Abstract
PURPOSE:
The use of pro-osteogenic growth factors, such as BMP2, in human adipose-derived stem cell (ASC) osteogenesis is well described. Because these growth factors work via signal transduction pathways, such as the mitogen-activated protein kinase (MAPK) cascade, a study of the relationship between MAPK signaling and ASC osteogenesis was conducted.
METHODS:
ERK, JNK, and p38MAPK activation were measured in ASCs osteo-induced using either dexamethasone or vitamin D3 and correlated to mineralization. Activation and mineralization were also measured without dexamethasone or using the glucocorticoid, cortisone. The expression of the MAPK phosphatase, MKP1, and its relationship to mineralization was also assessed. The effect of decreasing MAPK activation on mineralization through the use of exogenous inhibitors was examined along with siRNA-knockdown and adenoviral overexpression of ERK1/2. Finally, the effect of ERK1/2 overexpression on ASCs induced on PLGA scaffolds was assessed.
RESULTS:
ASC mineralization in dexamethasone or vitamin D3-induced ASCs correlated with both increased ERK1/2 and JNK1/2 activation. ASCs induced without dexamethasone also mineralized, with JNK1/2 signaling possibly mediating this event. No link between cortisone induction and MAPK signaling could be ascertained. ASCs treated with ERK, JNK, or p38MAPK inhibitors showed decreased osteogenic gene expression and diminished mineralization. Mineralization levels were also affected by viruses designed to inhibit or augment ERK1/2 expression and activity. Finally, ASC mineralization appeared to be a balance between MAPK kinase activity and MKP1.
CONCLUSIONS:
It is likely that MAPK signaling plays a significant role in ASC osteogenesis, affecting differentiation in kinase- and stage-specific manners.
Keywords: MAPK signaling, ERK1/2, JNK1/2, osteogenesis, adipose-derived stem cells
INTRODUCTION
In the field of regenerative medicine, the adipose-derived stem cell (ASC) with its osteogenic potential has become a popular cellular component of many in vitro and in vivo tissue engineering protocols [1–9]. Osteogenic induction of ASCs can be achieved through the use of simple agents such as dexamethasone or 1,25-dihydroxyvitamin D3/VD [1, 10–13] or with growth factors such as bone morphogenic protein-2 (BMP2). BMP2, in particular, has been used to increase the low osteogenic capacity of ASCs in many in vivo model systems [2, 4, 14, 15] and has transitioned to the clinic over 20 years ago [16, 17]. Unfortunately, the clinical use of BMP2 in bone regeneration is often contraindicated when dealing with young patients undergoing maxillofacial repair [18] and a recent study has suggested that BMP2 may not affect ASC osteogenic potential [19, 20]. Therefore, manipulating a growth factor’s downstream signaling pathway as an alternative strategy in promoting osteogenesis might allow ASCs to be used in younger patients.
The induction of MAPK signaling by growth factors, such as BMP2, is well described [21–25]. The MAPK cascade can be divided into: 1) the extracellular signal-related protein kinase/ERK1/2 path, 2) the jun N-terminal kinase/JNK path, 3) the p38MAPK path and 4) the ERK5 pathway (Supplemental Figure S1) [26, 27]. Each of these paths is composed of a linear, three-tiered series of kinases known as the MAP kinase kinase kinase/MAPKKK (MAPK3 or MKK), the MAP kinase kinase/MAPKK (MAPK2 or MEK) and the MAPK. Dual phosphorylation of the terminal MAPK is performed by the “upstream” MEK family of kinases – a group of kinases that are phosphorylated by members of the MKK family. Specificity is a hallmark of MAPK signaling, with each MKK and MEK showing negligible activity on substrates other than their target kinase. It is an elegant system whereby stimuli such as growth factors, cellular stress, heat shock, and inflammatory cytokines are transduced from the cell membrane (where the MKK is found) into the nucleus (or other organelle) via the terminal MAPK. The link between MAPK signaling and mesodermal fate decisions and differentiation, including osteogenic commitment, matrix synthesis, and calcification is extensive [28–32]. MAPK signaling has been studied in mesenchymal stem cells (MSCs) following dexamethasone or VD3 induction of osteogenesis [31, 33–35]. In ASCs, MAPK signaling has been studied in osteogenesis and adipogenesis [36–39], in ASCs pre-conditioned by hypoxia for chondrogenesis [40], and in ASCs maintained under three-dimensional osteogenic conditions [41].
The ability of osteogenic growth factors to act via MAPK signaling may make it possible to mediate osteogenesis in ASCs without the use of upstream factors. However, before this would be possible, the role of ERK, JNK, and p38MAPK signaling in ASC osteogenesis needs to be understood. Therefore, kinase activation and ASC mineralization, as an indicator of osteogenesis, were assessed in this study under conventional osteogenic conditions (i.e. dexamethasone or VD3 induction) and after treating cells with MAPK inhibitors and siRNA. Mineralization levels were also measured after kinase overexpression by ASC monolayers and in cells seeded onto three-dimensional scaffolds. Taken together, this data suggest that ERK1/2 and JNK1/2 signaling are involved ASC osteogenesis.
METHODS
Materials:
Human ASC populations (n=15) were isolated from human lipoaspirates under an approved IRB protocol [1, 42] and were cultured as described in the supplemental data section. Confirmation of osteogenic potential is also presented in this section (Supplemental Figure 2). Cell culture reagents for maintenance and expansion of ASCs (high glucose DMEM, PBS, antibiotics/antimycotics) were purchased from Mediatech Cellgro (Manassas, VA). Inductive factors for osteogenic differentiation were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). FBS was purchased from Gemini Bioproducts (West Sacramento, CA) or Omega Scientific (Tarzana, CA). Real time PCR reagents were purchased from Applied Biosystems (Foster City, CA). Antibodies to unphosphorylated ERK1/2, JNK1/2 and p38MAPK were purchased from Invitrogen Life Technologies (Carlsbad, CA) and EMD Millipore (Billerica, MA). Polyclonal antibodies to the dual phosphorylated forms of ERK1/2, JNK1/2 and p38MAPK were also purchased from Invitrogen. A monoclonal β-actin antibody was purchased from Abcam (Cambridge, MA). MAPK signaling inhibitors were purchased from EMD Biosciences/Calbiochem (Gibbstown, NJ).
