Abstract
The preS antigen of hepatitis B virus (HBV) corresponds to the N-terminal polypeptide in the large (L) antigen in addition to the small (S) antigen. The virus-like particle (VLP) of the S antigen is widely used as a vaccine to protect the population from HBV infection. The presence of the S antigen and its antibodies in patient blood has been used as markers to monitor hepatitis B. However, there is very limited knowledge about the preS antigen. We generated a preS VLP that is formed by a chimeric protein between preS and hemagglutinin (HA), and the matrix protein M1 of influenza virus. The HBV preS antigen is displayed on the surface of preS VLP. Asn112 and Ser98 of preS in VLP were found to be glycosylated and O-glycosylation of Ser98 has not been reported previously. The preS VLP shows a significantly higher immunogenicity than recombinant preS, eliciting robust anti-preS neutralizing antibodies. In addition, preS VLP is also capable of stimulating preS-specific CD8+ and CD4+ T cell responses in Balb/c mice and HBV transgenic mice. Furthermore, preS VLP immunization provided protection against hydrodynamic transfection of HBV DNA in mice. The data clearly suggest that this novel preS VLP could elicit robust immune responses to the HBV antigen, and can be potentially developed into prophylactic and therapeutic vaccines.
Keywords: Hepatitis B virus, preS, IFN-γ, Glycosylation, Prophylactic efficacy, Therapeutic potential
1. Introduction
Under the current immunization and antiviral programs, hepatitis B virus (HBV) infection remains a major global public health problem. Approximately 2 billion people have been infected worldwide during their lifetime, and more than 350 million are chronic carriers of the virus. HBV infection may cause acute and chronic hepatitis, which leads to liver cirrhosis (LC) and hepatocellular carcinoma (HCC). Current HBV vaccines on the market are based on the virus-like particle (VLP) of the Santigen, and effectively prevent most people from acquiring HBV infection. However, almost 5–10% people vaccinated with the available vaccines fail to mount an adequate antibody response to offer protection. As an additional antigen on infectious HBV particles, preS could also provide B and T cell epitopes which may promote the humoral and cellular responses and enhance the seroprotection rate by overcoming non-responsiveness to the S antigen-only vaccines (Milich, 1988).
There are three envelope proteins in the HBV virion, S, M and L. The preS protein is part of the L protein, a 163 amino acid extension at the N-terminus of the S protein (genotype A). preS may be further divided as preS1 (a.a.1–108) and preS2 (a.a.109–163). In the M protein, only preS2 is present at the N-terminus in addition to the amino acids that are common among the S, M and L proteins. Two well-known functions are related to preS. First of all, preS includes the region that interacts with the specific host receptor (Yan et al., 2012). Previous studies have mapped the interaction motif to be within the first 48 amino acids of preS (Glebe et al., 2005). The other important function of preS is that in the preS region, there are highly immunogenic sites as B and T cell epitopes. It has been reported that preS could induce humoral responses in mice which were nonresponsive to the S antigen, indicating that preS represents a potential antigen for novel HBV vaccine candidates (Milich, 1988). Notably, humoral response may play a major role in preventing HBV spreading to uninfected cells. In addition, it is generally believed that a proper CD4+ helper T cell response is a prerequisite for an adequate humoral response. Furthermore, T cell responses may help extend the longevity of humoral immunity. However, how these preS epitopes are related to virus clearance is not very clear. It is widely accepted that the CD8+ T cell response is primarily responsible for HBV clearance in both cytopathic and noncytopathic manner, and the HBV-specific CD8+ T cells are able to clear HBV from infected hepatocytes by secretion of antiviral cytokines, such as IFN-γ, and TNF-α.
VLPs resemble authentic native viruses in structure and morphology, but are non-infectious, because they assemble without containing the genetic material (Grgacic and Anderson, 2006). Compared to individual proteins or peptides, VLPs significantly improve humoral responses by presenting conformational epitopes more similarly as the native virus. Owing to their highly repetitive surface, VLPs are capable of eliciting robust B cell responses in the absence of adjuvants by efficiently cross-linking specific receptors on B cells. Besides, VLPs could also induce potent cytotoxic T lymphocyte (CTL) responses in immunized animals.
In this study, we have designed a preS VLP that consists of influenza M1 protein and the transmembrane domain and cytoplasmic tail of influenza hemagglutinin (H A) as the scaffold. The HBV preS antigen is displayed on the surface of VLP when it is fused with the HA fragment. We assessed the immunogenicity of preS VLP in mice. Here we report that immunization with preS VLP induced both potent humoral and cellular immune responses in Balb/c mice and HBV transgenic mice, and protected mice from hydrodynamic transfection of HBV DNA, indicating its potential as an effective prophylactic vaccine candidate or a potential therapeutic vaccine against HBV infection.
2. Materials and methods
Materials and methods are provided in the Supporting Information.
