Abstract
Background:
In 2015, ACIP recommended 16–23 year olds may be vaccinated with serogroup B meningococcal (MenB) vaccine based on individual clinical decision-making (Category B).
Objective:
To assess among US pediatricians (Peds) and family physicians (FPs): 1) practices regarding MenB vaccine delivery; 2) factors influencing a decision to recommend vaccine; 3) factors associated with discussing MenB vaccine at 16–18 year-old routine visits.
Design/Methods:
We surveyed a nationally representative sample of Peds and FPs by e-mail and internet from 10–12/2016.
Results:
The response rate was 72% (660/916). During routine visits, 51% of Peds and 31% of FPs reported always/often discussing MenB vaccine. Among those who discussed often/always, 91% recommended vaccination; among those who never/rarely discussed, 11% recommended. 73% of Peds and 41% of FPs currently administered MenB vaccine. While many providers reported not knowing about factors influencing recommendation decisions, serogroup B meningococcal disease outbreaks (89%), disease incidence (62%), and effectiveness (52%), safety (48%), and duration of protection of MenB vaccine (39%) increased likelihood of recommending while the Category B recommendation (45%) decreased likelihood. Those somewhat/not at all aware of MenB vaccine [Risk Ratio 0.32 (95% CI 0.25–0.41)] and those practicing in an HMO [0.39 (0.18–0.87)] were less likely whereas those aware of disease outbreaks in their state [1.25 (1.08–1.45)] were more likely to initiate a discussion about MenB vaccine.
Conclusion(s):
Primary care physicians have significant gaps in knowledge about serogroup B meningococcal disease and MenB vaccine and this appears to be a major driver of the decision not to discuss the vaccines.
Keywords: Serogroup B meningococcal vaccine, Serogroup B meningococcal disease, Primary care practice
Introduction
Meningococcal disease has an overall case-fatality ratio of approximately 10–15% and permanent severe sequelae are common among survivors,1–3 heightening the importance of preventing this deadly infection. At the same time, meningococcal disease caused by any serogroup is uncommon in the United States and has been decreasing4 since the mid-1990s due to a variety of reasons, including natural decline and, among adolescents and young adults, use of quadrivalent meningococcal conjugate vaccine against serogroups A, C, W and Y (MenACWY). MenACWY vaccine has been licensed in the United States since 2005 and is recommended for routine use among adolescents 11–18 years.
Meningococcal disease caused by serogroup B is also uncommon with 130 cases in 2016, of which 41 were in those aged 16–23 years.5 However, numerous highly publicized outbreaks have occurred on college campuses since 2009. Two serogroup B meningococcal (MenB) vaccines have been licensed by the Food and Drug Administration (FDA) in the United States and approved for use in persons aged 10–25 years: MenB-FHbp (Trumenba®) and MenB-4C (Bexsero®). Both vaccines were licensed under an accelerated approval process because of the outbreaks occurring on college campuses. FDA approval was based on safety and on demonstration of inferred efficacy by antibody responses to selected serogroup B meningococcal strains rather than clinical effectiveness.4 In October of 2015, the Advisory Committee on Immunization Practices (ACIP) recommended 16–23 year olds may be vaccinated with MenB vaccine, with a preferred age for administration of 16–18 years (Category B recommendation).4 In contrast, a Category A (routine) recommendation had already been made in June of 2015 for persons aged ≥10 years at increased risk for serogroup B meningococcal disease.6
The definitions of Category A and B recommendations were based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework7 which had been used to guide decision-making by ACIP since 2012. Category A recommendations are made for all persons in an age or risk factor-based group, while for Category B recommendations the decision whether to vaccinate was to occur “in the context of a clinician-patient interaction” with consideration of the balance between desirable and undesirable effects of the vaccine in question.7 The language for ACIP’s Category B recommendation for MenB vaccines incorporated the wording “may be administered” and the accompanying rationale identified the low prevalence of disease as well as insufficient data about vaccine effectiveness and safety as reasons for not making a Category A recommendation. CDC did not provide additional guidance on how to implement the ACIP recommendation. The American Academy of Family Physicians (AAFP) in February 2016 included frequently asked questions about MenB vaccine on its website, providing some guidance to their members, but no specific talking points REF.8 The American Academy of Pediatrics’ (AAP) Committee on Infectious Diseases published similar recommendations to ACIP’s almost one year later in September 2016, one month prior to our survey. AAP encourages pediatricians to discuss the benefits, risks and costs with patients and families and then “work with them to determine what is in their best interest.”9 (REF) No national data are available about how state or local public health agencies advised implementing the MenB vaccine recommendations for healthy adolescents and young adults.
Given the relatively new ACIP recommendations for use of MenB vaccine and the fact that this is the first widespread use of a Category B recommendation, it was unclear how MenB vaccine would be adopted. The objectives of this study were to assess: 1) current practices regarding MenB vaccine delivery in primary care; 2) reported influences on the decision to recommend or not recommend MenB vaccine to healthy adolescent patients; and 3) factors related to initiating a discussion about MenB vaccine at well visits for adolescents 16–18 years.