SEM-EDX analysis of ASC extracellular mineralization:
Elemental composition of ECM deposits upon OM induction was determined using Scanning Electron Microscopy Energy-Dispersive X-ray (SEM-EDX) analysis. ASC monolayer cultures (n=8) were induced as in OM for 21 days and fixed in 4% paraformaldehyde at room temperature for 30 min. Following washing with PBS and distilled water, the extracellular deposits were dehydrated using increasing grades of ethanol (50% to 100%, 60 min each grade) and immersed in hexamethyldisilazone (HMDS, EM Sciences, PA) for two h to preserve structure, following by air-drying overnight. An area around an ECM deposit was cut using a soldering iron, mounted onto scanning electron microscopy (SEM) stubs using double-sided carbon tape, and gold-coated. SEM-EDX analysis was performed using an EDX spectrometer (Noran System 7 X-ray Microanalysis System, ThermoFisher). Multiple points were selected within each analyzed mineral deposit, and elemental calcium, phosphorus, sodium, and potassium measured.
Western Analysis of MAPK Activation:
ASC populations were maintained in non-inductive CM or induced with the following: 1) OM plus dexamethasone (n=15), 2) OM without this glucocorticoid (OM/NoDexa; n=8), 3) OM/NoDexa containing 0.01 μM vitamin D3 (OM/VD; n=6) or 4) OM/NoDexa containing 0.1 μM hydroxycortisone (OM/Cortisone; n=6). Samples were harvested after three and five weeks. Mineral content of extracted ECM deposits and the cellular lysates used for western blotting were measured prior to western analysis [10]. Phosphorylation of ERK1/2, JNK1/2 and p38MAPK was measured via western blotting as an indication of kinase activation. Equivalent amounts of ASC lysates from each harvested time point were loaded onto SDS-PAGE gels (12% NuPAGE, Invitrogen), separated electrophoretically and transferred to nitrocellulose membranes (0.45 μM, Whatman Protran Nitrocellulose). The membranes were probed with both kinase-specific and phosphorylation-specific primary antibodies. The primary antibodies were detected with HRP-conjugated secondary antibodies. Band densities were quantified by conventional densitometry (Image J). Unphosphorylated kinase expression was normalized to β-actin as an internal control for total protein loading. Phosphorylated kinase levels were then normalized to their unphosphorylated counterpart. Western analysis was performed at least twice for each ASC population.
Role of MAPK phosphatases in ASC osteogenesis:
Expression levels of the ERK/JNK phosphatase MAPK-phosphatase 1 (MKP-1) were measured via Western blotting in osteogenic ASC populations and normalized to CM controls. To functionally assess MKP-1, ASCs were treated with either 1 μM or 10 μM sodium orthovanadate (an MKP inhibitor) for the first 48 hours of OM induction or continuously for 21 days. Samples were harvested and ASC mineralization measured biochemically [10]. Levels were compared with OM controls.
Functional assessment of MAPK signaling in ASC osteogenesis using MAPK inhibitors:
MEK-ERK signaling was studied using the following inhibitors: raf-1 Kinase Inhibitor (an inhibitor to the MEKK, raf-1), U0126 (MEK1/2 inhibitor), PD98059 (MEK/ERK1/2 inhibitor), Tyrphostin/AG126 (ERK1/2 inhibitor), and the ERK2 inhibitor ERK2 Activation Inhibitor Peptide I/ERK2 Inhibitor. p38MAPK signaling was assessed using SB203580 and SB202190. JNK1/2 signaling was studied using SP600125 and JNK Inhibitor III. Raf-1 Kinase Inhibitor, U0126, SB203580, and SP600125 were first solubilized in DMSO and working stocks made by diluting in 70% ethanol as a vehicle. Working stocks of JNK Inhibitor III, SB202190, Tyrphostin, and PD98059 were prepared directly in DMSO as a vehicle, while a working stock of ERK2 Inhibitor was prepared in water. All controls were treated with OM/vehicle to account for any effects of the solubilizing agent. The lowest inhibitor concentration that decreased kinase activation relative to controls (as confirmed by western blotting, Supplemental Fig. S3) was then used to assess its effect on ASC osteogenesis. ASCs (n=9) were plated overnight in CM in multi-well culture dishes at 75% confluency. The cells were treated with OM + inhibitor (or OM/vehicle) in four ways: 1) for the first 24 hours of induction, followed by OM induction for a total of 21 or 35 days, 2) at the onset of matrix synthesis – from 7–14 days followed by OM induction for a total of 21 days, 3) at the onset of matrix mineralization – from 14–21 days followed by OM induction for a total of 21 days or 4) continuously for 21 or 35 days. For treatment conditions 2–4, inhibitors were changed every 72 hours. Cells were harvested and matrix mineralization was quantified biochemically [10]. Mineralization was compared with OM/vehicle controls.
Real-time analysis of ASC Osteogenesis:
OM-induced and inhibitor-treated ASC samples (n=8) were harvested after 21 days and cDNA was synthesized from 1 μg total RNA using Taqman Gold reagents (Applied Biosystems). Real-time PCR reactions were prepared using the Quantitect Probe PCR Kit (Qiagen) and run on an ABI 7700 Fastcycler. Customized primer/probe mixes to Runx-2, Osteonectin (ON), Osteopontin (OP), Osteocalcin (OC) and Alkaline Phosphatase (AP) were purchased from Applied Biosystems (FAM-labelled probes, TAMRA quencher). Primer information is given in Supplemental Table S1. For each primer/probe mix, regression lines were calculated using serial dilutions prepared from known cDNA amounts. Expression levels were determined using these regression values (ΔCt method) and were normalized using GAPDH and 18S rRNA. OM + inhibitor expression levels were compared with OM controls and OM gene expression levels were compared with CM controls.