3. Results
3.1. Construction and preparation of preS VLP
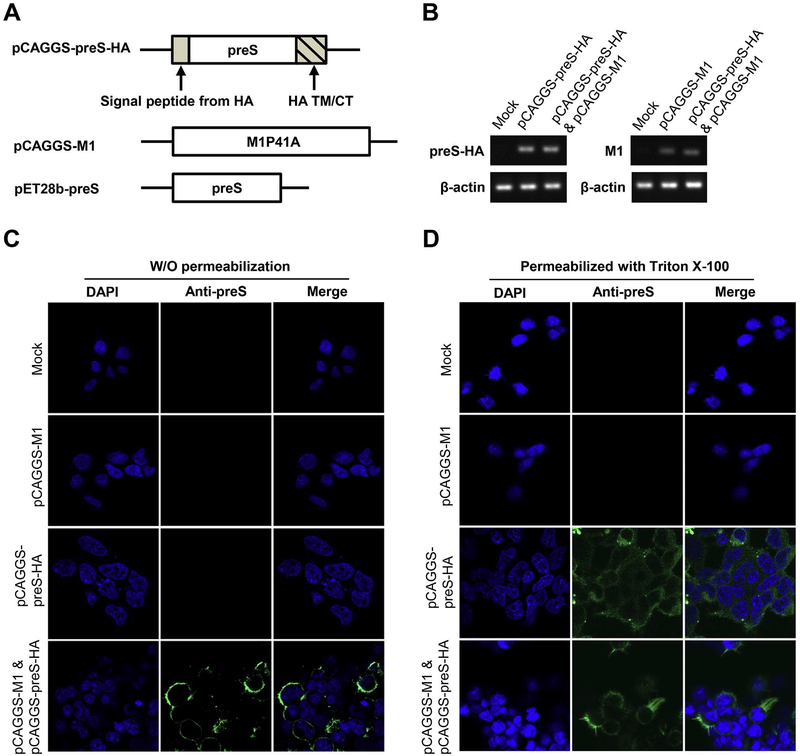
Co-expression of influenza virus M1 and HA releases a VLP decorated with HA antigen (Galarza et al., 2005). In order to generate a VLP that is decorated with HBV preS antigen, we constructed a chimeric protein that has the preS sequence fused at the N-terminus of a HA fragment that includes its transmembrane domain and the cytoplasmic tail (Fig. 1A). The signal peptide from HA was also added in front of the preS sequence which may be removed after the chimeric protein is expressed. The amino acid 41 of M1 was mutated to Ala to enhance the release of the VLP (Campbell et al., 2014). After transfection of 293T cells with pCAGGS-M1, pCAGGS-preS-HA, or both plasmids, respectively, total RNAs were extracted and analyzed for the transcription levels of M1 and preS-HA. The qRT-PCR results suggested that both M1 and preS-HA genes had been transcribed adequately in 293T cells 48 h after transfection (Fig. 1B). After co-transfection of pCAGGS-M1 and pCAGGS-preS-HA into 293T cells, expression of the preS antigen was readily detected by immunofluorescent microscopy (Fig. 1C and D). If the M1 protein was not co-expressed, the preS-HA chimeric protein appeared to be unable to expose the preS antigen on the exterior of the cellular membrane because the preS antigen was only detectable after the cellular membrane was permeabilized with Triton X-100 (Fig. 1C and D).
Fig. 1. Construction and production of preS VLP.
(A) Schematic representation of pCAGGS-preS-HA and pCAGGS-Ml used for preS VLP production, and pET28b-preS used for the expression of recombinant preS antigen. (B) qRT-PCR analysis was used to confirm the transcription of preS-HA and M1. (C, D) Immunofluorescent imaging of preS-HA expressing cells. (C) 293T cells were transfected with mock control, pCAGGS-M1, pCAGGS-preS-HA, or both plasmids. Cells were not permeabilized with Triton X-100 before staining. The nuclei were stained w ith DAPI (blue), and the preS antigen was stained with purified polyclonal rabbit anti-preS antibody, detected with Alexa Fluor® 488-Conjugated goat anti-rabbit secondary antibody. (D) The cells were the same as those in panel C, except that the cells were permeabilized with Triton X-100 before staining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
There has been an interesting observation of L antigen topology (Prange and Streeck, 1995; Lambert et al., 2004; Bruss, 2007). It was found that the preS region of the L antigen may be at both the side of cytosol or the side of lumen of ER membrane (Prange and Streeck, 1995). The association of the preS region with the S region in the L antigen is critical to the preS topology of the L antigen (Lambert et al., 2004). In our construct of VLP, the S region of the L antigen is not present and the N-terminus of preS may not be myristoylated. preS in the chimeric protein is completely in the cytosol side when preS-HA is expressed alone. In co-expression, M1 allows preS to be presented on the exterior of the cytoplasmic membrane, likely through its interactions with the cytoplasmic tail of HA. In addition, M1 also plays the critical role of assembly and budding of VLP (see below).
3.2. Purification and characterization of preS VLP
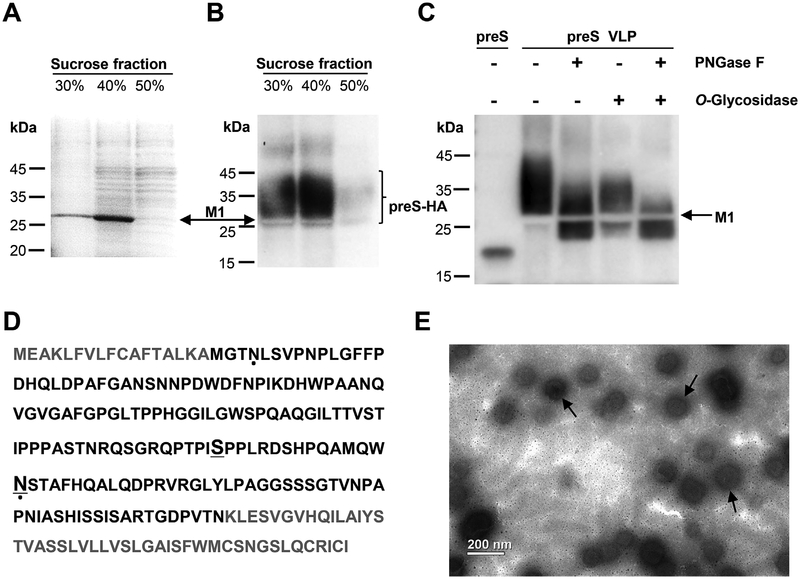
Next, we set out to purify the preS VLP from supernatant of the transfected 293T cells. Recombinant preS was also produced in E. coli (Lian et al., 2007) (Fig. S1) and was used to generate specific polyclonal antibodies. The culture media were collected from the cells 72 h post-cotransfection of pCAGGS-M1 and pCAGGS-preS-HA. The culture media were laid on a sucrose gradient and subjected to ultracentrifugation. A sample was collected from each of the 30%, 40% or 50% sucrose fractions. By SDS-PAGE and western blot analysis, the protein corresponding to the preS-HA antigen was identified (Fig. 2A and B). Based on western blot analysis, the secreted preS-HA spans a region of much larger molecular weight than the calculated molecular weight of the preS-HA chimeric protein. We concluded that the secreted forms of preS-HA are glycosylated, consistent with previous reports of the L antigen (Hassemer et al., 2017; Schmitt et al., 2004; Lambert and Prange, 2007). A band consistent with the molecular weight (27.8 kDa) of M1 was shown in Fig. 2A. This could account for the appearance of an unstained region (about 27.8 kDa) in western blot due to that M1 occupies the position (Fig. 2B and C). The sample was further characterized by liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS). The M1 sequence identified by MS analysis covered above 90% of the full length M1 sequence (Fig. S2). A negative stain electron micrograph showed that the sucrose fractions contain virus-like particles (Fig. 2E). These data showed that VLPs composed of M1 and preS-HA proteins were successfully made and purified.