Methods
We conducted a survey between October and December of 2016 among pediatricians (Peds) and family physicians (FPs) who were part of sentinel networks within each specialty. The human subjects review board at the University of Colorado Denver approved this study.
Study Population
This survey was conducted as part of a collaboration with the Centers for Disease Control and Prevention (CDC) to perform rapid turnaround surveys to assess physician attitudes about vaccine-related issues. We developed national networks of primary care physicians by recruiting from AAP and AAFP. We conducted quota sampling10 to ensure network physicians were similar to the AAP and AAFP memberships with respect to region, practice location, and practice setting. Exclusion criteria included practicing <50% in primary care, not practicing in the United States, or being in training. We have previously demonstrated that survey responses from network physicians compared to those of physicians randomly sampled from American Medical Association databases had similar demographic characteristics, practice attributes, and attitudes about a range of vaccination issues.10 No incentives are provided to the participating physicians.
Survey Design
We developed the survey in collaboration with CDC, and with input from AAP and AAFP. A national advisory panel of Peds and FPs pre-tested the survey; it was then piloted among 45 Peds and 13 FPs nationally and further modified based on this feedback. The survey began by specifying the two MenB vaccines with trade names, clarifying that MenB vaccine currently has a Category B recommendation from ACIP for use in healthy adolescents and young adults, and then specified that the survey was about MenB vaccine, not MenACWY vaccine. We used four-point Likert scales for assessing reported frequency of initiating discussions regarding MenB vaccine and recommendation practices at different ages. Although MenB vaccine is not recommended for 11–12 year olds, we included this age group in order to assess whether there was confusion about whether MenB vaccine should be given at the same time as the first MenACWY vaccine. We also asked about how each of a list of factors affected likelihood of recommending MenB vaccine using a five-point scale.
Survey Administration
We surveyed physicians by Internet (Verint, Melville, New York, www.verint.com) or, if they preferred, by mail. We sent the Internet group an initial e-mail with up to 8 reminders, and we sent the mail group an initial mailing and up to 2 reminders. We sent Internet survey non-respondents a mail survey in case of problems with e-mail correspondence. We patterned the mail protocol on Dillman’s tailored design method.11
Statistical Analysis
We pooled Internet and mail surveys for analyses because studies have shown that physician attitudes are similar when obtained by either method.18–20 We compared respondents with non-respondents using t-test and chi-square analyses and compared Ped and FP responses using chi-square and Mantel-Haenszel chi-square tests. We conducted a multivariable analysis with the dependent variable of “always/almost always or often” initiating a discussion about MenB vaccine during routine well visits for adolescents 16–18 years. Because the outcome was common (42% of the cohort) we used a log binomial model to generate relative risks. Independent variables included provider and practice characteristics, whether MenB vaccine is administered at the office, level of awareness regarding MenB vaccine, and awareness of outbreaks of meningococcal disease or serogroup B meningococcal disease specifically. We used a cut-off of p<0.25 for inclusion of variables into the model. Our multivariable model used a backwards elimination procedure in which the least significant predictor in the model was eliminated sequentially. At each step, estimates were checked to make sure other variables were not affected by dropping the least significant variable. This resulted in retention of only those factors that were significant at p <0.05 in the final model. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
Results
The overall response rate was 72% (660/916), 79% (374/475) among Peds and 65% (286/441) among FPs. Table 1 compares respondents and non-respondents within each specialty and describes additional characteristics available only for respondents. Some differences were seen between respondents and non-respondents by region among Peds and by provider gender and practice setting among FPs.
