Abstract
OBJECTIVE.
To characterize health professional schools by their vaccination policies for acceptable forms of evidence of immunity and exemptions permitted.
METHODS.
Data were collected between September 2011 and April 2012 using an Internet-based survey e-mailed to selected types of accredited health professional programs. Schools were identified through accrediting associations for each type of health professional program. Analysis was limited to schools requiring ≥1 vaccine recommended by the Advisory Committee on Immunization Practices (ACIP): measles, mumps, rubella, hepatitis B, varicella, pertussis, and influenza. Weighted bivariate frequencies were generated using SAS 9.3.
RESULTS.
Of 2,775 schools surveyed, 75% (n =2,077) responded; of responding schools, 93% (1947) required ≥1 ACIP-recommended vaccination. The proportion of schools accepting ≥1 non–ACIP-recommended form of evidence of immunity varied by vaccine: 42% for pertussis, 37% for influenza, 30% for rubella, 22% for hepatitis B, 18% for varicella, and 9% for measles and mumps. Among schools with ≥1 vaccination requirement, medical exemptions were permitted for ≥1 vaccine by 75% of schools; 54% permitted religious exemptions; 35% permitted personal belief exemptions; 58% permitted any nonmedical exemption.
CONCLUSIONS.
Many schools accept non–ACIP-recommended forms of evidence of immunity which could lead some students to believe they are protected from vaccine preventable diseases when they may be susceptible. Additional efforts are needed to better educate school officials about current ACIP recommendations for acceptable forms of evidence of immunity so school policies can be revised as needed.
Cases of vaccine-preventable diseases (VPDs) have been attributed to unvaccinated or nonimmune healthcare personnel (HCP) working in close contact with patients in healthcare settings.1,2 HCP have a significantly higher risk of acquiring VPDs than adults working in non-healthcare settings.3 Ensuring HCP are immunized against VPDs is an effective method to prevent cases of VPDs among susceptible patients and HCP working at healthcare facilities.4–8 The Advisory Committee on Immunization Practices (ACIP) routinely publishes and revises recommendations for HCP immunizations. Currently, the ACIP recommends that HCP receive vaccinations for measles, mumps, rubella (MMR), hepatitis B, varicella, pertussis, and seasonal influenza.9 The ACIP also recommends acceptable forms of evidence of immunity for VPDs for which HCP vaccination is recommended, which vary by vaccine and include provider-verified disease history, laboratory evidence of immunity, or provider-verified documentation of a completed vaccine series.
National compliance levels with ACIP vaccination recommendations for HCP remain below targeted goals. The Healthy People 2020 goal of 90% for hepatitis B and influenza vaccination coverage for HCP has not yet been reached.10 In 2012, 65% of HCP received at least 3 doses of hepatitis B vaccine during their lifetime and during the 2012–2013 influenza season, 72% of HCP received an influenza vaccination.11,12
Students and trainees working in healthcare settings are included in the ACIP definition of HCP as they may encounter similar risks to nontrainees in healthcare facilities during their training.9 Unvaccinated or nonimmune students training to become HCP can jeopardize the health of patients when they participate in clinical rotations or practical training components. Multiple reports have implicated HCP students as the source for VPD outbreaks.13–16 Outbreaks among students expose school communities and patients to VPDs, lead to high costs to investigate and control outbreaks, and cause interruptions in student training to prevent subsequent cases.
Although some information on vaccination coverage rates and school vaccination policies is available for students in medical, osteopathic medical, and baccalaureate nursing programs, information on students in other health professional programs is limited.17 The ACIP recommendations outline ideal vaccination practices and have been shown to influence the vaccination policies of 55% of health professional schools.18 This paper describes school compliance with ACIP and the CDC’s Healthcare Infection Control Practices Advisory Committee guidance for accepted types of evidence of immunity, the types of vaccination exemptions accepted by each school, and the school characteristics associated with policies for vaccination exemptions and accepted types of evidence of immunity.