Fig. 2. Characterization of preS VLP.
(A) SDS-PAGE analysis of fractions from sucrose gradient centrifugation. The major protein component is in the 40% sucrose fraction. The position of M1 is indicated by the arrow. (B) Western blot analysis with purified polyclonal rabbit anti-preS antibody. Lanes 1 and 2 showed the presence of the preS-HA antigen in fractions from sucrose gradient centrifugation. The unstained region corresponds to M1. (C) The sample in 40% sucrose fraction was treated with Peptide -N-Glycosidase F (PNGase F) and/or O Glycosidase, and the preS antigen was detected by western blot. (D) Identification of glycosylated sites in preS-HA. The preS domain in preS-HA is colored black. Glycosylated residues identified in this study are underlined. Glycosylated residues which have been reported previously are indicated by dots (Lambert and Prange, 2007). (E) An electron micrograph showing preS virus-like particles (preS VLP). Magnification, 11,000 ×.
To map glycosylation sites, we used Peptide -N-Glycosidase F (PNGase F) and/or O -Glycosidase to treat the 40% sucrose fraction, respectively. When preS VLP was treated with PNGase F to remove N-linked glycans, and O -Glycosidase to remove O-linked glycans, or both, a band with reduced molecular weight was observed (Fig. 2C). The molecular weight of the band is consistent with that of preS-HA. When O -Glycosidase was used alone, a smaller portion of deglycosylated preSHA was observed. The remaining protein also seemed to have a lower molecular weight, suggesting that the majority of preS-HA in VLP was modified with both N-linked and O-linked glycans. This is consistent with the pattern when both glycosidases were used to treat preS VLP. When PNGase F was used alone, on the other hand, a more significant amount of deglycosylated preS-HA was observed, suggesting a good portion of preS-HA in VLP contains only N-Linked glycans. preS VLP was further characterized by LC-MS/MS and analyzed by Byonic. Both preS-HA and M1 sequences identified by MS analysis covered above 85% of the full length sequences (Fig. S2). Asn112 and Ser98 within the preS domain were predicted to be modified by N-glycan and O-glycan, respectively (Fig. 2D, and Table S1). N-glycosylation of Asn4 and Asn112 has been reported previously (Lambert and Prange, 2007). However, we did not identify peptides containing glycosylated Asn4, which may be due to the experimental condition of MS. Interestingly, O-glycosylation of Ser98 has not been reported previously, the physiological role of O-glycosylated Ser98 remains to be elucidated.
3.3. preS VLP elicits robust neutralizing antibodies in Balb/c mice
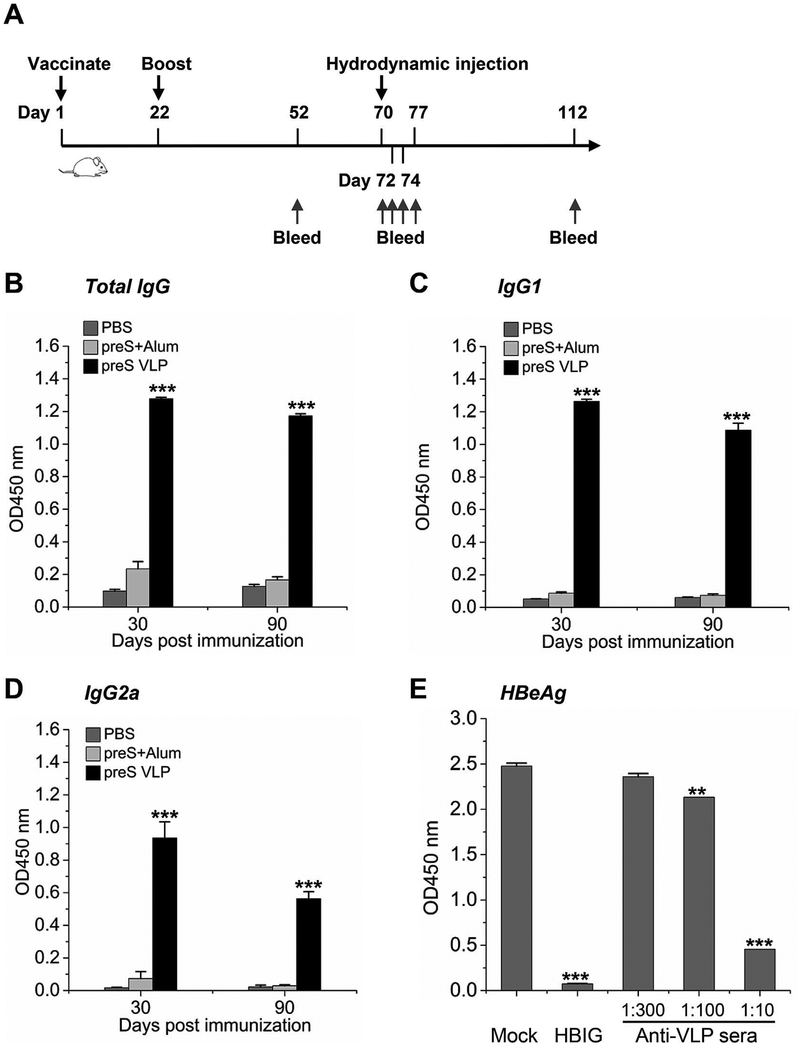
In order to investigate humoral immunogenicity of preS VLP, Balb/c mice were immunized each with 10 μg of preS VLP total protein, or recombinant preS (Lian et al., 2007) with alum adjuvant, respectively (Fig. 3A). PBS was used as a mock control. A booster was given on day 22. Blood samples were collected from each mouse on day 52 and 112, and sera were prepared from these samples. All serum samples were diluted by various folds before the serum antibody titers were determined by ELISA (Fig. S5). The data revealed that preS VLP is more potent than recombinant preS protein in generating anti-preS antibodies, even without the use of any adjuvant (Fig. 3B–D). In particular, preS VLP elicited high level anti-preS total IgG including both anti-preS IgG1 (Th2 isotype) and IgG2a (Th1 isotype), indicating a balanced Th1/Th2 response against preS VLP.
Fig. 3. preS VLP elicits superior neutralizing antibodies compared to recombinant preS vaccination.