Table 1:
Respondent and Non-Respondent Characteristics
| Characteristic | Peds | FP | ||
|---|---|---|---|---|
| Respondents (n=374) |
Non-Respondents (n=101) |
Respondents (n=286) |
Non-Respondents (n=155) |
|
| Age in years, mean (SD) | 50 (11) | 51 (11) | 55 (8) | 56 (9) |
| Male, % | 36 | 39 | 52** | 63** |
| Region, % | ||||
| Midwest | 22* | 20* | 30 | 25 |
| Northeast | 23* | 11* | 14 | 12 |
| South | 34* | 47* | 34 | 42 |
| West | 21* | 23* | 22 | 21 |
| Location of Practice, % | ||||
| Urban | 54 | 52 | 38 | 36 |
| Suburban | 45 | 47 | 52 | 56 |
| Rural | 1 | 1 | 9 | 8 |
| Setting, % | ||||
| Private practice | 80 | 77 | 65** | 76** |
| Hospital/clinic | 17 | 18 | 25** | 15** |
| HMO | 3 | 6 | 10** | 8* |
| Proportion of patients age 16-23 years old, % | ||||
| <10% | 15 | N/A | 57 | N/A |
| 10-19% | 39 | N/A | 31 | N/A |
| ≥20% | 46 | N/A | 12 | N/A |
| Proportion of Black or African American patients, % | ||||
| 0-24% | 78 | N/A | 83 | N/A |
| 25-49% | 18 | N/A | 13 | N/A |
| ≥50 | 4 | N/A | 4 | N/A |
| Proportion of Non-Hispanic white patients, % | ||||
| 0-24% | 19 | N/A | 14 | N/A |
| 25-49% | 29 | N/A | 19 | N/A |
| ≥50% | 52 | N/A | 67 | N/A |
| Proportion of patients with Medicaid or CHIP, % | ||||
| 0-24% | 43 | N/A | 66 | N/A |
| 25-49% | 27 | N/A | 21 | N/A |
| ≥50% | 30 | N/A | 13 | N/A |
| Proportion of patients with private insurance, % | ||||
| 0-24% | 21 | N/A | 19 | N/A |
| 25-49% | 18 | N/A | 25 | N/A |
| ≥50% | 61 | N/A | 56 | N/A |
Peds = pediatricians; FP = family physicians
p<0.05 for overall comparison of respondents and non-respondents within Peds
p<0.05 for overall comparison of respondents and non-respondents within FP
Current practices regarding MenB vaccine delivery
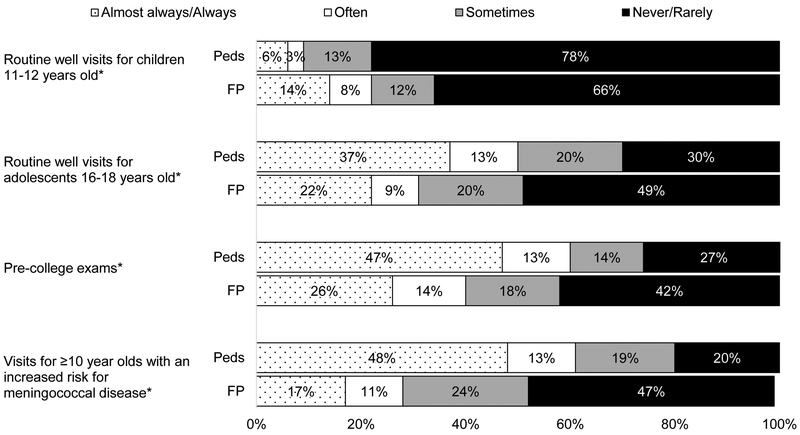
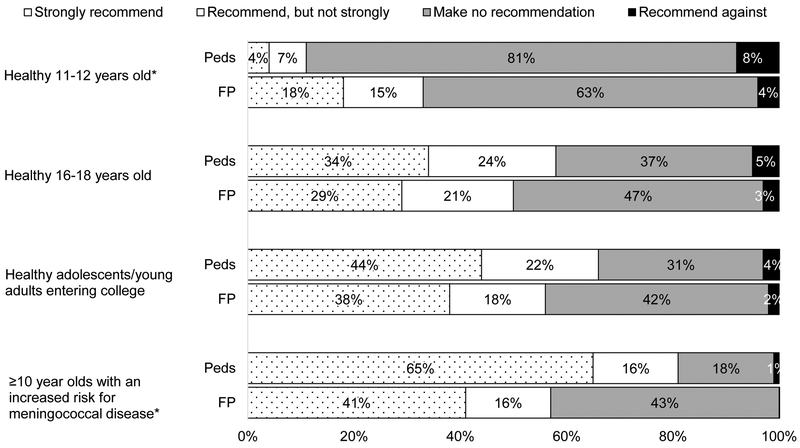
As shown in Figure 1, 50% of Peds and 31% of FPs reported always or often initiating a discussion about MenB vaccine during routine visits for 16–18 year olds and slightly more initiated discussions during pre-college physical exams. Peds were more likely to initiate discussions than FPs among all age groups examined with the exception of 11–12 year olds. Among Peds, 34% reported strongly recommending and 24% recommending but not strongly to 16–18 year olds; comparable percentages for FPs were 29% and 21%, respectively (Figure 2). Strong recommendations for those “entering college” were approximately 10 percentage points higher than for the 16–18 year old age group for both specialties. Overall, slightly higher percentages of providers in both specialties reported recommending MenB vaccine compared with initiating a discussion, although initiating was highly correlated with recommending. Among those who reported initiating a discussion always or often during routine visits for 16–18 year olds, 91% recommended MenB vaccine (66% strongly); among those who never or rarely initiated a discussion, only 11% recommended MenB vaccine (3% strongly). Not all who recommended the vaccine reported consistently initiating a discussion about it; among those who recommended the vaccine, 71% often/almost always/always initiated a discussion and another 21% sometimes did. Comparable percentages among those who made no recommendation were 6% and 16%, respectively. Eighty-one percent of Peds and 56% of FPs recommended to ≥10 year olds with an increased risk for meningococcal disease.
Figure 1: Frequency of Initiating a Discussion about MenB Vaccine (Peds n=374, FP=286).
Peds = Pediatricians, FP = Family Physicians
*p<0.001 for comparison between specialties (Mantel-Haenszel chi-squared)
Analyses exclude those who do not see patients in each age group: 1% of Peds and 9% of FP for 11–12 year olds, 1% of Peds and 6% of FP for 16–18 year olds, 6% of Peds and 4% of FP for pre-college exams, 16% of Peds and 11% of FP for ≥10 year olds with an increased risk for meningococcal disease. Some percentages do not add up to 100% due to rounding.