METHODS
Data collection and survey methods are described in detail elsewhere.18 Lists of accredited schools were provided by affiliated accrediting associations or were manually compiled from accrediting associations’ websites. An Internet-based survey was emailed to the staff member deemed most appropriate at accredited health professional schools between September 2011 and April 2012, including time for follow-up with nonresponders. Health professional program types surveyed included dental assistant, dental hygienist, dentistry, EMT/paramedic, medical assistant, nurse midwife, associate degree nursing, diploma nursing, practical nursing, occupational therapist, pharmacist, physical therapist, physician assistant, radiation therapy technologist, and certified or registered respiratory therapist. A simple random sample was drawn for program types with >300 schools, allowing for confidence intervals of ± 5% to estimate the percent of schools with each vaccine requirement, assuming α< 0.05 and power of 0.80. An assumed 30% nonresponse rate was used to adjust the sample sizes of these schools. For program types with ≤300 schools, all schools were surveyed.
Analysis was limited to schools that had at least 1 ACIP-recommended vaccination requirement for HCP. Schools were asked to report the types of exemptions accepted for any vaccine requirement and the forms of evidence of immunity (including types of exemptions) accepted for each vaccine requirement. Respondents could choose combinations of ACIP- and non–ACIP-recommended forms of evidence of immunity. ACIP-recommended acceptable forms of evidence of immunity at the time of the survey included a provider-signed immunization certificate for all vaccines; laboratory evidence of immunity for measles, mumps, rubella, varicella, and hepatitis B; provider-verified disease history for measles, mumps, and varicella; and birth before 1957 for measles, mumps, and rubella.9 Nonverified disease history and student self-report of vaccination are not recommended by ACIP as acceptable evidence of immunity for any vaccines. Types of vaccination exemptions permitted by schools included medical, religious, and personal belief; nonmedical exemptions were defined as religious and/or personal belief exemptions. Respondents reported whether their school’s vaccination policy was influenced by any of the following: ACIP/Centers for Disease Control (CDC) recommendations for HCP, ACIP/CDC recommendations for adults, ACIP/CDC recommendations for children and adolescents, American College Health Association recommendations, advice or judgment of the dean of the school, federal Occupational Safety and Health Administration (OSHA) requirements, health professional organizations, history of infection/disease outbreak at the school, Joint Commission on Accreditation of Healthcare Organizations (JCAHO) standards, policies of institutions at which students perform clinical rotations, or state laws (in general, not specified as pertaining to vaccinations).
Data were analyzed using SAS 9.3 (SAS Institute, Cary, NC). Weighted bivariate frequencies were used to characterize distributions of categorical variables. Rao-Scott design-adjusted χ2 tests, odds ratios (OR), and 95% confidence intervals (CI) were used to test for associations. Weighted multivariable logistic regression, using a backwards elimination strategy, was used to examine predictors for accepted forms of evidence of immunity and types of vaccine exemptions. Design-based sampling weights were calculated as the reciprocal of the probability of selection for each school. Differences in response rates by program types were accounted for by post-stratification adjustment. Significance was determined at a 2-sided α<0.05 level for all tests.
RESULTS
Of the 2,775 schools surveyed, the response rate was 75% (2,077 schools); of these schools, 93% (1,947) required at least 1 ACIP-recommended vaccination. Vaccinations required by responding schools ranged from 32% for influenza to 87% for MMR; only 19% of schools required all ACIP recommended vaccinations.18 Forms of evidence of immunity accepted for vaccine requirements by schools with at least 1 vaccination requirement varied by vaccine type (Table 1). One or more ACIP-recommended forms of evidence were accepted by 99% of schools with measles or mumps requirements, 98% of those with rubella or varicella requirements, 97% with a hepatitis B requirement, 92% with a pertussis requirement, and 90% with an influenza requirement. Schools accepting 1 or more non–ACIP-recommended forms of evidence also varied by vaccine: 42% for pertussis, 37% for influenza, 30% for rubella, 22% for hepatitis B, 18% for varicella, and 9% for measles and mumps. (Refer to Table 1 for additional results on the forms of evidence of immunity accepted by schools.)
