27.4 142.7 (71.4–224.8) |
<0.01 |
| TG (mg/dL) | 130 | 104.3±38.4 97.2 (33.0–208.0) |
172 | 99.1±40.8 90.1 (45.2–268.0) |
126 | 93.9±37.9 84.1 (40.6–274.1) |
<0.05 |
| Hematocrit (%) | 131 | 43.4 | 168 | 43.3 | 125 | 43.1 | >0.05 |
| Hemoglobin (g/dL) | 131 | 14.6±1.2 14.7 (12.0–17.0) |
168 | 14.5±1.1 14.3 (12.0–18.0) |
125 | 14.5±1.0 14.6 (12.0–17.0) |
>0.05 |
| FE (ug/dL) | 61 | 93.1±23.8 94.0 (38.2–137.5) |
115 | 91.6±33.9 87.5 (38.3–331.0) |
98 | 93.8±28.2 90.7 (45.4–201.2) |
>0.05 |
| FER (ng/dL) | 60 | 122.3±101.0 110.7 (12.0–671.5) |
115 | 125.5±99.5 93.6 (13.0–542.0) |
96 | 126.6±93.4 98.0 (16.8–419.3) |
>0.05 |
| tHcy (μmol /dL) | 111 | 13.4±4.6 12.8 (1.8–35.5) |
164 | 12.9±4.4 12.2 (2.0–31.3) |
124 | 12.3±4.0 11.6 (3.9–34.2) |
= 0.090 |
| Vitamin B12 (pg/ mL) | 109 | 375.8±181.2 345.3 (86.5–1163.0) |
163 | 390.3±202.0 343.7 (122.8–1650.0) |
124 | 386.4±172.4 350.2 (100.0–1402.0) |
>0.05 |
| sfolate (ng/ mL) | 111 | 10.1±4.2 8.8 (2.7–32.7) |
164 | 11.1±4.4 10.4 (3.9–29.3) |
124 | 11.9±4.2 11.7 (4.7–29.9) |
<0.001 |
| RBC folate (ng/ mL) | 91 | 345.9±117.2 326.9 (156.1–728.8) |
133 | 369.2±114.5 351.2 (184.2–862.6) |
113 | 384.2±105.5 379.1 (149.0–710.0) |
<0.01 |
| Vitamin D (25(OH)D) (mg/mL) | 109 | 25.6±10.3 24.1 (4.5–51.9) |
154 | 25.9±9.8 25.4 (4.3–64.4) |
120 | 26.0±10.5 25.5 (6.7–57.7) |
>0.05 |
Values are presented as the means±SD; n, median (minimum-maximum); no. of subjects. sfolate, serum folate; RBC folate; red blood cell folate; tHcy, total homocysteine; TC, total cholesterol; TG, triglycerides; HDL-c, HDL-cholesterol; LDL-c, LDL-cholesterol; FE, iron, FER, ferritin. Comparisons between physical fitness groups were analyzed by one-way ANOVA or the Kruskal-Wallis test, according to the normality of the variables. Level of significance p<0.05.
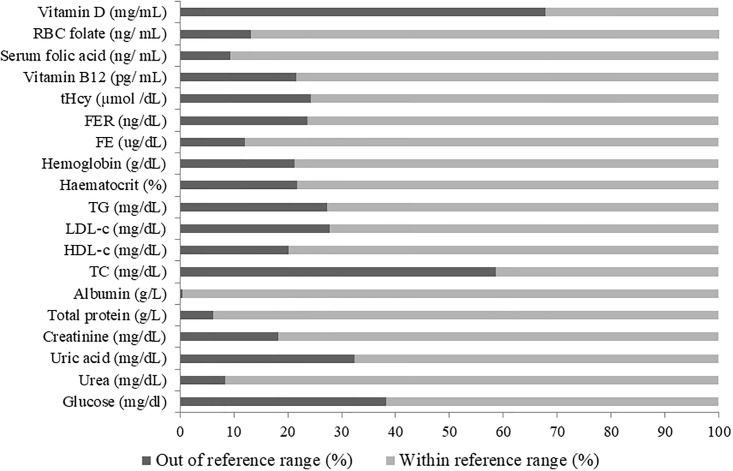
Fig 1 displays the percentage of each biomarker in and out of the reference range. A total of 67.9% and 58.6% of the sample presented low vitamin 25[OH]D concentrations and high TC concentrations, respectively, that were out of the reference range. Likewise, several blood markers including glucose (38.3%), uric acid (32.3%), LDL-c (27.7%) and tHcy (4.3%) that were higher than the reference range.
Fig 1. Percentage of each biomarker in and out of the reference range.
Values are presented as percentages (%). sfolate, serum folate; tHcy, total homocysteine; TC, total cholesterol; TG, triglycerides; HDL-c, HDL-cholesterol; LDL-c, LDL-cholesterol; FE, iron, FER, ferritin.