Figure 2: Current Practice Regarding Recommending MenB Vaccine (Peds n=374, FP=286).
Peds = Pediatricians, FP = Family Physicians
*p<0.001 for comparison between specialties (Mantel-Haenszel chi-squared)
Analyses exclude those who do not see patients in each age group: 10% of Peds and 1% of FP for 11–12 year olds, 6% of Peds and 1% of FP for 16–18 year olds, 3% of Peds and 3% of FP for healthy adolescents/young adults entering college, 9% of Peds and 12% of FP for ≥10 year olds with an increased risk for meningococcal disease. Some percentages do not add up to 100% due to rounding.
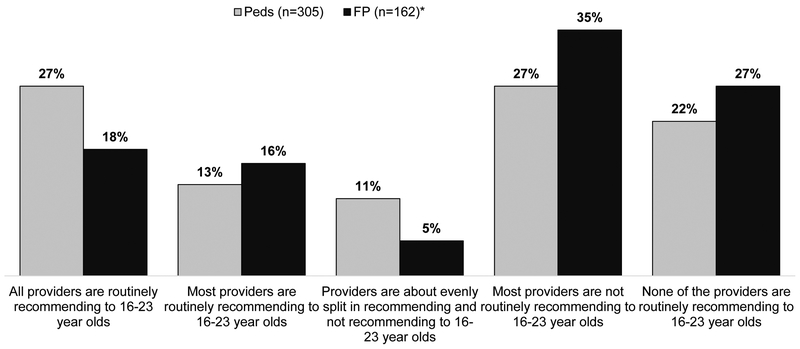
Seventy-three percent of Peds and 41% of FPs reported currently administering MenB vaccine in their practices; 2% of Peds and 11% of FPs did not know if MenB vaccine was being administered. Although 6% of Peds and 26% of FPs and who were not solo providers did not know the practices of other providers in their practice, the majority of physicians who reported knowing indicated that either a minority or none of the other providers were routinely recommending MenB vaccine to 16–23 year olds (Figure 3).
Figure 3. Agreement about Whether to Recommend MenB Vaccine for 16–23 Year Olds Among Providers in Practice.
*30 providers did not respond to this question
Peds = Pediatricians, FP = Family Physicians
Excluded response categories “I am the only provider in my office” (39 FP and 31 Peds) and “I don’t know other providers’ practices regarding recommending MenB vaccine to 16–23 year olds” (70 FP and 23 Peds).
Reported influences on decision to recommend or not recommend MenB vaccine to healthy adolescent patients
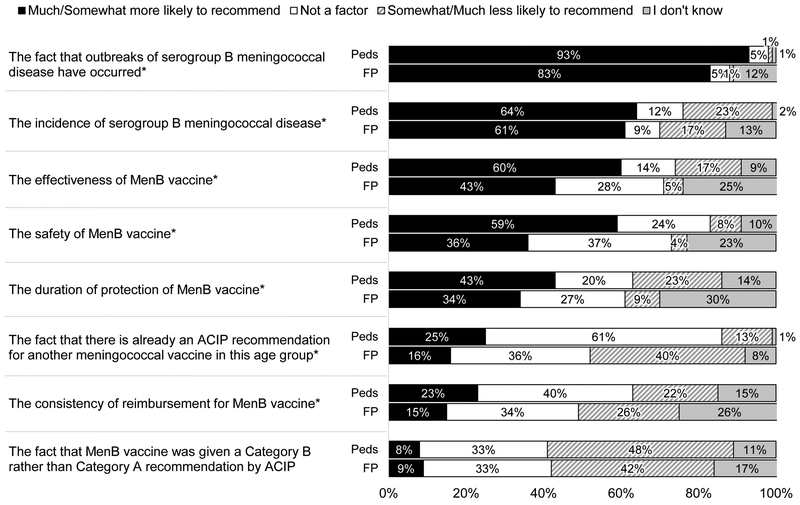
As shown in Figure 4, many providers, especially FPs, responded “I don’t know” to questions about factors influencing their likelihood of recommending MenB vaccine. The most commonly reported issues that were associated with a higher likelihood of recommending were the fact that serogroup B meningococcal disease outbreaks had occurred, the incidence of serogroup B meningococcal disease, the effectiveness and safety of MenB vaccine, and the duration of protection of MenB vaccine. The existence of a recommendation for another meningococcal vaccine (MenACWY) and consistency of reimbursement were related to a lower likelihood of recommendation. The fact that MenB vaccine was given a Category B as opposed to Category A recommendation by ACIP was the major issue associated with not recommending MenB vaccine identified by both specialties. Additional issues (not shown) endorsed as “not a factor” influencing the decision to recommend MenB vaccine by a majority included: the number of vaccines given to adolescents (“not a factor” for 76% of Peds, 59% of FPs), parents’ attitudes about MenB vaccine in our practice (59% and 52%), the fact that MenB vaccination requires multiple doses (79% and 53%), marketing by pharmaceutical companies for MenB vaccine (74% and 66%), and the time it would take me to discuss what a Category B recommendation means (69% and 50%).