shRNA knockdown of ERK1/2 activity:
Replication-deficient shRNA lentiviruses for siRNA-directed knockdown of ERK1/2 were created using the Block-iT Lentiviral RNAi Gateway Expression System (Invitrogen). Doubled-stranded ERK1 or ERK2 oligos (50-mer length) were designed using a proprietary algorithm (Invitrogen). Oligo sequences and their homology to ERK1/2 are given in Supplemental Table S2. Double-stranded oligos were cloned into a pLenti6-Block-iT/DEST shuttle vector for transfection into 293FT cells along with the requisite lentiviral packaging genes (gag, pol, rev, VSV-G; ViraPower Packaging mix – Invitrogen). Supernatants containing the replication-deficient shRNA-lentiviral ERK1/2 particles were harvested 48 hours following transfection and used to transduce ASCs. Knockdown of ERK1 and ERK2 by the resulting siRNA particles was confirmed after 72 hours and 14 days by western blotting prior to use. For functional assays, ASCs (n=4) at 75% confluency were transduced for 16–24 hours in CM supplemented with shRNA-lentiviral supernatants. The medium was removed and the cells induced for a total of 14 days or 21 days with OM. Matrix mineralization was quantified biochemically [10] and normalized to ASCs undergoing a mock transduction followed by osteo-induction for 14 or 21 days. Two shRNA ERK1/2 lentiviruses were tested and denoted as 1A5 and 2A1.
Adenoviral mediated overexpression of ERK1/2 expression:
Full-length coding sequences for human ERK1 or mouse ERK2 were purchased as plasmids from Genecopoeia (ERK1wt, ORFExpress Shuttle clone #GC-A0161) or Upstate Biotechnology (ERK2wt-pUSEamp, cat#21–112). Recombinant adenoviruses (AdERK1wt or AdERK2wt) were made at the UCLA Virology Core as follows: full-length open reading frames were cloned into the adenoviral shuttle vector pShuttle-CMV, followed by homologous recombination with the pAdEasy-1 vector (AdEasy XL Adenoviral Vector System, Agilent Technologies, Santa Clara, CA). Expression constructs were digested with PacI to expose the ITRs, transfected into a 293AD cell line (ATCC), and the cells were cultured for 48 h to allow development of the cytotoxic effect. Viral particles were purified from the supernatant using a commercial kit (Agilent Technologies) and a TCID50 titration performed. ASC monolayers were then transduced for 16–24 hours in CM with either AdERK1wt or AdERK2wt at multiplicities of infection (MOIs) of 1, 10, or 100. The transduction medium was removed and the monolayers induced for 21 days in OM. Overexpression of ERK1 and ERK2 was confirmed by western blotting and matrix mineralization was quantified biochemically [10]. ERK1/2 expression and mineralization levels were compared with controls transduced under mock conditions and induced in OM. ASCs transduced overnight with AdERK1wt or AdERK2wt at an MOI of 10 and induced in OM for 72 hours, were seeded at a density of 1 × 106 cells onto 3D PLGA scaffolds coated with hydroxyapatite [43] to assess the effect of ERK1/2 overexpression on three-dimensional ASC osteogenesis. Scaffolds seeded with mock-transduced ASCs were also cultured under the same conditions. All scaffolds were OM-induced in vitro for 21 days, and the cells were harvested for western analysis by soaking the scaffolds for several minutes in a RIPA buffer with mild agitation. Harvested cell lysates were analyzed for ERK1/2 overexpression by western blotting. The de-cellularized scaffolds were fixed for 10 min with 4% paraformaldehyde prepared in 1× PBS, washed with distilled water and then air-dried. Matrix mineralization was detected by staining the scaffolds for 60 sec with 1% silver nitrate. The silver nitrate was removed and the scaffolds rinsed with water, then dried. Scaffolds were imaged using conventional light microscopy and matrix mineralization observed after ERK1/2 overexpression compared with mock-transduced controls.
Statistical Analysis:
Multiple ASC populations (n=15) were used in this study. A minimum of three ASC populations was used for each assay, with each population assayed at least twice. For each assay, the experimental groups were compared with control ASCs and the mean change ± standard deviation groups were determined using a t-test (one-tailed) or analysis of variance (ANOVA) as indicated in the text. A p value < 0.05 was considered significant.
RESULTS
Functional confirmation of MAPK signaling during ASC osteogenesis: MAPK activation levels
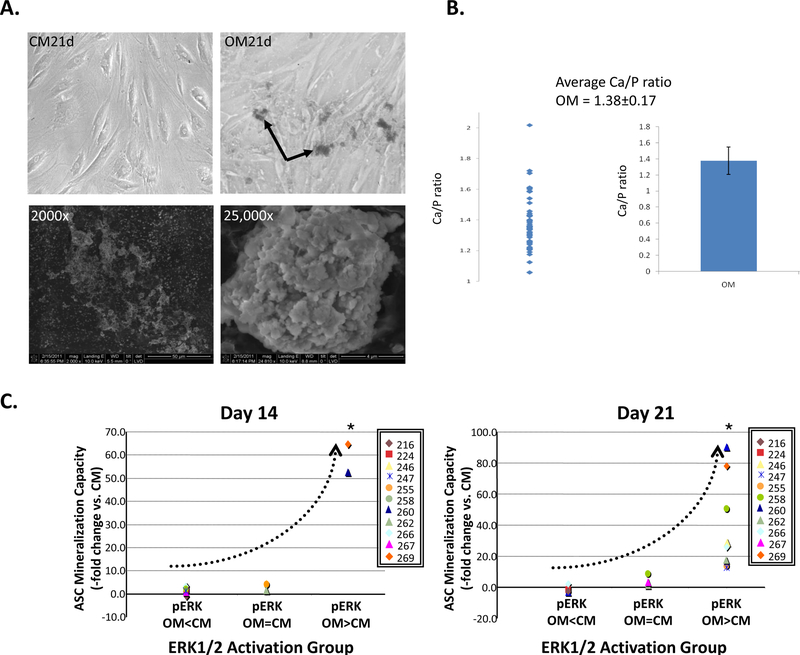
The initial microarray and real-time PCR analysis of several osteogenic ASC populations identified the MAPK cascade as a potential signaling pathway involved in ASC osteogenesis (Supplemental Fig. S4 and accompanying text). To functionally study MAPK signaling and ASC osteogenesis, the expression and activities of ERK1/2, JNK1/2 and p38MAPK were assessed in multiple ASC populations (n=15) to detect trends in kinase activity. When viewed under the light microscope, OM-induced ASC populations produced granular, extracellular deposits (Fig. 1A) that stained for phosphate using a von Kossa stain (see Supplemental Fig. 2A for a representative population). Biochemical analysis of these deposits [10] confirmed their calcium content (data not shown), while energy-dispersive X-Ray (EDX) analysis measured a calcium to phosphorus ratio of 1.38±0.17 (Fig. 1B). Having confirmed the presence of calcium phosphate within these deposits, they were subsequently used to identify osteogenic ASC populations.