Table 1.
Evidence of Immunity for Required Vaccinations in 2011–2012 Accepted by Schools with ≥1 Vaccination Requirements for Students (n= 1,947)
| Forms of Evidence of Immunity, % (95% CI)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Immunity requirement |
No. of schools with requirement |
≥1 ACIP- recommended forms of evidence |
Provider-signed immunization certificate |
Laboratory evidence |
Provider- verified disease history |
Birth before 1957 |
Nonverified disease history |
Student self- report of vaccination |
≥1 Non ACIP- recommended form of evidence |
| n | |||||||||
| Measles | 1,859 | 99 (98–99) | 84 (82–86) | 71 (69–74) | 29 (26–31) | 21 (19–23) | 7 (6–8) | 3 (2–4) | 9 (8–11) |
| Mumps | 1,832 | 99 (98–99) | 84 (82–86) | 71 (68–73) | 28 (26–30) | 20 (18–22) | 7 (6–8) | 4 (3–4) | 9 (8–11) |
| Rubella | 1,859 | 98 (97–98) | 83 (81–85) | 72 (70–75) | 27 (25–29) | 19 (17–21) | 6 (5–7) | 3 (2–4) | 30 (28–32) |
| Varicella | 1,556 | 98 (97–98) | 78 (76–80) | 76 (74–78) | 37 (34–39) | ... | 13 (11–15) | 8 (7–10) | 18 (16–20) |
| Hepatitis B | 1,768 | 97 (96–98) | 85 (83–86) | 72 (69–74) | 19 (17–20) | ... | 4 (3–5) | 4 (3–4.) | 22 (20–24) |
| Pertussis | 1,006 | 92 (90–93) | 92 (90–93) | 35 (32–38) | 16 (14–19) | ... | 4 (3–5) | 3 (2–4) | 42 (39–45) |
| Influenza | 634 | 90 (87–92) | 90 (87–92) | 29 (25–33) | 15 (12–17) | ... | 3 (2–4) | 4 (2–5) | 37 (33–40) |
NOTE. ACIP, Advisory Committee on Immunization Practices; CI, confidence interval.
Weighted percentages. Denominator is the number of schools with vaccination requirement and accepting at least one form of evidence; shaded cells indicate ACIP accepted forms of evidence for each vaccine. The non–ACIP-recommended evidence column shows the proportion of schools accepting at least one of the types of immunity in the nonshaded cells for the vaccine specified. Provider-diagnosed disease is no longer recommended for measles and mumps by the ACIP as of 2011.26
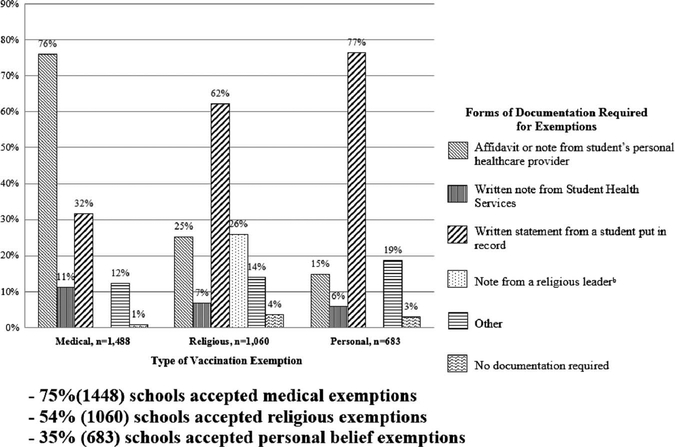
Of schools with at least 1 vaccination requirement, 79% (1,537) permitted an exemption for at least 1 vaccination requirement. For the 1,448 (75%) schools permitting medical exemptions, 76% accepted an affidavit or note from a student’s personal healthcare provider, and 32% accepted a written statement from a student (Figure 1). Of 1,060 (54%) schools permitting religious exemptions, 62% allowed a written statement from a student as a form of documentation, and 26% allowed a note from a religious leader. Of the 683 (35%) of schools permitting personal belief exemptions, 77% of schools accepted a written statement from a student, and 19% accepted another form of documentation.