Figure 4: Factors Influencing the Decision to Recommend MenB Vaccine to Healthy Adolescent Patients (Peds n=374, FP=286).
Peds = Pediatricians, FP = Family Physicians
*p≤0.001 for comparison between specialties (Mantel-Haenszel chi-squared)
Some percentages do not add up to 100% due to rounding.
Factors related to initiating a discussion about MenB vaccine at well visits for adolescents 16–18 years
As shown in Table 2, being aware of serogroup B meningococcal disease outbreaks that have occurred in their state was associated with always/almost always or often initiating a discussion of MenB vaccine for adolescents 16–18 years of age, while being only somewhat or not at all aware of MenB vaccine and practicing in a health maintenance organization (HMO) compared to a private practice setting were associated with lower frequency of initiating a discussion. FPs were much less likely to have been aware of MenB vaccine before taking the survey, and specialty and level of awareness were both independently associated with the outcome. However, when both variables were included in the model, specialty became non-significant, so only awareness of MenB vaccine was retained in the model. There was no evidence of a significant interaction between these two variables.
Table 2:
Multivariable Model Predicting Often/Almost Always/Always Initiating a Discussion Regarding MenB at Routine Well Visits for 16-18 Year Olds (n=638)
| Variable | Never / Rarely / Sometimes Col % (n) n=367 (58%) |
Often / Almost Always /Always Col % (n) n=271 (42%) |
Biv. p value |
MV RR (95% CI) |
|---|---|---|---|---|
| Practice Type | ||||
| Family Medicine | 50.1 (184) | 30.3 (82) | ||
| Pediatrics | 49.9 (183) | 69.7 (189) | <.0001 | |
| Gender | ||||
| Male | 45.0 (165) | 60.5 (164) | ||
| Female | 55.0 (202) | 39.5 (107) | 0.17 | |
| Setting | ||||
| Private practice | 68.9 (253) | 81.6 (221) | Ref. | |
| Hospital or clinic | 21.5 (79) | 16.6 (45) | 1.00 (0.81-1.23) | |
| HMO | 9.5 (35) | 1.9 (5) | <.0001 | 0.40 (0.18-0.89) |
| Census Location | ||||
| Urban | 48.0 (176) | 45.8 (124) | ||
| Suburban/Rural | 52.0 (191) | 54.2 (147) | 0.58 | |
| Region | ||||
| Midwest | 26.4 (97) | 25.1 (68) | ||
| Northeast | 20.2 (74) | 18.8 (51) | ||
| South | 32.7 (120) | 34.0 (92) | ||
| West | 20.7 (76) | 22.1 (60) | 0.92 | |
| Mean (sd) / Median age in years | 51.9 (10.1) / 53.0 | 51.7 (9.4) / 51.0 | 0.86 | |
| Number of providers in practice | ||||
| 1-5 | 47.8 (175) | 47.0 (127) | ||
| 6 or more | 52.2 (191) | 53.0 (143) | 0.85 | |
| Before taking this survey, how aware were you of the MenB vaccines described above? |
||||
| Very aware | 29.9 (105) | 76.7 (197) | Ref. | |
| Somewhat aware | 55.8 (196) | 21.0 (54) | 0.35 (0.27-0.45) | |
| Not at all aware | 14.3 (50) | 2.3 (6) | <.0001 | 0.19 (0.09-0.40) |
| I am aware of patient(s) in my practice who have had meningococcal disease |
||||
| Yes | 36.1 (127) | 41.3 (107) | ||
| No | 63.9 (225) | 58.7 (152) | 0.19 | |
| I am aware of serogroup B meningococcal outbreaks that have occurred in my state |
||||
| Yes | 23.3 (82) | 42.9 (111) | 1.25 (1.07-1.45) | |
| No | 76.7 (270) | 57.1 (148) | <.0001 | Ref. |
| I am aware of serogroup B meningococcal outbreaks that have occurred on college campuses in the U.S. |
||||
| Yes | 68.2 (240) | 84.9 (220) | ||
| No | 31.8 (112) | 15.1 (39) | <.0001 | |
| Proportion of patients that are 16-23 years old | ||||
| <10% | 39.4 (141) | 22.2 (59) | ||
| ≥10% | 60.6 (217) | 77.8 (207) | <.0001 | |
| Proportion patients Black or African American | ||||
| 0–24% | 80.2 (284) | 80.5 (210) | ||
| ≥25% | 19.8 (70) | 19.5 (51) | 0.94 | |
| Proportion patients Hispanic | ||||
| 0-24% | 80.2 (283) | 76.5 (198) | ||
| ≥25% | 19.8 (70) | 23.6 (61) | 0.27 | |
| Proportion patients with Medicaid or CHP | ||||
| 0-24% | 51.9 (182) | 53.4 (141) | ||
| ≥25% | 48.2 (169) | 46.6 (123) | 0.70 |
Discussion
This is the first national US survey of which we are aware that assesses reported practices related to MenB vaccine delivery since ACIP’s Category B recommendation for its use. Our findings indicate that half of Peds and about a third of FPs report often or always initiating a discussion about MenB vaccine for 16–18 year olds. Greater awareness about outbreaks of disease was associated with a higher likelihood of discussing the vaccine, while lower awareness about the vaccine and working in an HMO setting were associated with a lower likelihood. Those physicians who reported initiating a discussion almost always reported making a recommendation to vaccinate, whereas those who rarely initiated discussions were unlikely to recommend vaccination. Providers most often cited outbreaks of serogroup B meningococcal disease, incidence of disease, and effectiveness, safety and duration of protection of MenB vaccine as reasons increasing their likelihood of recommending MenB vaccine, although many providers reported lack of knowledge regarding these potential influences. The fact that MenB vaccine was given a Category B recommendation and inconsistency of reimbursement were factors many providers reported would make them less likely to recommend MenB vaccine.