Results from the general linear model investigating the associations between blood biomarkers and PF groups are shown in Table 3. Participants from the low PF group had lower vitamin B12 concentrations out of the reference range (both models, p<0.05). However, participants in the low PF group showed greater tHcy concentrations out of the reference range than those from the high PF group (both models, p<0.05). Furthermore, vitamin B12 deficiency and tHcy concentrations in males increased significantly in parallel with advanced age (p<0.05). Likewise, high total protein concentrations out of reference range were observed in the low PF (both models, p>0.05) and medium PF groups (both model, p>0.05) compared to the high PF group. Participants from the low PF group also had a higher likelihood of having creatinine concentrations out of the reference range than those from the high PF group (both models, p<0.001).
Table 3. Association between biomarkers and physical fitness.
| Dependent variable: Creatinine (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 1.431 | 0.000 | Male | 1.668 | 0.025 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.017 | 0.402 | Age (y) | 0.017 | 0.433 |
| Low fitness | -1.323 | 0.000 | Weight (kg) | 0.018 | 0.450 |
| Medium fitness | -0.696 | 0.062 | Height (cm) | 0.009 | 0.763 |
| High fitness | 0.000 | FFM (kg) | -0.035 | 0.560 | |
| Protein intake (g) | 0.007 | 0.288 | Low fitness | -1.381 | 0.000 |
| Medium fitness | -0.727 | 0.057 | |||
| High fitness | 0.000 | ||||
| Protein intake (g) | 0.008 | 0.277 | |||
| Dependent variable: Total protein (g/L) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 0.805 | 0.195 | Male | 0.607 | 0.360 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.005 | 0.894 | Age (y) | 0.038 | 0.364 |
| Low fitness | 2.002 | 0.061 | Low fitness | 2.058 | 0.063 |
| Medium fitness | 1.039 | 0.069 | Medium fitness | 1.048 | 0.082 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Protein intake (g) | 0.026 | 0.737 | GOT (U/L) | -0.013 | 0.846 |
| Vegetal intake (g) | -0.050 | 0.539 | GPT (U/L) | 0.030 | 0.066 |
| Animal intake (g) | 0.004 | 0.955 | GGT (U/L) | -0.001 | 0.963 |
| Dependent variable: Total cholesterol (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 1.092 | 0.000 | Male | 1.110 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.023 | 0.151 | Age (y) | 0.025 | 0.125 |
| Low fitness | 1.028 | 0.000 | Low fitness | 1.036 | 0.000 |
| Medium fitness | 0.546 | 0.038 | Medium fitness | 0.600 | 0.025 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Total cholesterol intake (mg) | 0.000 | 0.473 | Total cholesterol intake (mg) | 0.000 | 0.629 |
| SFA intake (g) | -0.106 | 0.037 | |||
| No lipid-lowering drugs | -0.962 | 0.000 | MUFA intake (g) | -0.109 | 0.033 |
| Yes lipid-lowering drugs | 0.000 | PUFA intake (g) | -0.148 | 0.025 | |
| Lipid intake (g) | 0.103 | 0.021 | |||
| No lipid-lowering drugs | -0.973 | 0.000 | |||
| Yes lipid-lowering drugs | 0.000 | ||||
| Dependent variable: HDL-cholesterol (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -1.829 | 0.000 | Male | -1.964 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.002 | 0.910 | Age (y) | 0.007 | 0.701 |
| Low fitness | -0.901 | 0.007 | Low fitness | -0.917 | 0.007 |
| Medium fitness | -0.391 | 0.231 | Medium fitness | -0.384 | 0.246 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Total cholesterol intake (mg) | -0.001 | 0.375 | Total cholesterol intake (mg) | -0.001 | 0.172 |
| No lipid-lowering drugs | -0.148 | 0.625 | SFA intake (g) | 0.091 | 0.119 |
| Yes lipid-lowering drugs | 0.000 | MUFA intake (g) | 0.068 | 0.225 | |
| PUFA intake (g) | 0.025 | 0.723 | |||
| Lipid intake (g) | -0.054 | 0.272 | |||
| No lipid-lowering drugs | -0.216 | 0.486 | |||
| Yes lipid-lowering drugs | 0.000 | ||||
| Dependent variable: LDL-cholesterol (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 0.297 | 0.158 | Male | 0.240 | 0.262 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.026 | 0.132 | Age (y) | 0.032 | 0.078 |
| Low fitness | 0.563 | 0.051 | Low fitness | 0.651 | 0.029 |
| Medium fitness | 0.488 | 0.065 | Medium fitness | 0.589 | 0.030 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Total cholesterol intake (mg) | 0.237 | 0.180 | Total cholesterol intake (mg) | -0.002 | 0.064 |
| No lipid-lowering drugs | -1.090 | 0.001 | SFA intake (g) | -0.079 | 0.165 |