Balb/c mice were immunized intramuscularly with preS VLP (n=6), recombinant preS protein (n=6), or PBS (n = 6). (A) Time schedule for preS VLP vaccination to prevent hydrodynamic transfection of HBV DNA. (B–D) The serum anti-preS titers were determined by ELISA. The plates were coated with 1 μg/mL purified recombinant preS. The immunization condition is labeled next to chart color codes. The sera were diluted by 100 folds in all the assays. (E) Neutralization of HBV in fectivity in HepG2/hNTCP cells by mouse anti-preS VLP sera or hepatitis B immunoglobulin (HBIG) (0.144 mg/mL). The mouse anti- preS VLP sera were diluted by various folds. HBeAg values at one week post infection were measured using an ELISA kit. The data are represented as mean ± SEM.
To further test whether anti-preS VLP sera could block HBV from infecting human hepatocytes, in vitro infection experiments were conducted. Using hepatitis B immunoglobulin (HBIG) as a positive control, the anti-preS VLP sera from three mice clearly prevented HBV from infecting HepG2/hNTCP cells, as demonstrated by a decreased level of HBeAg in the supernatant of cell culture (Fig. 3E). Collectively, these results indicate that preS VLP can stimulate anti-preS neutralizing antibodies in mice.
3.4. preS VLP provokes strong T cell responses in Balb/c mice
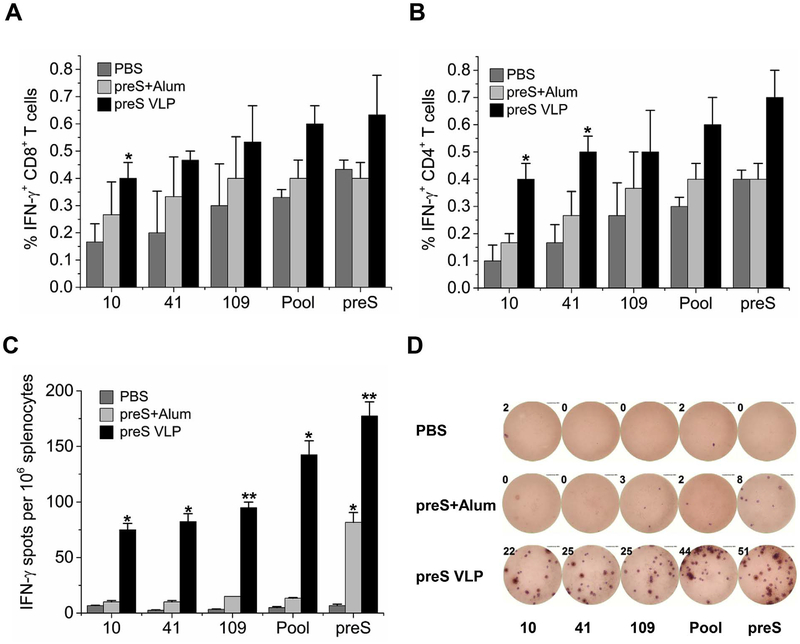
T cell responses play a vital role in the induction of humoral immunity, and are crucial to effectiveness of a therapeutic HBV vaccine (Celis et al., 1984; Chisari et al., 2010). To evaluate whether preS contained vital immunogenic epitopes to elicit strong T cell responses, we used preS VLP and preS protein to immunize Balb/c mice. Splenocytes were harvested and stimulated with preS-specific T cell peptides (Table 1) (Doh et al., 2003; Pajot et al., 2006; Ferrari et al., 1992). After stimulation for 6 h, T cell responses were determined using flow cyto-metric analysis (FACS) and ELISPOT (Fig. 4). FACS data showed that mice immunized with preS VLP elicited a higher percentage of preS-specific IFN-γ-producing CD4+ and CD8+ T cells than either the controls or those immunized with recombinant preS protein (Fig. 4A and B). ELISPOT also demonstrated that preS-specific IFN-γ-producing T cells are more abundant in mice immunized with preS VLP than either the controls or those immunized with recombinant preS protein (Fig. 4C and D). Since IFN-γ-producing T cells play a key role in controlling and clearing HBV in infected individuals, our results implicate that preS VLP can evoke potent preS-specific T cell responses that may be important in HBV clearance.
Table 1.
preS-specific T cell epitopes.
| Epitope | Residues | Amino acid sequence | Reference |
|---|---|---|---|
| 1 | preSl 10–19 | PLGFFPDHQL | (Ferrari et al., 1992) |
| 2 | preSl 41–56 | WPAANQVGVGAFGPGL | (Doh et al., 2003) |
| 3 | preS2 109–134 | MQWNSTAFHQALQDPRVRGLYLPAGG | (Pajot et al., 2006) |
Fig. 4. preS V LP induces stronger T cell responses than recombinant preS vaccination.
Balb/c mice were immunized intramuscularly with preS VLP (n = 6), recombinant preS protein (n = 6), or PBS (n = 6). 30 days postimmunization, splenocytes were isolated and analyzed for CD8, CD4, and IFN-γ expression by flow cytometry (A–B). CD8+ T cells (A) or CD4+ T cells (B) were gated, and IFN-γ-producing cells are presented as the percent average from each group. (C) Splenocytes were isolated and analyzed for IFN-γ expression using an IFN-γ ELISPOT assay. ELISPOT experiments were performed in triplicate wells per condition. (D) Representative images of ELISPOT from each group are shown. All values are presented as the average from each group, and error bars indicate ± SEM. Pool, indicates the mixture of peptide 10, 41 and 109, and preS indicates recombinant preS protein.