Whether our data indicate a level of discussion regarding MenB vaccine that is consistent with a Category B recommendation depends on one’s interpretation of how such a recommendation should be implemented. According to the original definition of a Category B recommendation based on the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach used by ACIP, such recommendations are supposed to result in individual clinical decision-making “in the context of a clinician-patient interaction.”7 In line with this interpretation, the AAP’s policy statement, as previously discussed, encouraged pediatricians to discuss the MenB vaccine with parents and patients.9 Others have stressed that a Category B recommendation is different from “no recommendation at all” and should consistently result in a discussion with parents and patients because individual decision-making “cannot occur if a patient does not know about the vaccine and the disease.”12
However, physicians may interpret “individual clinical decision-making” to reflect their own decision about whether to initiate a discussion of MenB vaccine, given their assessment of the risks and benefits of vaccinating, without involving parents or patients in decision-making. Providers may choose not to initiate a discussion with parents or patients if they do not intend to recommend MenB vaccine due to issues such as the low burden of serogroup B meningococcal disease in the United States or lack of data about the effectiveness of MenB vaccine, its duration of protection, or its safety.4 In our study, the fact that those providers who reported initiating a discussion were overwhelmingly also likely to recommend MenB vaccine, whereas those not initiating a discussion were very unlikely to recommend, is consistent with this second interpretation. Providers not initiating a discussion may not think the time required to discuss MenB vaccine is justified by the risks posed by the disease or the benefits offered by these vaccines. Alternatively, they may have a low level of awareness regarding the disease or MenB vaccine and feel insufficiently knowledgeable to have an informed discussion about pros and cons of vaccination. They also may have been entirely unaware of the ACIP recommendation for MenB vaccination. Why providers working in an HMO setting were less likely to initiate a discussion is unclear. This may reflect different decisions regarding the cost benefit ratio of MenB vaccination in an HMO as opposed to other practice settings or may reflect more centralized decision processes in HMO settings.
Our data demonstrate a lack of familiarity with many aspects of serogroup B meningococcal disease and MenB vaccine among primary care providers. For example, the incidence of serogroup B meningococcal disease, the vaccines’ effectiveness, and the duration of protection afforded by the vaccines were among the top five reasons supporting a higher likelihood of provider recommendation. In truth, the low incidence of serogroup B meningococcal disease might be expected to be a likely reason for not recommending these vaccines. The vaccines’ effectiveness against clinical disease had not been demonstrated at the time, but was inferred based on an immunologic marker of protection, and the two licensed vaccines were not expected to provide protection against all serogroup B strains circulating.4 In addition, duration of protection provided by MenB vaccines is unknown and studies have shown a rather steep decline in antibodies for both vaccines, suggesting protection may in fact be short-lived.13,14 Finally, sizable portions of respondents, especially FPs, reported they “didn’t know” how these factors influenced recommendation decisions. Providers were also more likely to recommend MenB vaccine at pre-college visits rather than routine 16–18 year old health maintenance visits, as recommended by ACIP, possibly as the result of the extensive publicity around college outbreaks of serogroup B meningococcal disease. They were more likely to initiate a discussion regarding the vaccine if they were aware of serogroup B meningococcal disease outbreaks in their state, despite the fact that most cases are not outbreak related.9 Approximately 10% of Peds and one-third of FPs reported recommending MenB vaccine to healthy 11–12 year olds, indicating confusion with timing for the first MenACWY vaccine. In addition, 19% of Peds and 43% of FPs reported making no recommendation regarding MenB vaccine for children aged ≥10 years at increased risk for meningococcal disease. MenB vaccination in this group is a Category A, rather than Category B recommendation, therefore this is an important misunderstanding among primary care physicians. These findings suggest a need to develop methods of better highlighting differential recommendations for the same vaccine in different patient groups.