| Yes lipid-lowering drugs | 0.000 | MUFA intake (g) | -0.129 | 0.024 | |
| PUFA intake (g) | -0.015 | 0.904 | |||
| Lipid intake (g) | 0.009 | 0.061 | |||
| No lipid-lowering drugs | -1.130 | 0.001 | |||
| Yes lipid-lowering drugs | 0.000 | ||||
| Dependent variable: Triglycerides (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -0.436 | 0.056 | Male | -0.557 | 0.022 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.026 | 0.132 | Age (y) | 0.006 | 0.745 |
| Low fitness | -0.720 | 0.013 | Low fitness | -0.694 | 0.020 |
| Medium fitness | -0.387 | 0.170 | Medium fitness | -0.312 | 0.274 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Total cholesterol intake (mg) | 0.000 | 0.803 | Total cholesterol intake (mg) | -0.001 | 0.209 |
| No lipid-lowering drugs | 0.437 | 0.079 | SFA intake (g) | -0.116 | 0.053 |
| Yes lipid-lowering drugs | 0.000 | MUFA intake (g) | -0.166 | 0.007 | |
| PUFA intake (g) | -0.123 | 0.104 | |||
| Lipid intake (g) | 0.141 | 0.009 | |||
| No lipid-lowering drugs | 0.423 | 0.095 | |||
| Yes lipid-lowering drugs | 0.000 | ||||
| Dependent variable: Total homocysteine (μmol /dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -1.284 | 0.000 | Male | -1.287 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | -0.046 | 0.014 | Age (y) | -0.047 | 0.014 |
| Low fitness | -0.723 | 0.030 | Low fitness | -0.716 | 0.033 |
| Medium fitness | -0.334 | 0.283 | Medium fitness | -0.329 | 0.285 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Vitamin B12 intake (ug) | 0.035 | 0.080 | Vitamin B12 intake (ug) | 0.034 | 0.113 |
| Folate intake (ug) | |||||
| Yes Renin-angiotensin system drug | 0.542 | 0.053 | Yes Renin-angiotensin system drug | 0.541 | 0.053 |
| No Renin-angiotensin system drug | 0.000 | No Renin-angiotensin system drug | 0.000 | ||
| Yes beta blocker | 0.352 | 0.445 | Yes beta blocker | 0.355 | 0.442 |
| No beta blocker | 0.000 | No beta blocker | 0.000 | ||
| Dependent variable: Vitamin B12 (pg/mL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -0.747 | 0.004 | Male | -0.749 | 0.004 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | -0.053 | 0.006 | Age (y) | -0.053 | 0.005 |
| Low fitness | -0.847 | 0.020 | Low fitness | -0.757 | 0.028 |
| Medium fitness | -0.509 | 0.099 | Medium fitness | -0.539 | 0.091 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Protein intake (g) | 0.005 | 0.468 | Vitamin B12 intake (ug) | 0.019 | 0.265 |
| Folate intake (ug) | 0.000 | 0.692 | |||
| Dependent variable: Glucose (mg/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -10.962 | 0.002 | Male | -1.738 | 0.015 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | -0.019 | 0.245 | Age (y) | -0.012 | 0.492 |
| Low fitness | -0.433 | 0.282 | Low fitness | -0.275 | 0.513 |
| Medium fitness | -0.307 | 0423 | Medium fitness | -0.022 | 0.957 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Carbohydrate intake (g) | 0.000 | 0.896 | Weight (kg) | 0.064 | 0.185 |
| Mono & Disaccharides intake (g) | 0.004 | 0.616 | BMI (kg/m2) | -0.227 | 0.011 |
| Polysaccharides intake (g) | -0.002 | 0.756 | FFM (kg) | -6-323 | 0.108 |
| TBW (KG) | 8.634 | 0.108 | |||
| Yes antidiabetic drug | 4.366 | 0.000 | Yes antidiabetic drug | 4.339 | 0.000 |
| No antidiabetic drug | 0.000 | No antidiabetic drug | 0.000 | ||
| Dependent variable: Uric acid (mg/dL) | |||||
| Model 1 | β | p-value | |||
| Male | -2.249 | 0.000 | |||
| Female | 0.000 | ||||
| Age (y) | -0.005 | 0.783 | |||
| Low fitness | -0.135 | 0.663 | |||
| Medium fitness | -0.420 | 0.145 | |||
| High fitness | 0.000 | ||||
| Protein intake (g) | 0.006 | 0.261 | |||
| Dependent variable: Urea (mg/dL) | |||||
| Model 1 | β | p-value | |||
| Male | -0.650 | 0.096 | |||
| Female | 0.000 | ||||
| Age (y) | -0.038 | 0.165 | |||
| Low fitness | -0.213 | 0.639 | |||
| Medium fitness | 0.059 | 0.897 | |||
| High fitness | 0.000 | ||||
| Protein intake (g) | -0.018 | 0.005 | |||
| Dependent variable: Hematocrit (%) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -2.568 | 0.000 | Male | -1.746 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Low fitness | -0.429 | 0.249 | Low fitness | -0.608 | 0.195 |
| Medium fitness | -0.480 | 0.134 | Medium fitness | -0.497 | 0.211 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Red blood cells (10^6/mm3) | -5.559 | 0.000 | |||