Figure 1: ASC mineralization is associated with increased ERK1/2 activation.
Panel A: Production of extracellular mineral deposits (arrows) by ASCs maintained for 21 days in non-inductive CM or inductive OM (top panels). Scanning electron microscopy (SEM) of the extracellular deposits is shown below (2000× and 25,000× magnifications). Panel B: SEM energy dispersive X ray (SEM EDX) microscopy analysis confirms the presence of calcium and phosphorus within the deposits of several ASC populations (n=8). Panel C: ERK1/2 activation (pERK) following OM induction of the indicated ASC populations for either 14 or 21 days was categorized as being either: 1) lower than that of CM controls (pERK OM<CM), 2) equivalent to controls (pERK OM=CM) or 3) significantly higher than controls (pERK OM>CM). Mineralization of these populations compared with controls (ASC Mineralization Capacity – fold change vs. CM) are shown with respect to these three pERK categories. For clarity, the average pERK1/2 level for each ASC population was plotted without its standard deviation. The trend in ERK1/2 activation and mineralization is shown (dotted line). ANOVA confirmed significant differences between the three activation groups.
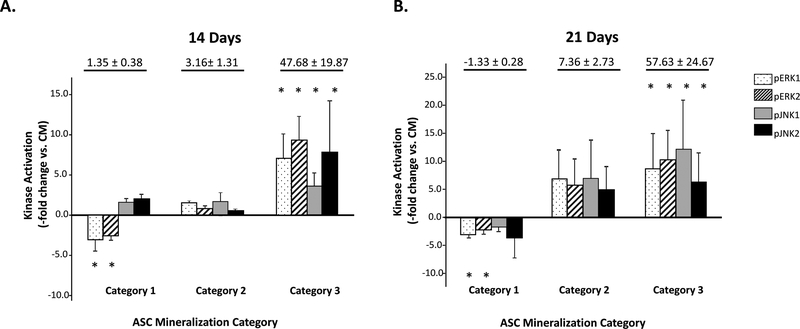
Activation/phosphorylation levels of ERK, JNK, and p38MAPK were then measured in these populations and compared with non-induced controls. No difference in the expression of unphosphorylated ERK1/2, JNK1/2, or p38MAPK proteins relative to controls was measured at any induction point (data not shown). However, OM induction was associated with significant changes in kinase activity. When ERK1/2 activation levels were measured after 2–3 weeks induction and compared with non-induced controls, three distinct kinase groups emerged: ASC populations with decreased pERK1/2 levels, ASCs with basal pERK1/2 levels, and ASCs with elevated pERK1/2 levels (Fig. 1C). Similar groupings were observed when pJNK1/2 levels were assessed. When matrix mineralization was examined with respect to pERK1/2 and pJNK1/2 levels, three distinct categories became apparent (Fig. 2 and Supplemental Table S3). In Category 1, negligible mineralization was associated with decreased or basal ERK1/2 or JNK1/2 activation. In Category 2, moderate mineralization levels (2 to 10-fold increase) were associated with increased activation of either ERK1/2 or JNK1/2 kinases. However, there was a substantial amount of variability in ERK1/2 and JNK1/2 expression in this category. In Category 3, enhanced mineralization (over 10-fold increase) coincided with significant increases in both pERK1/2 and pJNK1/2 levels relative to controls. When ASC populations from Category 1 and 3 were examined for p38MAPK activation, no obvious correlation between ASC mineralization and p38MAPK activation was observed (Supplemental Fig. S5).
Figure 2: ASC mineralization correlates to ERK1/2, JNK1/2 activation.
ASC populations (n=15) after OM induction for 14 (Panel A) or 21 days (Panel B) are shown categorized based on mineralization and activation level of ERK1/2 and JNK1/2 (pERK1/2, pJNK1/2) versus non-induced controls (Kinase Activation – fold change vs. CM). ASCs were determined to have: 1) negligible mineralization versus CM controls (Category 1); 2) moderate mineralization versus controls (Category 2) or 3) enhanced mineralization versus controls (Category 3). TAverage mineralization levelfor each Category is given at the top of the graph. * indicates a significant difference in kinase activation vs controls (p<0.05; t-test). ANOVA also confirmed a statistically significant difference between the Category 1 and Category 3 activation levels.
MAPK signaling, mineralization and dexamethasone
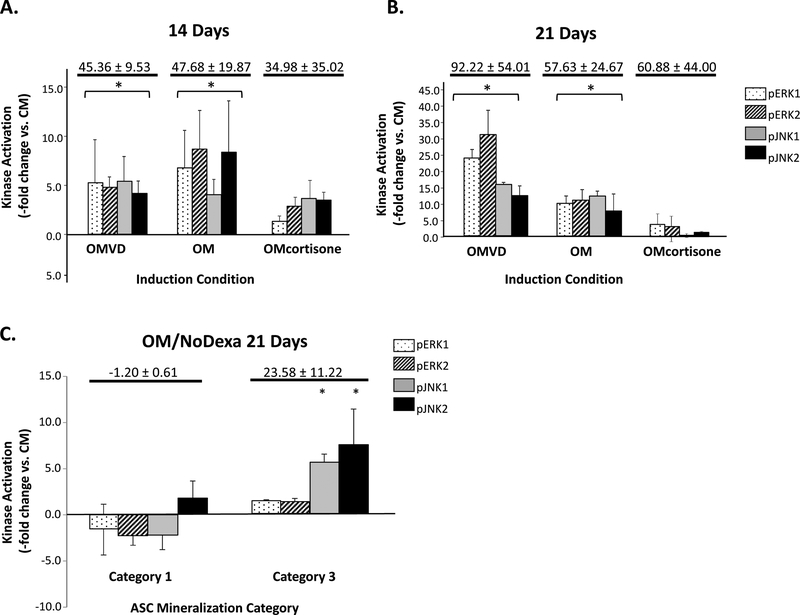
To determine if increased MAPK activation and augmented mineralization were a dexamethasone-induced artifact rather than osteogenesis, Category 3 ASC populations were induced for up to 3 weeks with 1,25-dihydroxyvitamin D3 (VD3). Like dexamethasone, VD3 induction resulted in increased extracellular mineral deposition versus controls (data not shown) together with increased ERK1/2 and JNK1/2 activation (Fig. 3A). ASCs were also induced in OM containing hydroxycortisone in lieu of dexamethasone to assess for any non-specific glucocorticoid effect. Although some of these ASC populations mineralized, a definitive link between mineralization and increased MAPK signaling could not be made (Fig. 3B). Finally, ASCs were cultured for 21 days in OM without dexamethasone (OM/NoDexa). Despite lacking dexamethasone, many ASC populations underwent mineralization, albeit at a lower level than that of their OM counterparts (an average of 2.25-fold lower vs. OM, data not shown). In these populations, the extent of mineralization was associated with JNK1/2 activity (Fig. 3C).