FIGURE 1.
Vaccination exemptions accepted and required forms of documentation among schools with ≥1 vaccination requirements for students, (n = 1,947).a
aWeighted percentages. Denominator is the total number of programs with each type of exemption; programs could choose more than 1 form of documentation. Responses may sum to more than 100%. For at least 1 vaccine type, 58.0% of programs accepted any nonmedical exemption.
bThis response is only applicable for religious exemptions.
For the multivariable models assessing predictors of schools requiring only ≥1 ACIP- and no non–ACIP-recommended forms of evidence versus only requiring ≥1 non–ACIP- and no ACIP-recommended forms of evidence of immunity for each type of vaccine, the final models included number of years required to complete a program, type of school, and influences on a school’s vaccination policy. Schools with program lengths ≤1 year, compared with those with 2-year durations, were less likely to require ACIP-recommended forms of evidence of immunity for measles, mumps, and rubella (OR, 0.6; 95% CI, 0.4–0.9), and hepatitis B (OR, 0.7; 95% CI, 0.5–0.9) (Table 2). Schools reporting that ACIP/CDC recommendations for HCP influenced their vaccination requirements were more likely to require ACIP-recommended forms of evidence of immunity for hepatitis B (OR, 1.5; 95% CI, 1.2–1.9), pertussis (OR, 1.4; 95% CI, 1.1–1.9), and influenza (OR, 1.6; 95% CI, 1.1–2.3) than schools not influenced by the recommendations.
Table 2.
Association Between School Characteristics and Schools Requiring ACIP-Recommended Evidence of Immunity
| Requiring ≥1 ACIP Recommended Forms of Evidence versus Requiring ≥1 Non-ACIP-Recommended Forms of Evidence of Immunitya | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measles, Mumps, and Rubella, n =1,823 |
Hepatitis B, n= 1,767 |
Varicella, n= 1,555 |
Pertussis, n =1,006 |
Influenza, n =634 |
|||||||||||
| School characteristics | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Years required for program completion | .02b | .04b | .11 | .06 | .70 | ||||||||||
| ≤1 yr | 0.6 | 0.4–0.9 | .02b | 0.7 | 0.5–0.9 | .01b | 0.8 | 0.6–1.1 | .20 | 0.9 | 0.6–1.2 | .41 | 0.9 | 0.6–1.4 | .64 |
| ≥3 yr | 1.2 | 0.7–1.9 | .52 | 0.9 | 0.6–1.2 | .29 | 1.3 | 0.9–1.8 | .20 | 1.4 | 1.0–1.9 | .06 | 1.2 | 0.7–1.9 | .56 |
| 2 yr (referent) | 1.0 | ... | ... | 1.0 | ... | ... | 1.0 | ... | ... | 1.0 | ... | ... | 1.0 | ... | ... |
| Type of school | |||||||||||||||
| Public | 1.5 | 1.0–2.2 | .03b | 1.2 | 0.9–1.6 | .18 | 1.0 | 0.7–1.3 | .79 | 1.3 | 0.9–1.8 | .10 | 1.6 | 1.1–2.4 | .03b |
| Private (referent) | 1.0 | ... | 1.0 | ... | 1.0 | ... | ... | 1.0 | ... | ... | 1.0 | ... | ... | ||
| Influences on school’s vaccination requirements, yes vs no (referent) | |||||||||||||||
| ACIP/CDC Recommendations for HCP | 1.0 | 0.7–1.4 | .84 | 1.5 | 1.2–1.9 | <.01b | 1.0 | 0.7–1.3 | .71 | 1.4 | 1.1–1.9 | .01b | 1.6 | 1.1–2.3 | .02b |
| ACIP Recommendations for Adults | 1.6 | 0.9–2.6 | .07 | 0.8 | 0.5–1.1 | .18 | 0.8 | 0.5–1.2 | .22 | 0.8 | 0.5–1.2 | .23 | 0.7 | 0.4–1.2 | .15 |
NOTE. ACIP, Advisory Committee on Immunization Practices; OR, odds ratio; CI, confidence interval; CDC, Centers for Disease Control; HCP, healthcare personnel; JCAHO, Joint Commission on Accreditation of Healthcare Organizations.