| Dependent variable: Hemoglobin (g/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -2.408 | 0.000 | Male | -1.677 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.029 | 0.157 | Age (y) | 0.028 | 0.221 |
| Low fitness | -0.575 | 0.100 | Low fitness | -0.527 | 0.171 |
| Medium fitness | -0.433 | 0.192 | Medium fitness | -0.434 | 0.232 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Red blood cells (10^6/mm3) | -3.137 | 0.000 | |||
| Dependent variable: Iron (ug/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 0.405 | 0.307 | Male | 0.432 | 0.293 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | -0.010 | 0.714 | Age (y) | -0.012 | 0.660 |
| Low fitness | -0.176 | 0.729 | Low fitness | -0.326 | 0.528 |
| Medium fitness | -0.069 | 0.874 | Medium fitness | -0.204 | 0.646 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Iron intake (mg) | -0.008 | 0.163 | B12 intake (mg) | 0.010 | 0.714 |
| Folate intake (ug) | -0.003 | 0.033 | |||
| Iron intake (mg) | -0.008 | 0.152 | |||
| Dependent variable: Ferritin (ng/dL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | -1.310 | 0.000 | Male | -1.336 | 0.000 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | -0.001 | 0.951 | Age (y) | 0.000 | 0.986 |
| Low fitness | 0.184 | 0.665 | Low fitness | 0.211 | 0.626 |
| Medium fitness | 0.067 | 0.843 | Medium fitness | 0.073 | 0.831 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Iron intake (mg) | 0.032 | 0.114 | B12 intake (mg) | 0.010 | 0.641 |
| Folate intake (ug) | 0.000 | 0.858 | |||
| Iron intake (mg) | 0.029 | 0.149 | |||
| Lipid intake (g) | -0.053 | 0.284 | |||
| Dependent variable: Serum folate (ng/mL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 0.202 | 0.581 | Male | 0.130 | 0.725 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.013 | 0.651 | Age (y) | 0.015 | 0.586 |
| Low fitness | 0.658 | 0.173 | Low fitness | 0.632 | 0.192 |
| Medium fitness | 0.343 | 0.387 | Medium fitness | 0.372 | 0.350 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Folate intake (ug) | -0.001 | 0.240 | Folate intake (ug) | -0.001 | 0.202 |
| Vitamin B12 intake (ug) | 0.030 | 0.368 | |||
| Dependent variable: RBC folate (ng/mL) | |||||
| Model 1 | β | p-value | Model 2 | β | p-value |
| Male | 0.784 | 0.034 | Male | 0.730 | 0.051 |
| Female | 0.000 | Female | 0.000 | ||
| Age (y) | 0.018 | 0.479 | Age (y) | 0.019 | 0.440 |
| Low fitness | 0.051 | 0.904 | Low fitness | 0.017 | 0.967 |
| Medium fitness | 0.086 | 0.826 | Medium fitness | 0.097 | 0.803 |
| High fitness | 0.000 | High fitness | 0.000 | ||
| Folate intake (ug) | -0.001 | 0.404 | Folate intake (ug) | -0.001 | 0.358 |
| Vitamin B12 intake (ug) | 0.017 | 0.496 | |||
| Dependent variable: Vitamin D 25[OH]D (ng/mL) | |||||
| Model 1 | β | p-value | |||
| Male | 0.235 | 0.300 | |||
| Female | 0.000 | ||||
| Age (y) | -0.016 | 0.351 | |||
| Low fitness | 0.221 | 0.442 | |||
| Medium fitness | 0.101 | 0.705 | |||
| High fitness | 0.000 | ||||
| Vitamin D intake (ug) | 0.005 | 0.318 | |||
| Calcium intake (mg) | 0.000 | 0.688 | |||
β; standardized coefficients. BMI; body mass index; FFM, fat free mass; SFA; saturated fatty acids; MUFA; mono-unsaturated fatty acids; PUFA; polyunsaturated fatty acids; GOT; Glutamic oxalacetic transaminase, GPT; Glutamic-pyruvic transaminase, GGT; Gamma glutamyl transpeptidase; RBC folate, red blood cell folate; TBW, total body water. Level of significance p<0.05.
Concerning the blood lipid profile, more participants in the low PF group than in the high PF group had TC levels above the reference range (both models, p<0.001). Males had higher concentrations of TC than females (p<0.001). Additionally, when considering lipid and fatty acid intake in the model, these associations remained significant (p<0.05). Similarly, participants from the low (both models, p<0.001) and medium (both models, p<0.05) PF groups had greater HDL-c concentrations out of the reference range than those from the high PF group. Considering drugs related to blood lipid modifications, participants who regularly consumed lipid-reducing drugs presented higher concentrations out of the reference range for TC and LDL-c than participants who did not take these drugs (both models, p<0.01). In contrast, more individuals in the high PF group than in the low PF group had TG concentrations out of the reference range (both models, p<0.05), and this significant association remained when lipids and monounsaturated fatty acids were considered as cofounders (model 2, p<0.05).