Figure 3: ERK1/2 and JNK1/2 activation mediate ASC osteogenesis in alternate osteoinduction conditions.
Activation levels of ERK1/2 and JNK1/2 following osteogenic induction of Category 3 ASC populations (n=6) for either 14 (Panel A) or 21 days (Panel B) is shown after conventional OM induction, in addition to induction with OM containing 1,25-dihydroxyvitamin D3 (OMVD) or hydroxycortisol (OMcortisone) in lieu of dexamethasone. ERK1/2 and JNK1/2 activation levels (pERK1/2, pJNK1/2) are shown normalized to non-induced controls (Kinase Activation -fold change vs. CM). Activation levels of ERK1/2 and JNK1/2 following 21 days induction of Category 1 and 3 ASC populations (n=8) in OM lacking dexamethasone (OMNoDex) are shown compared with non-induced controls (Panel C). Average mineralization levels versus controls are given for each condition. * indicates a significance difference (p<0.05; t-test) versus CM controls.
ASC osteogenesis and MAPK phosphatase expression
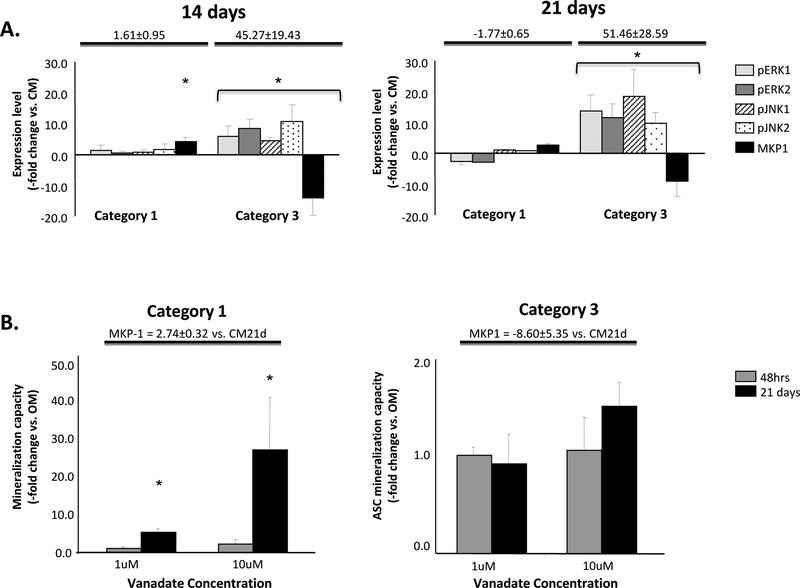
Kinase phosphorylation levels are affected by MAPK phosphatases [44–46]. In light of our Western data, expression levels of the MAPK phosphatase 1 MKP1 were measured in Category 1 (low capacity) and Category 3 populations (enhanced capacity). Nonsignificant changes in ERK1/2 and JNK1/2 activation, together with elevated levels of MKP-1 expression, were measured in Category 1 populations, with decreased MKP-1 expression and elevated ERK1/2 and JNK1/2 activities found in Category 3 populations (Fig. 4A). A preliminary examination of another ERK/JNK phosphatase, MKP3, also indicated a reciprocal relationship between phosphatase expression and kinase phosphorylation/activation (data not shown). Low mineralizing Category 1 ASCs treated with sodium orthovanadate, an MKP expression inhibitor [47] demonstrated significant mineralization, with continuous administration with 10 μM vanadate over 21 days being the most effective (Fig. 4B). Increased ERK1/2 activation compared with non-treated controls was also measured in these cells (ERK1 = 12.4±4.8 fold increase; ERK2 = 7.5±3.9 fold increase). In Category 3 populations with basal levels of MKP-1 expression, vanadate did not effect on mineralization (Fig. 4B) or ERK1/2 activation (data not shown).
Figure 4: MKP1 expression level upon ASC osteogenesis.
Panel A: Normalized expression of the MAPK phosphatase, MKP-1 (Expression level, -fold change vs CM), together with activation levels of ERK1/2 and JNK1/2 versus controls are shown for Category 1 and Category 3 ASCs induced with OM for 14 and 21 days. Mineralization versus non-induced controls are given at the top of each graph. Panel B: The effect on mineralization following treatment of these ASCs with 1 μM or 10 μM of the phosphatase inhibitor sodium orthovanadate either for the first 48 hours of OM induction or continuously for 21 days is shown. Mineralization is presented compared with non-treated OM controls (-fold change vs. OM). MKP-1 expression levels are indicated at the top of the graph. * indicates a significance difference (p<0.05; t-test) between groups.