Additional variables controlled for in the regressions include history of infection/disease outbreak, JCAHO, policies of institutions where students perform clinical rotations, American College Health Association, school dean, Occupational Safety and Health Administration, health profession organization, and state laws.
Statistically significant at α<0.05.
The final multivariable models for the outcome of a school accepting a medical, religious, or personal exemption included school type and the influences on a school’s vaccination policy. Schools reporting that their vaccination policies were influenced by the ACIP recommendations for HCP were more likely to accept a medical exemption (OR, 1.4; 95% CI, 1.1–1.8) (data not presented). Of those schools indicating their vaccination policy was influenced by the American College Health Association, personal exemptions were less likely to be accepted (OR, 0.5; 95% CI, 0.3–0.8).
DISCUSSION
Overall, vaccination policies at health professional schools varied in their level of adherence to the ACIP recommendations for acceptable forms of evidence of immunity for HCP. A few schools (7%) did not have any vaccination requirements, while many schools accepted inadequate forms of evidence of immunity for vaccinations that were required for their students. Having no required vaccinations or accepting non–ACIP-recommended forms of evidence of immunity may lead some students, school officials, and clinical institutions to falsely believe students are immune to VPDs when they may actually be susceptible and may contribute to the spread of VPDs.
The majority of surveyed schools permitted at least 1 form of nonmedical exemption for at least 1 vaccine. Any type of vaccination exemption may increase the risk of an outbreak by allowing nonimmune HCP to spread a VPD among colleagues, patients, and family members who may not be immune. However, nonmedical exemptions pose a larger threat to community-level immunity, as they make up the majority of exemptions and the rates of these types of exemptions among kindergarten students have increased in past years for some areas.19,20 From January to August of 2013, 82% of measles cases (mainly import-associated) occurred in unvaccinated persons; the majority of these cases had claimed philosophical exemptions to vaccination.21
Our study identified significant influences on the outcomes of whether schools accept ACIP-recommended forms of evidence of immunity and specific vaccination exemptions. The ACIP recommendations for HCP were only a significant influence for schools accepting ACIP-recommended forms of evidence of immunity for hepatitis B, pertussis, and influenza, and for schools accepting medical exemptions. Schools influenced by the American College Health Association were less likely to accept a personal exemption. This finding may reflect the Association’s recommendations for institutional prematriculation immunizations, which are consistent with the ACIP’s recommendations and discourage the use of nonmedical exemptions for vaccines required for students.22 Despite the fact that healthcare facilities often require vaccinations for HCP, we found few significant associations between the influence of the policies of institutions where students perform clinical rotations and school vaccination policies. Although no common influence was found to be associated with whether schools accept ACIP-recommended forms of evidence of immunity and specific vaccination exemptions, our study helps to illuminate potential mechanisms for disseminating the ACIP recommendations for HCP and schools.