The rather substantial differences in awareness regarding MenB vaccine and in delivery practices seen between Peds and FPs reflect prior literature. Similar to previous studies regarding childhood15–20 and adolescent vaccines,21–26 FPs were much less likely to have been aware of MenB vaccine before our survey and were less likely to report initiating a discussion or recommending MenB vaccine or to administer MenB vaccine in their office. The multivariable model suggests that lack of knowledge regarding MenB vaccine was a major contributing factor in not initiating vaccine discussions. FPs were also more likely to report they didn’t know about many of the factors queried as reasons for recommending or not recommending MenB vaccine. As discussed in previous literature, FPs may have different attitudes and practices than Peds related to the fact that they may see fewer or less severe cases of certain childhood diseases, have more competing demands given their focus on both children and adults, and may face more barriers related to vaccine financing and vaccine supply.16,27
There are strengths and limitations to our data. We surveyed large, nationally representative samples of Peds and FPs and achieved high response rates. The responses of our sentinel physicians may not be fully generalizable, especially since participating providers are aware they are going to be surveyed about vaccine-related issues. However, previous work has demonstrated the sampling methods described yield similar responses to the most commonly employed method of sampling physicians nationally.10 Non-respondents may have had different views than respondents, although the high response rates somewhat mitigate against this source of bias. The survey was conducted a year after the Category B recommendations were made and results might differ if a longer timeframe after the recommendations had been used. Finally, physicians’ reported frequency of initiating a discussion about MenB vaccine or recommending it were based on self-report rather than direct observation and responses may reflect social desirability bias.
Primary care physicians are responding to the new Category B recommendation for MenB vaccine in a variety of ways, which might be expected from this type of recommendation. Our data suggest that lack of knowledge about serogroup B meningococcal disease or awareness of MenB vaccine may be a primary motivation for not initiating a discussion for many, rather than clinician or parent/patient assessment of the risk and benefit of these vaccines. Many primary care physicians do not appear to be familiar enough with the data required to have a well-informed discussion with parents and patients about the pros and cons of MenB vaccination in healthy adolescents. In addition, sizable percentages are unaware of the routine recommendation for MenB vaccination in children aged ≥10 years at increased risk. Lack of awareness about MenB vaccine may not be surprising given the competing demands of primary care, the low prevalence of serogroup B meningococcal disease, and the relative newness of the recommendation. Our data highlight the challenges providers face with implementing recommendations for vaccination based on individual clinical decision-making when they have limited experience with a disease and limited knowledge of a new vaccine. Since category B recommendations are likely to continue to occur in certain situations, it will be key for national clinical organizations such as the AAP and AAFP to provide as specific guidance as possible about how to implement Category B recommendations for different vaccines, including talking points, to assist in the complex decision making that such a recommendation requires.
Acknowledgments
FUNDING SOURCE: This investigation was funded by the Centers for Disease Control and Prevention (grant #U01IP000849).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS:
- AAFP
American Academy of Family Physicians
- AAP
American Academy of Pediatrics
- CDC
Centers for Disease Control and Prevention
- ACIP
Advisory Committee on Immunization Practices
- MenB
Serogroup B meningococcal vaccine
- Peds
Pediatricians
- FPs
Family Physicians
Footnotes
CONFLICT OF INTEREST: The authors have no conflict of interests.
FINANCIAL DISCLOSURE: The authors have no financial disclosures.
DISCLOSURES: Portions of this article were presented at the Pediatric Academic Societies’ Annual Meeting; San Francisco, CA, May 6–9, 2017.
WHAT’S KNOWN ON THIS SUBJECT: In 2015, the Advisory Committee on Immunization Practices recommended 16–23 year olds may be vaccinated with serogroup B meningococcal vaccine based on individual clinical decision-making (Category B). Little is known about how primary care physicians are adopting these recommendations.
WHAT THIS STUDY ADDS: A minority of physicians are discussing MenB vaccine during routine 16–18 year-old visits. Significant gaps in knowledge about serogroup B meningococcal disease and MenB vaccine exist and appear to be a major driver of decisions not to discuss the vaccines.
TABLE OF CONTENTS SUMMARY: This study examines how primary care physicians are adopting serogroup B meningococcal vaccine after it received a category B recommendation by ACIP in 2015.
CONTRIBUTOR’S STATEMENT: Dr. Kempe conceptualized and designed the study, contributed to the data collection instrument design, drafted the initial and final manuscript; Drs. Allison, O’Leary and Hurley and Ms. MacNeil, Lindley and Albert assisted in study design and creation of the data collection instrument and reviewed and revised the manuscript; Dr. Crane conceptualized and designed the study, designed the data collection instrument, and reviewed and revised the manuscript; Ms Beaty contributed to the study design, carried out the initial and further analyses, and reviewed and revised the manuscript; Ms. Brtnikova contributed to the study design and data collection instrument design, coordinated and supervised all data collection, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
References
- 1.Kaplan SL, Schutze GE, Leake JA, et al. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics. 2006;118(4):e979–984. [DOI] [PubMed] [Google Scholar]
- 2.Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010;50(2):184–191. [DOI] [PubMed] [Google Scholar]
- 3.MacNeil JR, Blain AE, Wang X, Cohn AC. Current Epidemiology and Trends in Meningococcal Disease-United States, 1996–2015. Clin Infect Dis 2017. [DOI] [PubMed] [Google Scholar]
- 4.MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of Serogroup B Meningococcal Vaccines in adolescents and young adults: Recommendations of the ddvisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep 2015;64(41):1171–1176. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report, 2016. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf. Accessed April 3, 2018.