Functional confirmation of MAPK activation in ASC osteogenesis: Use of exogenous MAPK Inhibitors
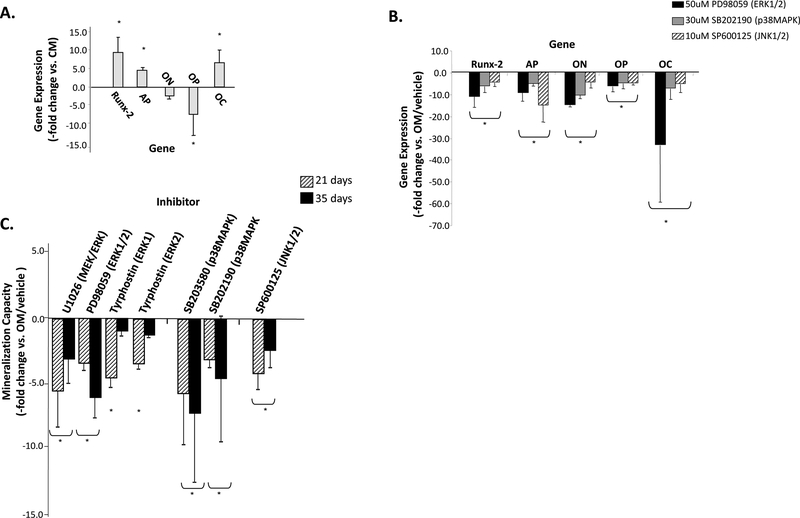
Category 3 ASCs were then treated with OM containing inhibitors to kinase activation and their effect on Runx-2, AP, ON, OP and OC gene expression were compared by real-time PCR with OM controls. The average mineralization potential of these OM-induced Category 3 ASC populations was 43.3-fold higher compared with controls and elevated expression of Runx-2 (9.5-fold), AP (4.5-fold) and OC (6.0-fold) was measured, together with decreases in ON and OP (Fig. 5A). Decreases in each of these five osteogenic markers was measured after continuous treatment of ASCs for 21 days in OM plus the MEK/ERK inhibitor PD98059, the p38MAPK inhibitor SB202190, or the JNK1/2 inhibitor SP60015 (Fig. 5B).
Figure 5: Inhibition of MAPK activity may inhibit ASC osteogenesis.
Panel A - Expression of the osteogenic genes Runx-2, AP, OP, and OC after ASC osteo-induction was quantified after 21 days and expressed relative to non-induced controls (Gene Expression, -fold change vs. CM) to confirm ASC osteogenic capacity. Panel B - The effect of continuous administration of the indicated MAPK inhibitors on the expression of these osteogenic genes is shown relative to untreated OM controls containing inhibitor vehicle (-fold change vs. OM/vehicle). Panel C – Decreased mineralization versus controls was measured in osteo-induced ASCs treated either continuously for 21 or 35 days with the indicated MAPK inhibitors (ASC Mineralization Capacity, -fold change vs. OM/vehicle). The kinase affected by each inhibitor is given in parentheses. SP600125 was administered during the first seven days of induction to minimize cell death. * indicates a significance difference (p<0.05; t-test) between groups. MAPK inhibitors not significantly affecting mineralization versus controls are not shown.
Inhibitor treatment of Category 3 ASCs also affected osteogenic potential. A dose-dependent effect on kinase phosphorylation was measured for each inhibitor and optimal inhibitor dose and efficacy was confirmed using phospho-specific Westerns (Supplemental Fig. S3). Proliferation/MTT assays also confirmed that each inhibitor did not affect ASC proliferation (data not shown). Inhibitors were initially administered continuously over the entire OM induction period of 21 or 35 days. Both PD98059 and U0126 significantly decreased mineralization versus untreated OM controls at these time points (Fig. 5C). Decreased mineralization was also measured after 3 weeks using Tyrphostin at doses for ERK1 or ERK2 inhibition. However, ASCs continuously treated with an upstream raf-1 Kinase inhibitor demonstrated no change in osteogenic potential at either assay point (data not shown). Decreased mineralization was also measured after 3 and 5 weeks of p38MAPK inhibition using SB203580, or SB202190 and when the JNK1/2 inhibitor SP600125 was used. JNK Inhibitor III was unable to significantly affect ASC mineralization, a likely consequence of its inability to decrease JNK1/2 phosphorylation levels (data not shown). In some ASC populations, SP600125 at 10 μM increased cell death levels as induction time increased (data not shown). These levels decreased when SP600125 concentrations were lowered to 1 μM. However, this concentration had no significant effect on kinase activation or ASC osteogenesis and was not used (data not shown). Thus, ASCs were treated with SP600125 at 10 μM for only the first 7 days of induction. When AP activity was measured after 3 weeks as an alternate marker of osteogenesis, PD98059, SB203580, and SB202190 significantly decreased activity compared with controls (Supplemental Fig. S6).
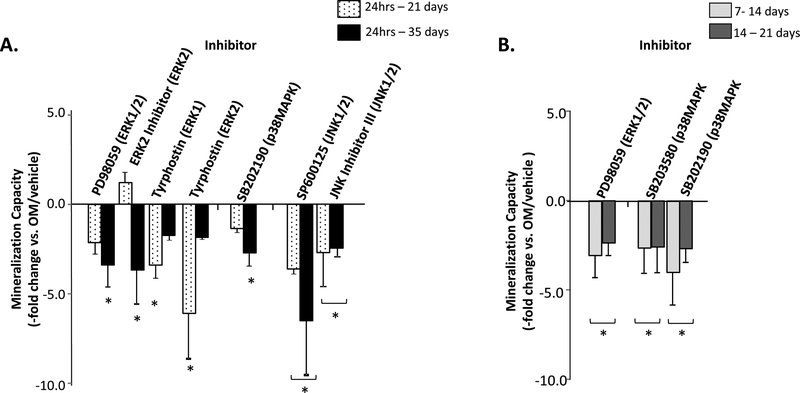
Inhibitors were also added during the first 24 hours of OM induction to assess MAPK signaling in osteogenic commitment, at the onset of matrix synthesis (7–14days) and at the onset of matrix mineralization (14–21 days) (Fig. 6). Mineralization after 24 hour addition was assessed after 21 and 35 days, while the 7–14 and 14–21 day treatment conditions were assessed after 21 days. No significant effect on mineralization was found using raf-1 Kinase Inhibitor or U0126 under each of these restricted induction conditions (data not shown). Decreased ASC mineralization was measured when ERK1/2 activation was inhibited for the first 24 hours using PD98059, Tyrphostin, or ERK2 Inhibitor, with cultures showing decreases in mineral content after either 21 days (Tyrphostin), 35 days (ERK2 Inhibitor), or both (PD98059). When these inhibitors were used either from 7–14 days or 14–21 days, only PD98059 decreased mineralization (Fig. 6). Only the 24-hour administration of SP600125 and JNK Inhibitor III significantly inhibited mineralization. Finally, with the exception of the SB202190-treated samples measured at 5 weeks, inhibiting p38MAPK for the first 24 hours did not affect ASC mineralization. A more consistent effect was seen when the two p38MAPK inhibitors were given during the matrix synthesis and onset of mineralization time points.