Vaccination requirements are especially important for vaccines that require multiple doses or repeat vaccinations (ie, influenza) to ensure that students remain immune to VPDs. It is important to have documentation of when students complete vaccine series, when they receive repeat vaccinations, and their laboratory test results. Recent outbreaks of measles, pertussis, and hepatitis B among HCP have been reported due to lack of vaccination or no evidence of immunity to these diseases.23–25 These outbreaks highlight the importance of requiring adequate forms of evidence of immunity as well as having up-to-date and well-organized vaccination tracking systems. Many health professional schools still use paper medical records and lack systematic methods that could increase students’ vaccine uptake.18 When possible, vaccination records should be managed in secure and computerized systems and should document immunity status of VPDs, vaccinations administered during employment, and any adverse events after vaccination.9 Tracking vaccinations among students is useful for identifying students that are susceptible to particular diseases as well as aiding with vaccination compliance and outbreak response.1,15
In this study, we were unable to assess specifically how state vaccination laws and clinical rotation site policies for HCP affect school vaccination policies; the number of students fulfilling a school’s immunity requirements and the specific language used by each school to define a requirement were not assessed. This information could have helped us better identify organizations that could be targeted to strengthen school vaccination policies. Another limitation is that survey respondents originated from a variety of occupations and may have been unfamiliar with the types of evidence of immunity. The survey also was unable to capture whether schools conducted pre- versus post-vaccination serologic testing for hepatitis B as part of their student vaccination policy; therefore, we were unable to determine how schools defined “laboratory evidence” as a form of evidence of immunity. The survey question regarding the types of evidence of immunity accepted may have been misinterpreted by respondents; they were first asked about accepted vaccine exemption types for any vaccine, then they were asked about the types of evidence of immunity accepted for each vaccine. For respondents who had previously indicated that 1 or more types of exemptions were accepted for any vaccine, these types of evidence included vaccination exemptions. Some schools allowed different types of exemptions for each vaccine; this was not common, as schools usually have the same vaccination policy for all required vaccines. We were unable to determine the extent to which potentially susceptible students were misclassified as immune to VPDs because we could not measure how often each type of evidence of immunity was submitted by students. Additionally, we did not assess the frequency of claimed vaccine exemptions. Notably, changes have been made in the forms of evidence of immunity that are recommended by the ACIP since the time the survey was completed; provider-diagnosed disease is no longer acceptable for measles and mumps.26 Also, the survey did not assess how school vaccination policies were updated as ACIP recommendations change over time; therefore, we do not know whether schools continue to accept the forms of evidence of immunity they indicated on the survey.
Vaccination programs for health professional students that follow the ACIP recommendations for HCP may help prevent excess cases of VPDs. It is important that health professionals are vaccinated before they enter the workforce. Ensuring that students are adequately immunized against VPDs is important for both their personal health and for the health of patients in the event of disease exposure at the worksite. Vaccination exemptions permitted and the types of evidence of immunity accepted by each school may influence overall vaccination coverage rates and susceptibility among HCP students. Contributing factors that have been suggested to improve vaccination programs among healthcare professional schools include establishing mandatory vaccine requirements, developing policies for noncompliance, monitoring compliance among students, and removing vaccination barriers.27 Additional efforts are needed to better educate school policy officials about current ACIP vaccination recommendations to strengthen immunization programs at health professional schools and to ensure that future HCP are adequately immunized.
ACKNOWLEDGMENTS
The authors acknowledge the professional and accrediting organizations that provided assistance to compile the survey sample frame and encouraged their members to participate. We also thank Sheri Hester, Freneka Minter, Deborah McFalls, and Brenda Koch of the Oak Ridge Institute for Science and Education (ORISE) for compiling the survey contact list and leading active follow-up with nonresponders. We are grateful for the technical support provided by John Stamper and Michael Locke for the survey.
Financial Support. This publication was supported by Cooperative Agreement No. U36/CCU300430 from the Centers for Disease Control and Prevention and the Association of Schools and Programs of Public Health. S.B.D. received salary support from the Association of Schools and Programs of Public Health. The findings and conclusions of this publication do not necessarily represent the official views of the CDC or the Association of Schools and Programs in Public Health.
Footnotes
Conflict of Interest.