Discussion
This study has shown that there are significant associations between PF levels and several blood biomarkers related to disease risk. Our main results revealed that participants from the low PF group showed lower vitamin B12 and TG concentrations out of the reference range and higher concentrations out of the reference range for tHcy, creatinine, TC, HDL-c and LDL-c. A high percentage of the sample population presented also with low vitamin 25[OH]D and high TC, glucose, uric acid, LDL-c and tHcy concentrations independent of PF. To the best of the authors´ knowledge, there are no studies analyzing the relationship between blood biomarker concentrations within and out of the reference range and PF groups in Spanish older adults through this holistic approach. In this sense, participants who showed concentrations out of the reference range were also considered as having concentrations related to disease risk because long periods with biomarker levels out of the reference range could have a negative effect on health.
Regarding B-vitamins, subclinical deficiency and deficiency of these vitamins are common in the older adult population [15,37], and both tend to increase with age [38]. A total of 51.8% of the studied population presented subclinical deficiency considering as a cut-off point a concentration less than 352.3 pg/mL [15] (data not shown). Severe vitamin B12 deficiency causes an irreversible degeneration of the nervous system. Vitamin B12 and folate are involved as coenzymes of numerous regulating enzymes [39].
The relationships among vitamin B12, sfolate, RBC folate and tHcy are an ongoing research areas, especially if PF and PA are considered [1]. Likewise, no single blood marker has been described that could effectively evaluate the aging process [40]. Surprisingly, participants from the low PF group had lower vitamin B12 concentrations out of the reference range. Additionally, participants included in the low PF group presented significantly high tHcy concentrations out of the reference range, but no association was found among sfolate and RBC folate and PF levels in our study. Joubert et al. indicated that participants from the active and high PF groups presented a significantly higher concentration of tHcy than the sedentary group, as measured by means of treadmill VO2 max [41]. Alomari et al. observed an inverse association between PA levels and tHcy. Additionally, they reported a lower concentration of tHcy in subjects with higher vs. lower PA adjusting for vitamin B12 [42]. In this sense, Murakami et al. also did not observe differences in tHcy after controlling for age, sex, and folate intake according to PA groups [43]. In our study, with advancing age (in males), vitamin B12 deficiency and tHcy concentrations increased. Kuo et al. observed that high tHcy concentrations were inversely associated with cardiovascular fitness in females but not in males [44]. Likewise, Schoor et al. observed that females in the highest quartile of tHcy had a significantly lower PF than those in the lowest quartile [12]. A recent systematic review suggested positive results of increasing folic acid and vitamin B12 supplementation and regular physical exercise to prevent hyperhomocysteinemia [45].
Vitamin D deficiency has been previously published in all age stages [24,46,47] and is still an unsolved public health problem. In our population, a total of 67.9% of participants presented vitamin D concentrations below 29.99 ng/mL. Vitamin D is considered a marker of improved muscle condition, mainly in healthy older adults with lower PA as measured by means of PA questionnaires [27]. Al-Eisa et al. found that participants with low PA showed significantly lower 25[OH]D serum concentrations than active participants [27]. However, in our study there was no association between PF groups and vitamin D.
Another interesting finding in our study was that the low PF group presented a higher risk of high TC, high LDL-c, low HDL-c and low TG concentrations out of the reference ranges than the high PF group. These results are in accordance with the findings of other authors [48,49]. Furthermore, LDL-c and TG showed a significant relationship with PF groups when lipid intake was included into the statistical analysis but not with TC intake. These results confirm the relationship between lipid intake suggested by Siri-Tarino et al. [50]. Dvorak et al. observed that older adult subjects with high cardiorespiratory fitness, independent of their PA levels, presented lower concentrations of TG, TC, total HDL-c and low LDL-c [51].
In our study, uric acid and urea were analyzed, but there were not significant differences between PF groups, and there was also no association between PF and concentrations out of the reference range. These results could suggest that both blood biomarkers might increase after intensive exercise [1,52,53] but not as a consequence of performed PA during their lives. Low concentrations of total protein are associated with malnutrition [54]. A significant relationship was obtained between total protein and PF groups, and a major tendency for total protein concentrations out of the reference range was observed between low and medium PF groups compared to the high PF group. Furthermore, participants in the low PF group showed a significantly higher risk of presenting concentrations out of the reference range for creatinine than participants in the high PF group in our study.
Hematocrit, hemoglobin and red blood cells have been related to significant higher possibilities of adverse health-status measurements (i.e., multiple morbidities, cognitive impairment, disability and mortality) [55]. Nevertheless, in our study, we could not find these associations between hematocrit and hemoglobin and PF groups. This could be because similar concentrations were obtained in all PF groups. Low hematocrits have been observed in trained persons mainly due to an increased plasma volume [56].
Several biomarkers are key in biological pathways and have been related to cardiovascular disease, and it is clearly established that recurrent exercise modifies a wide number of blood biomarkers. Dietary intake was considered in the analysis due to its fundamental role in decreasing the progression of chronic disease [57].