Figure 6: Restricted inhibition of MAPK activity inhibits ASC mineralization.
Panel A – Mineralization levels following treatment for the first 24 hours of induction with the indicated MAPK inhibitors was measured after 21 or 35 days of osteogenesis and compared with non-treated controls (ASC Mineralization Capacity, -fold change vs. OM/vehicle). Panel B – Inhibitors were also added for 7 days to induce cells either at the start of matrix synthesis (7–14 days) or matrix mineralization (14–21 days) and then OM induction continued until 21 days. The ERK1/2 inhibitor Tyrphostin was administered at concentrations affecting either ERK1 or ERK2. * indicates a significance difference (p<0.05; t-test) between groups. MAPK inhibitors not significantly affecting mineralization versus controls are not shown.
Functional confirmation of ERK1/2 signaling in ASC osteogenesis: shRNA knockdown
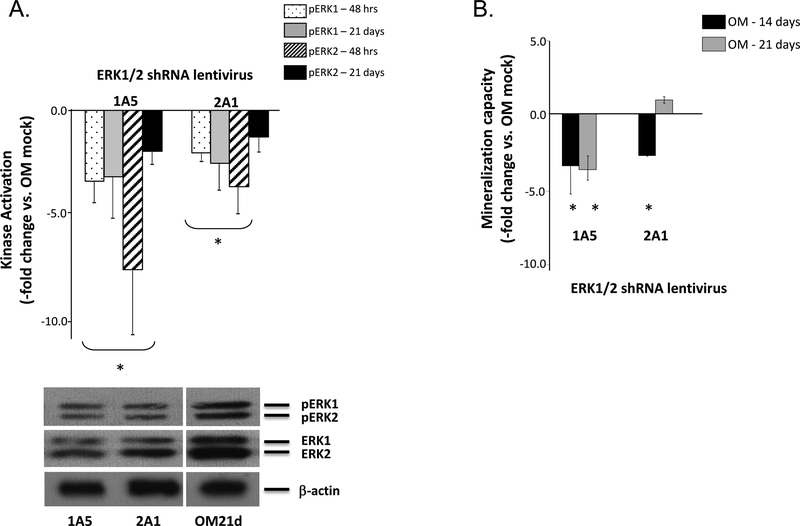
Because the ERK1/2 inhibitors used in this study can inhibit other kinases, specific knockdown of ERK1/2 was achieved using shRNA lentiviruses designed to produce ERK1/2 specific siRNA. The overall scheme of siRNA-mediated silencing is shown in Supplemental Figure S7. The shRNA oligos 1A5 and 2A1 showed extensive homology to both ERK1 and 2 (Supplemental Table S2). Therefore, the 1A5 and 2A1 siRNAs knocked down both ERK isoforms. Western blotting confirmed decreased ERK1/2 activation at 48 hours and 21 days post-transduction for the 1A5 and 2A1 shRNA lentiviruses (Fig. 7A). Both lentiviruses significantly decreased mineralization versus mock-transduced OM controls when measured after either 14 or 21 days (Fig. 7B). When osteogenic induction was increased to 4 weeks, a substantial drop in mineralization was also measured (approximately 25-fold decrease vs. OM mock controls, data not shown). ERK1/2 knockdown using shRNA adenoviruses also resulted in significant decreases in mineralization at these two time points (data not shown).
Figure 7: siRNA inhibition of ERK1/2 activity decreases ASC mineralization.
Panel A – ERK1/2 activation levels following transduction with the shRNA lentiviruses 1A5 or 2A1 is shown after 21 days induction in OM. Activation levels were normalized to OM-induced controls transduced under mock conditions (Kinase Activation, -fold change vs. OM mock). A representative western blot of pERK1/2 and ERK1/2 expression 21 days after lentiviral transduction is shown. Panel B – Mineralization levels by the transduced ASCs were measured after 14 or 21 days induction and normalized to controls (Mineralization Capacity, -fold change vs. OM Mock). * indicates a significance difference (p<0.05; t-test) between groups
Functional confirmation of ASC osteogenesis and ERK1/2 signaling: Overexpression of ERK1/2 isoforms
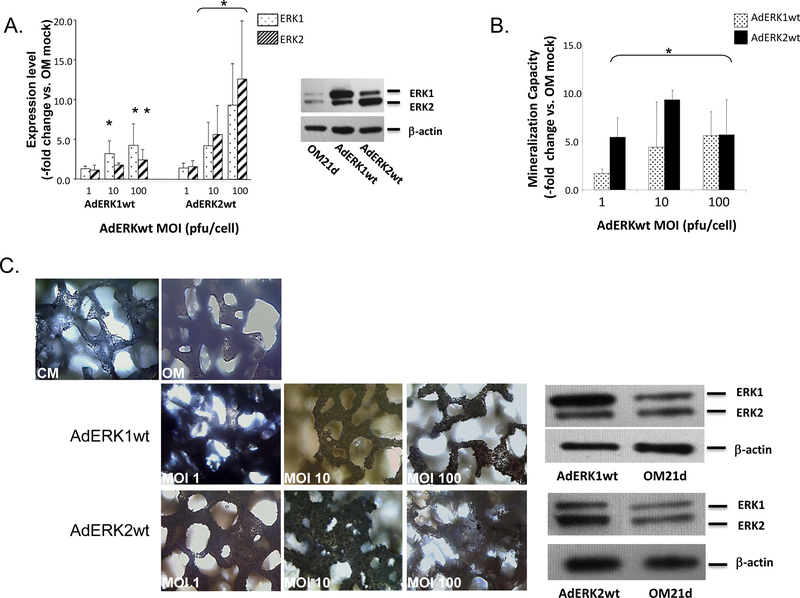
Mineralization was also measured after overexpressing ERK1/2 isoforms using recombinant adenoviruses (AdERK1wt and AdERK2wt). Western blotting confirmed an MOI-dependent effect on kinase overexpression (Fig. 8A). At an MOI of 1, no significant increase in either ERK1 or ERK2 expression was measured following transduction with the respective adenoviruses. Increasing the AdERK1wt MOI to 10 resulted in increased ERK1 expression. However, increasing the MOI to 100 increased both ERK1 and ERK2 expression. The same non-specific effect resulted after increasing the AdERK2wt MOI to either 10 or 100. AdERK1wt and AdERK2wt-transduced ASCs demonstrated increased mineralization compared with mock-transduced OM controls. Again, MOI-dependent effects were noted (Fig. 8B). Consistent with its failure to augment ERK1 expression, AdERK1wt viruses at an MOI of 1 did not significantly increase ASC mineralization capacity, whereas increasing the MOI to 10 or 100 did. In contrast, although AdERK2wt transduction at MOI 1 did not significantly increase ERK2 isoform expression, this low MOI did increas ASC mineralization. Mineralization levels induced by AdERK2wt almost doubled when the MOI was increased from 1 to 10. No further increase in mineralization was measured when the MOI was increased to 100. However, in many populations significant apoptosis was associated with this high MOI and may be the reason for the lack of increased mineralization (data not shown).