- 6.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR, Centers for Disease C. Use of Serogroup B Meningococcal Vaccines in Persons Aged >/=10 Years at Increased Risk for Serogroup B Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep 2015;64(22):608–612. [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed F. U.S. Advisory Committee on Immunization Practices (ACIP) Handbook for Developing Evidence-based Recommendations, Version 1.2. 2013; https://www.cdc.gov/vaccines/acip/recs/grade/downloads/handbook.pdf. Accessed July 5, 2017.
- 8.American Academy of Family Physicians. Meningococcal Disease Vaccine. 2016; https://www.aafp.org/patient-care/public-health/immunizations/disease-population/meningococcal.html. Accessed April 4, 2018.
- 9.Committee On Infectious Diseases. Recommendations for Serogroup B Meningococcal Vaccine for Persons 10 Years and Older. Pediatrics. 2016;138(3). [DOI] [PubMed] [Google Scholar]
- 10.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. EvalHealth Prof 2008;31(1):43–64. [DOI] [PubMed] [Google Scholar]
- 11.Dillman DA, Smyth J, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Desgin Method, 3rd Edition. Vol 3rd New York, NY: John Wiley Co.; 2009. [Google Scholar]
- 12.Marshall GS, Tan L. Understanding the Category B Recommendation for Serogroup B Meningococcal Vaccine. Pediatrics. 2017;139(5). [DOI] [PubMed] [Google Scholar]
- 13.Santolaya ME, O’Ryan M, Valenzuela MT, et al. Persistence of antibodies in adolescents 18–24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine. Hum Vaccin Immunother 2013;9(11):2304–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richmond PC, Marshall HS, Nissen MD, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012;12(8):597–607. [DOI] [PubMed] [Google Scholar]
- 15.Kempe A, Babbel C, Wallace GS, et al. Knowledge of interim recommendations and use of Hib vaccine during vaccine shortages. Pediatrics. 2010;125(5):914–920. [DOI] [PubMed] [Google Scholar]
- 16.Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the United States. Pediatrics. 2009;124(5):e809–816. [DOI] [PubMed] [Google Scholar]
- 17.Nelson NP, Allison MA, Lindley MC, et al. Physician Knowledge and Attitudes About Hepatitis A and Current Practices Regarding Hepatitis A Vaccination Delivery. Acad Pediatr 2017;17(5):562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MM, Ndiaye SM, Freed GL, Clark SJ. One-year uptake of pneumococcal conjugate vaccine: a national survey of family physicians and pediatricians. J Am Board Fam Pract 2003;16(5):363–371. [DOI] [PubMed] [Google Scholar]
- 19.Freed GL, Freeman VA, Clark SJ, Konrad TR, Pathman DE. Pediatrician and family physician agreement with and adoption of universal hepatitis B immunization. J Fam Pract 1996;42(6):587–592. [PubMed] [Google Scholar]
- 20.Schaffer SJ, Szilagyi PG, Shone LP, et al. Physician perspectives regarding pneumococcal conjugate vaccine. Pediatrics. 2002;110(6):e68. [DOI] [PubMed] [Google Scholar]
- 21.Allison MA, Hurley LP, Markowitz L, et al. Primary Care Physicians’ Perspectives About HPV Vaccine. Pediatrics. 2016;137(2):e20152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison MA, Cohn AC, Stokley S, et al. Timing of adolescent meningococcal conjugate vaccination attitudes and practices of pediatricians and family medicine physicians. AmJ Prev Med 2011;41(6):581–587. [DOI] [PubMed] [Google Scholar]
- 23.Schaffer SJ, Humiston SG, Shone LP, Averhoff FM, Szilagyi PG. Adolescent immunization practices: a national survey of US physicians. Arch Pediatr Adolesc Med 2001;155(5):566–571. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey AF, Cowan AE, Broder KR, Kretsinger K, Stokley S, Clark SJ. Adolescent Tdap vaccine use among primary care physicians. J AdolescHealth. 2009;44(4):387–393. [DOI] [PubMed] [Google Scholar]
- 25.Oster NV, Phillips-Tangum CA, Averhoff F, Howell K. Barriers to adolescent immunization: a survey of family physicians and pediatricians. J Am Board Fam Pract 2005;18(1):13–19. [DOI] [PubMed] [Google Scholar]
- 26.Davis MM, Broder KR, Cowan AE, et al. Physician attitudes and preferences about combined Tdap vaccines for adolescents. Am J PrevMed 2006;31(2):176–180. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary ST, Parashar UD, Crane LA, et al. Adoption of rotavirus vaccine by U.S. physicians: progress and challenges. AmJ Prev Med 2013;44(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]