The current study has some limitations. First, the cross-sectional design cannot determine the cause-effect relationship between PF and biomarkers. Second, all participants were volunteers; thus, the outcomes are applicable only for the study group. Despite the abovementioned limitations, the PHYSMED project has several strengths. Likewise, some results are well known (e.g., lipid markers); this study analyzed several blood markers, including PF as a health marker. Another strength is the inclusion of a battery of PF tests, which is a more objective and precise method than questionnaires. Additionally, clustering of PF produces an alternative approach to summarizing PF levels, and it allows a realistic, wide vision about behavioral patterns. Moreover, the strict standardization of the field work and the blood sample protocol among the cities that took part in the study was another strength.
In conclusion, the study shows new approaches regarding the relationship between blood biomarkers and different groups of PF. Blood markers of health were generally associated with high PF in Spanish older adults. Regular physical exercise modifies a broad variety of metabolic processes that are then reflected by particular variations in biomarkers; therefore, both PF and blood markers should be considered when analyzing health status in older subjects. The proposed blood markers could be used routinely considering also the PF groups, and public health strategies should be implemented regarding biomarkers at risk. Additionally, longitudinal studies are needed to predict the capabilities of blood markers according to PF.
Supporting information
HDL-Cholesterol; high density lipoprotein cholesterol. LDL-cholesterol; low density lipoprotein cholesterol.
(DOCX)
Acknowledgments
The authors are grateful to all subjects who took part in the PHYSMED study and to all researchers who set up the data for further analysis. Furthermore, our special thanks go to Rosa María Torres Herrera and Raquel Seco for their contribution to laboratory work and to Laura Barrios for her statistical assistance.
Data Availability
There are restrictions on the availability of data for the paper entitled “A grouping of blood markers analyses regarding physical fitness groups in Spanish older adults: a cross-sectional study from the PHYSMED project” by R Aparicio-Ugarriza, AE Díaz, G Palacios, MM Bibiloni, A Julibert, JA Tur, and M Gonzalez-Gross. The data belongs to the ImFINE Research Group of the Universidad Politécnica de Madrid and Research Group on Community Nutrition& Oxidative Stress of the University of the Balearic Islands (Spain), and contain confidential data from several patients, and its diffusion is restricted by agreement with Ethics Committee. However, under demand, authors interested in the data may contact us at nucox@uib.es and/or +34-971-173146. Ethics Committee contact: secretaria.adjunto.vinvestigacion@upm.es.
Funding Statement
This work was supported by the Institute of Health Carlos III (Projects 11/01791 CIBEROBN CB12/03/30038). Raquel Aparicio Ugarriza was supported with a predoctoral grant from the Universidad Politécnica de Madrid. The funders had no role in study design, data collectionand analysis, decision to publish, or preparation of the manuscript.
References
- 1.Palacios G, Pedrero-Chamizo R, Palacios N, Maroto-Sanchez B, Aznar S, Gonzalez-Gross M, et al. Biomarkers of physical activity and exercise. Nutr Hosp 2015. February 26;31 Suppl 3:237–244. [DOI] [PubMed] [Google Scholar]
- 2.Huh Y, Yang EJ, Lee SA, Lim JY, Kim KW, Paik NJ. Association between executive function and physical performance in older Korean adults: findings from the Korean Longitudinal Study on Health and Aging (KLoSHA). Arch Gerontol Geriatr 2011. May-Jun;52(3):e156–61. 10.1016/j.archger.2010.10.018 [DOI] [PubMed] [Google Scholar]
- 3.Raji MA, Kuo YF, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc 2005. September;53(9):1462–1468. 10.1111/j.1532-5415.2005.53457.x [DOI] [PubMed] [Google Scholar]
- 4.Alves LC, Leimann BCQ, Vasconcelos MEL, Carvalho MS, Vasconcelos AGG, Fonseca TCO et al. A influência das doenças crônicas na capacidade funcional dos idosos do Município de São Paulo. Cad Saúde Pública. Cad Saúde Pública 2007;23:1924–30. 3. [DOI] [PubMed] [Google Scholar]
- 5.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 2010. April;34(5):721–733. 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowa A, Tobiasz-Adamczyk B, Topor-Madry R, Poscia A, la Milia DI. Predictors of healthy ageing: public health policy targets. BMC Health Serv Res 2016. September 5;16 Suppl 5:289-016-1520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013;2013:657508 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura M, Mizuta C, Yamada Y, Okayama Y, Nakamura E. Constructing an index of physical fitness age for Japanese elderly based on 7-year longitudinal data: sex differences in estimated physical fitness age. Age (Dordr) 2012. February;34(1):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair SN, Kohl HW, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989. November 3;262(17):2395–2401. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjostrom M, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008. July 1;337:a439 10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega FB, Ruiz JR, Castillo MJ, Sjostrom M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond) 2008. January;32(1):1–11. [DOI] [PubMed] [Google Scholar]