3.5. preS VLP provides protection against hydrodynamic transfection of H B V D N A
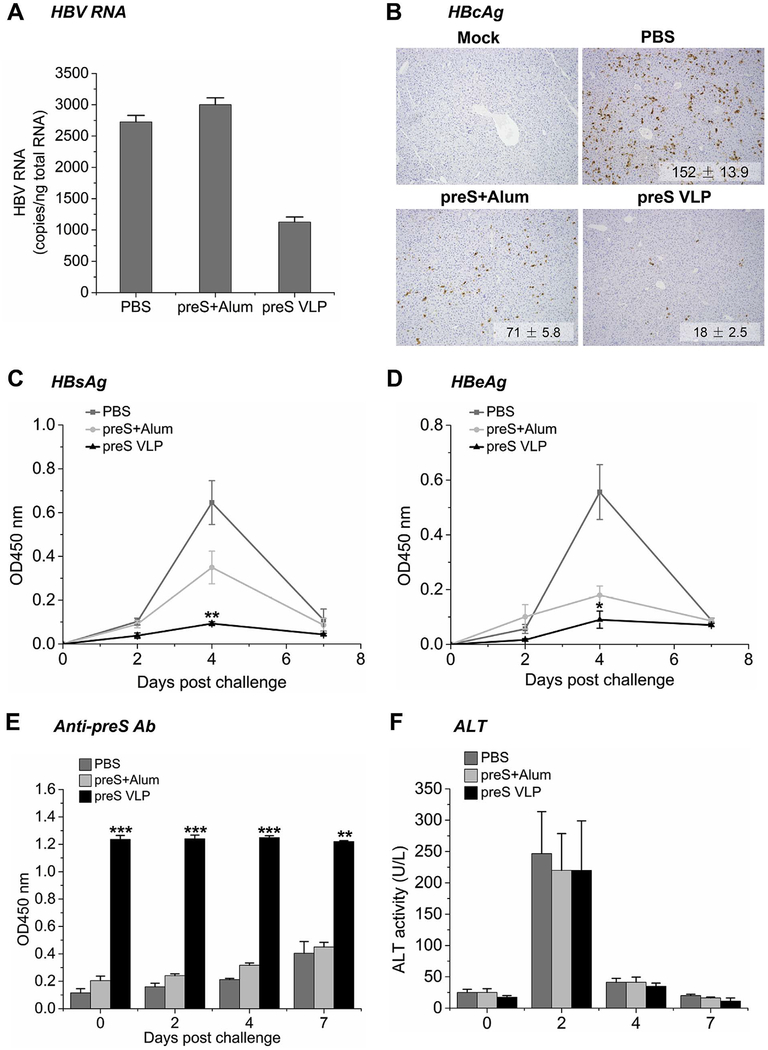
To test whether preS VLP-induced T cell responses played a role in protection against HBV infection, mice immunized with preS VLP or preS protein were hydrodynamically transfected with HBV DNA on day 70 of post-immunization (Fig. 3A). HBV replication was induced by hydrodynamic injection of pHBV1.3 plasmid (10 μg/mouse) that contains a 1.3-fold-overlength genome of HBV. Liver tissues were collected on day 77 for analysis of HBV RNA copies, viral antigen load, and humoral responses. HBV RNA copies from about 50 mg of each tissue sample were measured by qRT-PCR (Fig. 5A). The levels of HBV RNA in preS VLP-immunized animals were lower than that in animals immunized with recombinant preS. Liver sections stained for HBV core antigen indicated that the HBcAg-positive hepatocytes were eliminated almost entirely in preS VLP-immunized animals, but persisted in preS-immunized animals (Fig. 5B). We also used ELISA assays to detect HBV proteins including HBsAg and HBeAg in the serum samples collected from each immunized mouse on day 0, 2, 4 and 7 after hydrodynamic transfection of HBV DNA. As shown in Fig. 5C, HBsAg levels in preS VLP-immunized animals were nearly undetectable throughout 7 days, but rose to high levels in animals immunized with recombinant preS on day 4 after transfection. The serum levels of HBeAg also remained nearly undetectable in preS VLP-immunized animals over the course of 7 days. On the other hand, HBeAg levels in preS-immunized animals elevated on day 4 then dropped to nearly undetectable levels on day 7 after transfection (Fig. 5D). This phase of HBsAg and HBeAg clearance coincides with the development of anti-preS neutralizing antibodies (Fig. 5E). In line with previous study (Yang et al., 2002), serum alanine aminotransferase (ALT) activity increased significantly on day 2, indicative of successful transfection (Fig. 5F). Subsequently, the ALT activity of all mice returned to baseline by day 7 after transfection, implying that preS VLP did not cause liver damage. This was further supported by H&E staining, which suggested that no obvious necroinflammatory lesions were observed in liver section of mice from preS VLP group on day 7 after transfection (Fig. S3). Collectively, preS VLP prophylactically protects mice against hydrodynamic transfection of HBV DNA.
Fig. 5. Immunization with preS VLP offers protection against hydrodynamic transfection of HBV DNA.
Balb/c mice were immunized intramuscularly with preS VLP (n = 6), recombinant preS protein (n = 6), or PBS (n = 6). On day 70, HBV replication was induced by hydrodynamic injection of pT-HBV1.3 plasmid via tail vein (10 μg per mouse). (A) Liver-associated HBV RNA copies were measured by qRT-PCR. (B) Immunohistochemistry analysis of liver tissues. Quantifications represent average numbers of HBcAg-positive cells per 100 × field (n = 3 with 5 fields counted per sample). Representative images are shown. Magnification, 100 ×. Serum analysis for HBsAg (C), HBeAg (D) and anti-preS antibody titers (E) was completed by ELISA on days 0, 2, 4, and 7 post transfection. (F) Serum alanine aminotransferase (ALT) activity was determined with a Hitachi 7600 Autom atic Biochemistry Analyzer. All values are presented as the average from each group, and error bars indicate ± SEM.