All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Bonebrake AL, Silkaitis C, Monga G, et al. Effects of mumps outbreak in hospital, Chicago, Illinois, USA, 2006. Emerg Infect Dis 2010;16:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Hospital-acquired pertussis among newborns—Texas, 2004. MMWR Morb Mortal Wkly Rep 2008;57:600–603. [PubMed] [Google Scholar]
- 3.Kuster SP, Shah PS, Coleman BL, et al. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One 2011;6:e26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre M,Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc 2009;57:1580–1586. [DOI] [PubMed] [Google Scholar]
- 5.Wilde JA, McMillan JA, Serwint J, Butta J, O’Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999;281:908–913. [DOI] [PubMed] [Google Scholar]
- 6.Weber DJ, Rutala WA, Orenstein WA. Prevention of mumps, measles, and rubella among hospital personnel. J Pediatr 1991;119:322–326. [DOI] [PubMed] [Google Scholar]
- 7.Maltezou HC, Wicker S. Measles in healthcare settings. Am J Infect Control 2013;41:661–663. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L. Effect of influenza vaccination of health care personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis 2013;58:50–57. [DOI] [PubMed] [Google Scholar]
- 9.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Immunization of healthcare personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1–45. [PubMed] [Google Scholar]
- 10.Immunization and infectious diseases objectives. U.S. Department of Health and Human Services website. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23. Published 2012. Accessed January 22, 2013.
- 11.Williams WW, Lu PJ, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014;63:95–102. [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Influenza vaccination coverage among healthcare personnel—United States, 2012–2013 influenza season. MMWR Morb Mortal Wkly Rep 2013;62:781–786. [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews E, Armstrong G, Spencer T. Pertussis infection in a baccalaureate nursing program: clinical implications, emerging issues, and recommendations. J Contin Educ Nurs 2008;39: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poland GA, Nichol KL. Medical students as sources of rubella and measles outbreaks. Arch Intern Med 1990;150:44–46. [PubMed] [Google Scholar]
- 15.Storch GA, Gruber C, Benz B, Beaudoin J, Hayes J. A rubella outbreak among dental students: description of the outbreak and analysis of control measures. Infect Control 1985;6:150–156. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DS, Curtis CR, Schauer SL, et al. Surveillance for transmission and antibiotic adverse events among neonates and adults exposed to a healthcare worker with pertussis. Infect Control Hosp Epidemiol 2004;25:967–973. [DOI] [PubMed] [Google Scholar]
- 17.Lindley MC, Lorick SA, Spinner JR, et al. Student vaccination requirements of US health professional schools: a survey. Ann Intern Med 2011;154:391–400. [DOI] [PubMed] [Google Scholar]
- 18.Libby T Student vaccination requirements of US health professional schools: a national survey. J Allied Health 2014;43: 12–21. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Vaccination coverage among children in kindergarten—United States, 2012–2013 school year. MMWR Morb Mortal Wkly Rep 2013;62: 607–612. [PMC free article] [PubMed] [Google Scholar]
- 20.Richards JL, Wagenaar BH, Van Otterloo J, et al. Nonmedical exemptions to immunization requirements in California: a 16-year longitudinal analysis of trends and associated community factors. Vaccine 2013;31:3009–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Measles—United States, January 1–August 24, 2013. MMWR Morb Mortal Wkly Rep 2013;62:741–743. [PMC free article] [PubMed] [Google Scholar]
- 22.ACHA guidelines: recommendations for institutional premarticulation immunizations. American College Health Association website. http://www.acha.org/publications/docs/ACHA_RIPI_April_2014. pdf Published 2014 Accessed August 14, 2014.
- 23.Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006;355:447–455. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Hospital-associated measles outbreak—Pennsylvania, March–April 2009. MMWR Morb Mortal Wkly Rep 2012;61:30–32. [PubMed] [Google Scholar]
- 25.Chen SY, Anderson S, Kutty PK, et al. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis 2011;203: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older—United States, 2013. MMWR Surveill Summ 2013;62(Supplement 1). [PubMed] [Google Scholar]
- 27.Poland GA, Nichol KL. Medical schools and immunization policies: missed opportunities for disease prevention. Ann Intern Med 1990;113:628–631. [DOI] [PubMed] [Google Scholar]