- 12.van Schoor NM, Swart KM, Pluijm SM, Visser M, Simsek S, Smulders Y, et al. Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr 2012. February;66(2):174–181. 10.1038/ejcn.2011.151 [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C, Shephard RJ, Stephens T editor. Physical activity; fitness and health: International proceedings and consensus statement. Champaign III: Human Kinetics ed; 1994. [Google Scholar]
- 14.González-Gross M, Marcos A, Pietrzik K. Nutrition and cognitive impairment in the elderly. Br J Nutr 2001. September;86(3):313–2. [DOI] [PubMed] [Google Scholar]
- 15.Aparicio-Ugarriza R, Palacios G, Alder M, Gonzalez-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med 2015. July;53(8):1149–1159. 10.1515/cclm-2014-0784 [DOI] [PubMed] [Google Scholar]
- 16.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, et al. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 2004. May 13;350(20):2042–2049. 10.1056/NEJMoa032739 [DOI] [PubMed] [Google Scholar]
- 17.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 2005. October;82(4):806–812. 10.1093/ajcn/82.4.806 [DOI] [PubMed] [Google Scholar]
- 18.Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: An update. Arch Cardiovasc Dis 2009. December;102(12):847–854. 10.1016/j.acvd.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 19.Ravaglia G, Forti P, Maioli F, Montesi F, Rietti E, Pisacane N, et al. Risk factors for dementia: data from the Conselice study of brain aging. Arch Gerontol Geriatr 2007;44 Suppl 1:311–320. [DOI] [PubMed] [Google Scholar]
- 20.Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull 2008. June;29(2 Suppl):S143–72. 10.1177/15648265080292S119 [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA 2005. March 2;293(9):1082–1088. 10.1001/jama.293.9.1082 [DOI] [PubMed] [Google Scholar]
- 22.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP. Elevated homocysteine is a predictor of all-cause mortality in a prospective cohort of renal transplant recipients. Nephron Clin Pract 2010;114(1):c5–11. 10.1159/000242443 [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Sun K, Chen J, Liao Y, Qin Q, Ma A, et al. High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci (Lond) 2009. October 26;118(3):187–194. [DOI] [PubMed] [Google Scholar]
- 24.de Souza WN, Martini LA. The role of Vitamin D in obesity and inflammation at adipose tissue. J Obes Metab Res 2015;2:161–6. [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med 2007. July 19;357(3):266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology 2011. February;152(2):354–363. 10.1210/en.2010-1109 [DOI] [PubMed] [Google Scholar]
- 27.Al-Eisa ES, Alghadir AH, Gabr SA. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin Interv Aging 2016. May 4;11:513–522. 10.2147/CIA.S102892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, et al. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract 2012. Mar-Apr;18 Suppl 1:1–78. [DOI] [PubMed] [Google Scholar]
- 29.Park YM, Sui X, Liu J, Zhou H, Kokkinos PF, Lavie CJ, et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol 2015. May 19;65(19):2091–2100. 10.1016/j.jacc.2015.03.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikli R. & Jones C.J. Senior Fitness Test Manual. United States: Human Kinetics; 2001. [Google Scholar]
- 31.Pedrero-Chamizo R, Gomez-Cabello A, Delgado S, Rodriguez-Llarena S, Rodriguez-Marroyo JA, Cabanillas E, et al. Physical fitness levels among independent non-institutionalized Spanish elderly: the elderly EXERNET multi-center study. Arch Gerontol Geriatr 2012. Sep-Oct;55(2):406–416. 10.1016/j.archger.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 32.VV.AA. Eurofit para adultos: evaluación de la aptitud física en relación con la salud. Primera ed. Madrid: CSD; 1998.
- 33.Mataix J, Mañas M, Llopis J, Martínez de Victoria E, Juan J, Borregón A. Tablas de composición de alimentos españoles. 4th ed Granada: INTA-Universidad de Granada: Granada; 2004. [Google Scholar]
- 34.Ortega RM, López AM, Requejo AM, Carvajales PA. La composición de los alimentos Herramienta básica para lavaloración nutricional. Madrid: Editorial Complutense; 2004. [Google Scholar]
- 35.Feinberg M, Favier JC, Ireland-Ripert J. Répertoire general des aliments. París: Tec&Doc Lavoisier; 1995. [Google Scholar]
- 36.Ripoll L. La cocina de las Islas Baleares. 5th ed Palma de Mallorca: Ripoll Pub; 1992. [Google Scholar]
- 37.Clarke R, Grimley Evans J, Schneede J, Nexo E, Bates C, Fletcher A, et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004. January;33(1):34–41. [DOI] [PubMed] [Google Scholar]
- 38.Loikas S, Koskinen P, Irjala K, Lopponen M, Isoaho R, Kivela SL, et al. Vitamin B12 deficiency in the aged: a population-based study. Age Ageing 2007. 36:177–83. 10.1093/ageing/afl150 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Gross M. Health impact of vitamin b12 and folate deficiency. Aktuel Ernaehr Med 2005;S1:26–9. [Google Scholar]