3.6. preS VLP immunization stimulates potent humoral and cellular responses in HBV transgenic mice
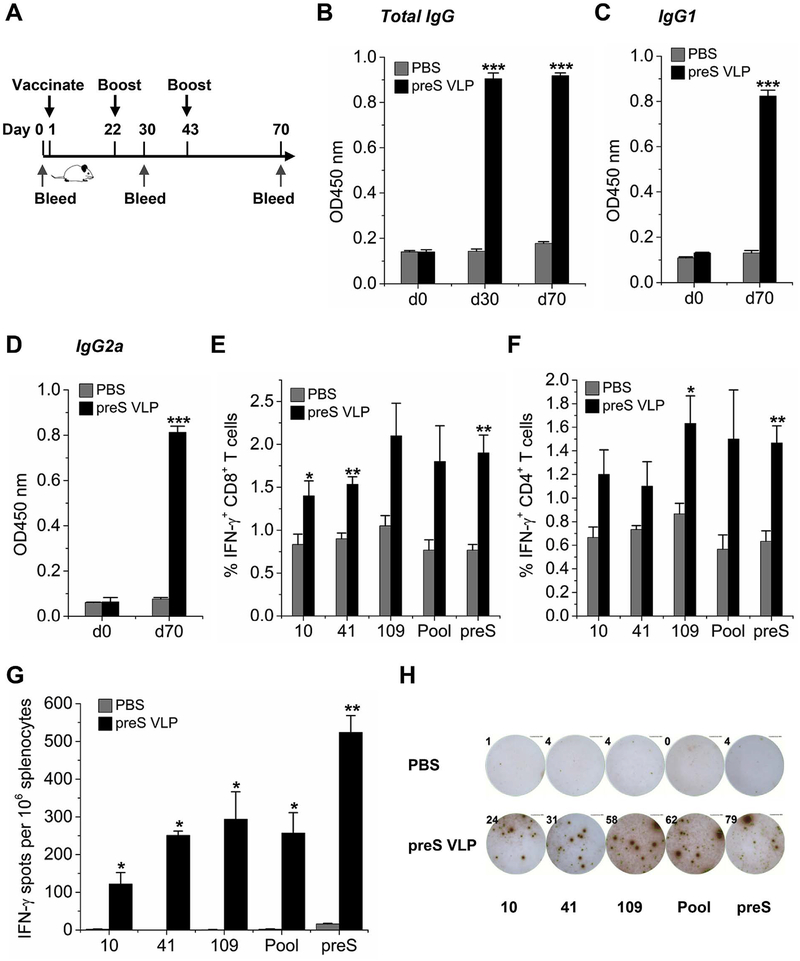
We next set out to investigate the therapeutic potential of preS VLP immunization by employing HBV transgenic mice as a model of chronic infection. HBV transgenic mice were first primed with preS VLP or PBS, and then were boosted on days 22 and 43, respectively. Despite that HBV transgenic mice have become tolerance to HBV (Allweiss and Dandri, 2016), preS VLP could still induce high levels of anti-preS total IgG including botHAnti-preS IgG1 (Th2 isotype) and IgG2a (Th1 isotype) (Fig. 6B–D), suggesting a balanced Th1/Th2 response against preS VLP. In addition, preS VLP immunization could stimulate a higher percentage of preS-specific IFN-γ-producing CD4+ and CD8+ T cells than the control by FACS analysis (Fig. 6E and F). Furthermore, ELI-SPOT results also showed that preS-specific IFN-γ-producing T cells are more plentiful in mice immunized with preS VLP than the control (Fig. 6G and H), implying the therapeutic potential of preS VLP. Taken together, preS VLP vaccine could induce preS-specific CD8+ and CD4+ T cell responses in HBV transgenic mice.
Fig. 6. Immunization with preS VLP induces robust anti-preS antibodies and T cell responses in HBV transgenic mice.
HBV transgenic mice were immunized intramuscularly with preS VLP (n = 6) or PBS (n = 6). (A) Time schedule for preS VLP as a vaccine to treat HBV transgenic mice. (B–D) The serum anti-preS titers were determined by ELISA. The plates were coated with 1 μg/mL purified recombinant preS. The immunization condition is labeled next to chart color codes. The sera were diluted by 100 folds in all the assays. CD8+ T cells (E) or CD4+ T cells (F) were gated, and IFN-γ-producing cells are presented as the percent average from each group. (G) On day 70, splenocytes were isolated and analyzed for IFN-γ expression by ELISPOT assays. ELISPOT experiments were performed in triplicate wells per condition. (H) Representative images of ELISPOT from each group are shown. All values are presented as the average from each group, and error bars indicate ± SEM.
4. Discussion
Previous studies of immune responses to the preS antigen, or preS1 and preS2, were largely carried out with recombinant proteins or synthetic peptides (Lempp and Urban, 2014; Cornelius et al., 2016; Chen et al., 2016; Sankhyan et al., 2016; Kim et al., 2015; Zhang et al., 2013; Toita et al., 2015). These polypeptides do not have the folded structure or glycosylation of preS found in HBV particles. The full extent of immune responses to the preS antigen may not have been recapitulated by these reports. We have successfully developed a unique system to produce preS VLP. In contrast to recombinant preS, preS VLP is able to evoke much higher levels of neutralizing antibodies in Balb/c mice, even without any adjuvants. This is likely attributed to that preS VLPs display highly repetitive, glycosylated and folded preS antigen on the surface. Importantly, the sera from mice immunized with preS VLP is capable of blocking HBV from infecting HepG2/hNTCP cells in vitro. Moreover, the preS VLP could provide protection against HBV in the hydrodynamic transfection model. Anti-preS1 antibodies were previously found to be effective in neutralization of HBV (Neurath et al., 1989). The identification of protective epitopes within the preS1 region reveals that preS1 specific antibodies neutralize the virus by blocking the binding of host NTCP receptor (Yan et al., 2012; Sankhyan et al., 2016). The potent antigenicity of preS VLP suggests that its preS antigen may have similar immunological properties of HBV particles.
Therapeutic vaccination requires multiple T cell responses, especially the CD8+ T cell responses (Celis et al., 1984; Chisari et al., 2010). It is generally believed that immunization with a therapeutic vaccine would stimulate the immune system to elicit a specific CD8+ T cell response that is capable of controlling viral infection. In particular, therapeutic vaccination is fascinating for HBV because individuals who become chronically infected have a weak and narrowly focused CD8+ T cell response (Barnaba et al., 1989; Nayersina et al., 1993). Compared to chronically infected individuals, there are greater numbers of HBV-specific IFN-γ secreting CD4+ and CD8+ T cells in individuals that resolve acute infections (Bertoletti and Gehring, 2006). Indeed, preS VLP induce potent CD4+ and CD8+ T cell responses both in Balb/c mice and HBV transgenic mice. The T cell epitopes of preS used in this study clearly reactivated specific T cell responses. In addition, humoral immune responses could also be induced by preS VLP in HBV transgenic mice. These findings suggest that preS VLP may have the therapeutic potential for chronic HBV infection.
Interestingly, the preS domain of VLP was identified to be N-gly- cosylated at Asn112 and O-glycosylated at Ser98. O-glycosylation of Ser98 has not been reported previously. Alignment of the primary sequences of preS from all HBV genotypes revealed that this residue is highly conserved except that a threonine is at position 98 in genotype E HBV preS (Fig. S4). Notably, the N-glycosylation of preS is crucial for secretion and stability of preS antigen (Lee et al., 2003). The physiological role of O-glycosylation of Ser98 and relationship between glycosylation and immunogenicity await further studies.