- 40.Wagner KH, Cameron-Smith D, Wessner B, Franzke B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016. June 2;8(6): 10.3390/nu8060338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joubert LM, Manore MM. The role of physical activity level and B-vitamin status on blood homocysteine levels. Med Sci Sports Exerc 2008. November;40(11):1923–1931. 10.1249/MSS.0b013e31817f36f9 [DOI] [PubMed] [Google Scholar]
- 42.Alomari MA, Khabour OF, Gharaibeh MY, Qhatan RA. Effect of physical activity on levels of homocysteine, folate, and vitamin B12 in the elderly. Phys Sportsmed 2016;44(1):68–73. 10.1080/00913847.2016.1135037 [DOI] [PubMed] [Google Scholar]
- 43.Murakami H, Iemitsu M, Sanada K, Gando Y, Ohmori Y, Kawakami R, et al. Associations among objectively measured physical activity, fasting plasma homocysteine concentration, and MTHFR C677T genotype. Eur J Appl Physiol 2011. December;111(12):2997–3005. 10.1007/s00421-011-1926-z [DOI] [PubMed] [Google Scholar]
- 44.Kuo HK, Yen CJ, Bean JF. Levels of homocysteine are inversely associated with cardiovascular fitness in women, but not in men: data from the National Health and Nutrition Examination Survey 1999–2002. J Intern Med 2005. October;258(4):328–335. 10.1111/j.1365-2796.2005.01546.x [DOI] [PubMed] [Google Scholar]
- 45.Han L, Liu Y, Wang C, Tang L, Feng X, Astell-Burt T, et al. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: A population-based study and systematic review. Clin Nutr 2016. November 19. [DOI] [PubMed] [Google Scholar]
- 46.Salminen M, Saaristo P, Salonoja M, Vaapio S, Vahlberg T, Lamberg-Allardt C, et al. Vitamin D status and physical function in older Finnish people: A one-year follow-up study. Arch Gerontol Geriatr 2015. Nov-Dec;61(3):419–424. 10.1016/j.archger.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Gross M, Valtuena J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, et al. Vitamin D status among adolescents in Europe: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 2012. March;107(5):755–764. 10.1017/S0007114511003527 [DOI] [PubMed] [Google Scholar]
- 48.Bakrania K, Edwardson CL, Bodicoat DH, Esliger DW, Gill JM, Kazi A, et al. Associations of mutually exclusive categories of physical activity and sedentary time with markers of cardiometabolic health in English adults: a cross-sectional analysis of the Health Survey for England. BMC Public Health 2016. January 12;16:25-016-2694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atienza AA, Moser RP, Perna F, Dodd K, Ballard-Barbash R, Troiano RP, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc 2011. May;43(5):815–821. 10.1249/MSS.0b013e3181fdfc32 [DOI] [PubMed] [Google Scholar]
- 50.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep 2010. November;12(6):384–390. 10.1007/s11883-010-0131-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab 2000. March;85(3):957–963. 10.1210/jcem.85.3.6432 [DOI] [PubMed] [Google Scholar]
- 52.Calderón Montero FJ, Benito Peinado PJ, Melendez Ortega A, González-Gross M. Control biológico del entrenamiento de resistencia. Rev Int Cienc Deporte 2006. 2: 65–87. [Google Scholar]
- 53.Testai FD, Gorelick PB. Inherited metabolic disorders and stroke part 2: homocystinuria, organic acidurias, and urea cycle disorders. Arch Neurol 2010. February;67(2):148–153. 10.1001/archneurol.2009.333 [DOI] [PubMed] [Google Scholar]
- 54.Batool R, Butt MS, Sultan MT, Saeed F, Naz R. Protein-energy malnutrition: a risk factor for various ailments. Crit Rev Food Sci Nutr 2015;55(2):242–253. 10.1080/10408398.2011.651543 [DOI] [PubMed] [Google Scholar]
- 55.Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, et al. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev 2011. October;132(10):496–502. 10.1016/j.mad.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 56.Mairbaurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol 2013. November 12;4:332 10.3389/fphys.2013.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gil A, Martinez de Victoria E, Olza J. Indicators for the evaluation of diet quality. Nutr Hosp 2015. February 26;31 Suppl 3:128–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HDL-Cholesterol; high density lipoprotein cholesterol. LDL-cholesterol; low density lipoprotein cholesterol.
(DOCX)
Data Availability Statement
There are restrictions on the availability of data for the paper entitled “A grouping of blood markers analyses regarding physical fitness groups in Spanish older adults: a cross-sectional study from the PHYSMED project” by R Aparicio-Ugarriza, AE Díaz, G Palacios, MM Bibiloni, A Julibert, JA Tur, and M Gonzalez-Gross. The data belongs to the ImFINE Research Group of the Universidad Politécnica de Madrid and Research Group on Community Nutrition& Oxidative Stress of the University of the Balearic Islands (Spain), and contain confidential data from several patients, and its diffusion is restricted by agreement with Ethics Committee. However, under demand, authors interested in the data may contact us at nucox@uib.es and/or +34-971-173146. Ethics Committee contact: secretaria.adjunto.vinvestigacion@upm.es.