Due to the additional B and T cell epitopes in HBV preS region, preS represents an attractive antigen for HBV vaccine candidates that are able to overcome non-responsiveness to the S antigen-based vaccines, or break immune tolerance in patients with chronic HBV infections (Milich, 1988; Shouval et al., 2015). Previous clinical trials have shown that vaccines containing preS/S are effective and even superior to traditional S protein containing vaccine (Krawczyk et al., 2014; Madalinski et al., 2004; Rendi-Wagner et al., 2006; Schumann et al., 2007). This may be due to the stimulation of preS-specific antibodies; however, the role of anti-preS antibodies was not evaluated in these studies. On the other hand, such epitopes may contribute to the better immunogenicity of these vaccines, although T cell immune responses induced by specific T cell epitopes from preS have not been defined.
The preS antigen has been exploited previously as candidates of prophylactic or therapeutic vaccines. When recombinant preS proteins were used in immunization, the antibody titers were not very high (Sylvan et al., 2009). The ability of neutralizing HBV by these antibodies is limited. More critically, no T cell responses against HBV were demonstrated (Raz et al., 1996; Shapira et al., 2001). This is consistent with that isolated proteins generally do not induce T cell responses because they usually are not internalized by antigen presenting cells (Pennock et al., 2016). In order to overcome the barrier, virus or yeast vectors were employed to express HBV antigens (Reynolds et al., 2015; King et al., 2014). However, there are safety concerns when a replicating vector is used despite their capability to induce strong T cell responses. In addition, these vectors may only be given once because the host will establish immunity against the vector. A recent study showed that administration of preS-based allergen-derived peptides with alum adjuvant induced preS-specific antibodies that inhibit hepatitis B infection in vitro (Cornelius et al., 2016). However, the stability of this peptide vaccine may be the limitation for clinical use. Furthermore, the preS in this peptide vaccine may not adopt a similar conformation as preS in HBV particles. Our design of preS VLP has the advantages of mimicking a virus particle and being able to interact with the antigen presenting cells to elicit both CD4+ and CD8+ T cell responses, and at the same time, it permits multiple doses and does not replicate any foreign microorganisms. In summary, preS VLP represents an effective vaccine candidate for prophylactic protection and potential therapeutic intervention against HBV infection.
Supplementary Material
Acknowledgements
We thank Professor John Steel of Emory University School of Medicine for providing the cDNAs of influenza virus used in this study. We thank Professor Andy (Qiqui) Yu Indiana University School of Medicine for discussions and comments.
Funding
This work was supported in part by the Natural Science Foundation of China Grants 21372023 and 81572198; MOST 2015DFA31590, the Shenzhen Science and Technology Innovation Committee JSGG20140519105550503, JCYJ20150331100849958, JCYJ2015040 3101146313, JCYJ20160301111338144, JCYJ20160331115853521 and JSGG20160301095829250; the Shenzhen Peacock Program KQTD201103.
Abbreviations:
- HBV
hepatitis B virus
- VLPs
virus-like particles
- M1
matrix protein of influenza virus
- HA
hemagglutinin of influenza virus
- ELISA
enzym e-linked immunosorbent assay
- ELISPOT
enzym e-linked immunospot assay
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBcAg
hepatitis B core antigen
- LC
liver cirrhosis
- HCC
hepatocellular carcinoma
- CTL
cytotoxic T lymphocyte
- FBS
fetal bovine serum
- PBS
phosphate buffered saline
- qPCR
quantitative polymerase chain reaction
- ALT
alanine amino transferase
- HBIG
hepatitis B immunoglobulin
- PNGase F
Peptide -N-Glycosidase F
- H&E
hematoxylin and eosin
- FACS
flow cytometry
Footnotes
Conflicts of interest
M.L. and X.C. are the inventors of the intellectual property. The other authors declare no conflict of interest with respect to this manuscript.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2017.11.007.
References
- Allweiss L, Dandri M, 2016. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J. Hepatol 64, S17–S31. [DOI] [PubMed] [Google Scholar]
- Barnaba V, Franco A, Alberti A, Balsano C, Benvenuto R, Balsano F, 1989. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J. Immunol 143, 2650–2655. [PubMed] [Google Scholar]
- Bertoletti A, Gehring AJ, 2006. The immune response during hepatitis B virus infection. J. Gen. Virol 87, 1439–1449. [DOI] [PubMed] [Google Scholar]
- Bruss V, 2007. Hepatitis B virus morphogenesis. World J. Gastroenterol 13, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Kyriakis CS, Marshall N, Suppiah S, Seladi-Schulman J, Danzy S, et al. , 2014. Residue 41 of the Eurasian avian-like swine influenza a virus matrix protein modulates virion filament length and efficiency of contact transmission. J. Virol 88, 7569–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E, Kung PC, Chang TW, 1984. Hepatitis B virus-reactive human T lymphocyte clones: antigen specificity and helper function for antibody synthesis. J. Immunol 132, 1511–1516. [PubMed] [Google Scholar]
- Chen Y, Bai Y, Guo X, Wang W, Zheng Q, Wang F, et al. , 2016. Selection of affinity-im proved neutralizing human scFv against HBV PreS1 from CDR3 VH/VL mutant library. Biologicals 44, 271–275. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Isogawa M, Wieland SF, 2010. Pathogenesis of hepatitis B virus infection. Pathol. Biol 58, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C, Schoneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, et al. , 2016. Immunotherapy with the PreS-based grass pollen allergy vaccine BM32 induces antibody responses protecting against hepatitis B infection. EBioMedicine 11, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh H, Roh S, Lee KW, Kim K, 2003. Response of primed human PBMC to synthetic peptides derived from hepatitis B virus envelope proteins: a search for promiscuous epitopes. FEMS Immunol. Med. Microbiol 35, 77–85. [DOI] [PubMed] [Google Scholar]
- Ferrari C, Cavalli A, Penna A, Valli A, Bertoletti A, Pedretti G, et al. , 1992. Fine specificity of the human T-cell response to the hepatitis B virus preS1 antigen. Gastroenterology 103, 255–263. [DOI] [PubMed] [Google Scholar]
- Galarza JM, Latham T, Cupo A, 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18, 244–251. [DOI] [PubMed] [Google Scholar]
- Glebe D, Urban S, Knoop EV, Cag N, Krass P, Grun S, et al. , 2005. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 129, 234–245. [DOI] [PubMed] [Google Scholar]
- Grgacic EV, Anderson DA, 2006. Virus-like particles: passport to immune recognition. Methods 40, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassemer M, Finkernagel M, Peiffer KH, Glebe D, Akhras S, Reuter A, et al. , 2017. Comparative characterization of hepatitis B virus surface antigen derived from different hepatitis B virus genotypes. Virology 502, 1–12. [DOI] [PubMed] [Google Scholar]
- Kim JH, Gripon P, Bouezzedine F, Jeong MS, Chi SW, Ryu SE, et al. , 2015. Enhanced humanization and affinity maturation of neutralizing anti-hepatitis B virus preS1 antibody based on antigen-antibody complex structure. FEBS Lett. 589, 193–200. [DOI] [PubMed] [Google Scholar]
- King TH, Kem mler CB, Guo Z, Mann D, Lu Y, Coeshott C, et al. , 2014. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One 9, e101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, et al. 2014. Induction of a robust T-and B-cell immune response in non-and low-re-sponders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine 32, 5077–5082. [DOI] [PubMed] [Google Scholar]
- Lambert C, Prange R, 2007. Posttranslational N -glycosylation of the hepatitis B virus large envelope protein. Virol. J 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Mann S, Prange R, 2004. Assessment of determinants affecting the dual topology of hepadnaviral large envelope proteins. J. Gen. Virol 85, 1221–1225. [DOI] [PubMed] [Google Scholar]
- Lee J, Park JS, Moon JY, Kim KY, Moon HM, 2003. The influence of glycosylation on secretion, stability, and immunogenicity of recombinant HBV pre-S antigen synthesized in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun 303, 427–432. [DOI] [PubMed] [Google Scholar]
- Lempp FA, Urban S, 2014. Inhibitors of hepatitis B virus attachment and entry. Intervirology 57, 151–157. [DOI] [PubMed] [Google Scholar]
- Lian M, Zhou X, Wei L, Qiu S, Zhou T, Li L, et al. , 2007. Serum levels of preS antigen (HBpreSAg) in chronic hepatitis B virus infected patients. Virol. J 4, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madalinski K, Sylvan SP, Hellstrom U, Mikolajewicz J, Dzierzanowska-Fangrat K, 2004. Presence of anti-preS1, anti-preS2, and anti-HBs antibodies in newborns immunized with Bio-Hep-B vaccine. Med. Sci. Monit 10, PI10–17. [PubMed] [Google Scholar]
- Milich DR, 1988. T- and B-cell recognition of hepatitis B viral antigens. Immunol. Today 9, 380–386. [DOI] [PubMed] [Google Scholar]
- Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht HJ, et al. , 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol 150, 4659–4671. [PubMed] [Google Scholar]
- Neurath AR, Seto B, Strick N, 1989. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 7, 234–236. [DOI] [PubMed] [Google Scholar]
- Pajot A, Michel ML, Mancini-Bourgine M, Ungeheuer MN, Ojcius DM, Deng Q, et al. , 2006. Identification of novel HLA-DR1-restricted epitopes from the hepatitis B virus envelope protein in mice expressing HLA-DR1 and vaccinated human subjects. Microbes Infect. 8, 2783–2790. [DOI] [PubMed] [Google Scholar]
- Pennock ND, Kedl JD, Kedl RM, 2016. T cell vaccinology: beyond the reflection of infectious responses. Trends Immunol. 37, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange R, Streeck RE, 1995. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 14, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Dagan R, Gallil A, Brill G, Kassis I, Koren R, 1996. Safety and immunogenicity of a novel mammalian cell-derived recombinant hepatitis B vaccine containing Pre-S1 and Pre-S2 antigens in children. Vaccine 14, 207–211. [DOI] [PubMed] [Google Scholar]
- Rendi-Wagner P, Shouval D, Genton B, Lurie Y, Rumke H, Boland G, et al. , 2006. Com parative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine 24, 2781–2789. [DOI] [PubMed] [Google Scholar]
- Reynolds TD, Buonocore L, Rose NF, Rose JK, Robek MD, 2015. Virus-like vesicle-based therapeutic vaccine vectors for chronic hepatitis B virus infection. J. Virol 89, 10407–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankhyan A, Sharma C, Dutta D, Sharma T, Chosdol K, Wakita T, et al. , 2016. Inhibition of preS1-hepatocyte interaction by an array of recombinant human antibodies from naturally recovered individuals. Sci. Rep 6, 21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Glebe D, Tolle TK, Lochnit G, Linder D, Geyer R, et al. , 2004. Structure of pre-S2 N- and O-linked glycans in surface proteins from different genotypes of hepatitis B virus. J. Gen. Virol 85, 2045–2053. [DOI] [PubMed] [Google Scholar]
- Schumann A, Fiedler M, Dahmen U, Grosse-Wilde H, Roggendorf M, Lindemann M, 2007. Cellular and humoral immune response to a third generation hepatitis B vaccine. J. Viral Hepat 14, 592–598. [DOI] [PubMed] [Google Scholar]
- Shapira MY, Zeira E, Adler R, Shouval D, 2001. Rapid seroprotection against hepatitis B follow ing the first dose of a Pre-S1/Pre-S2/S vaccine. J. Hepatol 34, 123–127. [DOI] [PubMed] [Google Scholar]
- Shouval D, Roggendorf H, Roggendorf M, 2015. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med. Microbiol. Immunol 204, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvan SP, Madalinski K, Hellstrom UB, 2009. Anti-preS responses influence the antiHBs response in newborns after vaccination with the third generation Sci-B-Vac vaccine. Vaccine 28, 446–451. [DOI] [PubMed] [Google Scholar]
- Toita R, Kawano T, Kang JH, Murata M, 2015. Applications of human hepatitis B virus preS domain in bio- and nanotechnology. World J. Gastroenterol 21, 7400–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. , 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1, e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PL, Althage A, Chung J, Chisari FV, 2002. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A 99, 13825–13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li X, Yi W, Li S, Hu C, Chen A, 2013. A monoclonal antibody specific to the non-epitope region of hepatitis B virus preS1 contributes to more effective HBV detection. Clin. Biochem 46, 1105–1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.