Figure 8: Overexpression of ERK1/2 increases ASC mineralization.
Panel A - Overexpression of ERK1 or ERK2 by OM-induced ASC monolayers transduced by recombinant ERK1 or ERK2 adenoviruses (AdERK1wt or AdERK2wt) at the indicated MOIs (pfu/cell) was confirmed after 21 days through western blotting and compared with OM mock controls (Expression, -fold change vs. OM mock). ERK1/2 overexpression is shown in a representative Western blot. Panel B- Mineralization by transduced ASCs was quantified after 21 days of OM induction and compared with controls (Mineralization Capacity, -fold change vs. OM mock). Panel C - 3D hydroxyapatite-coated PLGA scaffolds seeded with AdERK1wt- or AdERK2wt-transduced ASCs and cultured in OM for 21 days are shown after von Kossa staining to detect mineralization. Staining within a scaffold layer is shown compared with controls maintained in CM or OM. A representative Western blot showing isoform-specific overexpression of ERK1/2 following transduction with AdERK1wt or AdERK2wt at MOI 10 is shown on the right. For Panels A and B, * indicates a significance difference (p<0.05; t-test) between groups.
Role of ERK1/2 expression in 3D ASC osteogenesis
AdERK1wt and AdERK2wt-transduced ASCs were then seeded and induced for 21 days on PLGA scaffolds coated with hydroxyapatite [48] to assess if they could increase mineralization by ASCs in a 3D environment. With the exception of AdERK2wt-transduced ASCs at an MOI of 100, viral isoform-specific increases in kinase expression were measured in the AdERK1wt or AdERK2wt-transduced scaffolds (Table 1). As with 2D monolayers, AdERK2wt at MOI 100 increased expression of both ERK1 and ERK2, with a greater increase in the ERK2 isoform being measured. Because the scaffolds were coated with hydroxyapatite, determining extracellular mineralization levels was not possible biochemically. Therefore, the scaffolds were von Kossa stained and sections of the scaffold visualized under light microscopy. ASC-seeded scaffolds maintained in CM for three weeks showed little extracellular matrix deposition, demonstrating that the calcium phosphate composition of the scaffold is not detectable histologically (Fig. 8C). OM induction of the scaffolds produced a low level of extracellular mineralization, as staining levels were slightly increased. When compared with these OM controls, extracellular mineralization was significantly increased in scaffolds seeded with AdERK1wt or AdERK2wt-transduced cells at 10 and 100 MOI, and in scaffolds seeded with AdERK2wt-transduced cells at an MOI of 1. In these scaffolds, obvious dark brown to black mineral deposits were evident throughout the scaffold.
Table 1:
ERK1/2 Expression Levels by AdERKwt-transduced ASCs on 3D scaffolds
| Adenovirus | MOI (pfu/cell) | OM+AdERK1/2wt – 21 days (-fold change vs. OM mock) |
|
|---|---|---|---|
| ERK1 | ERK2 | ||
| AdERK1wt | 1 | 1.50±0.28 | 1.37±0.38 |
| 10 | 2.77±0.60 | 2.28±0.81 | |
| 100 | 3.58±0.64 | 2.04±0.28 | |
| AdERK2wt | 1 | 0.95±0.28 | 1.05±0.34 |
| 10 | 1.36±0.52 | 5.89±1.48 | |
| 100 | 12.17±5.45 | 33.78±8.00 | |
n=4;
statistical significance (p<0.05) shown in bold
DISCUSSION
Enhanced ERK1/2 and JNK1/2 activation is associated with ASC mineralization
The present study investigated the role of ERK, JNK, and p38MAPK signaling on ASC osteogenesis. The signaling pathways involved in ASC osteogenesis were initially identified using a small, focused microarray and confirmed through real-time PCR. Osteogenesis was associated with changes in the expression of numerous MAPK signaling genes. Although MAPK activity and osteogenesis has been studied in osteoblasts and bone marrow stem cells [49, 50], a functional link between MAPK signaling and osteogenesis has only recently been suggested [39]. Since this seminal paper, a role for p38MAPK signaling in TGFβ1-mediated osteogenesis has been described in rat ASCs [51] and osteo-induction of human ASCs has been linked to JNK1/2 signaling [52]. Therefore, the current study involved a series of functional studies to examine the link between MAPK signaling and ASC osteogenesis.
We first screened numerous ASC populations to determine their osteogenic capacity. Mineralization was observed as granular extracellular deposits that increased in number and size as osteo-induction progressed. The ASC populations producing these deposits demonstrated higher mineralization levels versus controls, while basal mineralization similar to non-induced controls were measured in ASC populations failing to produce these deposits. EDX analysis of the deposits measured an atomic calcium to phosphorus (Ca/P) ratio of approximately 1.40. Although in vivo apatite has values closer to 1.67 [53], lower Ca/P ratios of 1.41–1.47 have been measured in newly formed dental enamel [54]. Ratios of 1.49 have also been measured in murine osteoblast cultures producing nodular mineralization patterns [55]. Aggregates with poor crystallinity and low Ca/P ratios have also been recorded in chicken osteoblast cultures [56]. Thus, the lower Ca/P ratio measured in the granular deposits produced by our ASC cultures may be consistent with poor crystalline apatite - a consequence of the artificial conditions inherent to in vitro culture conditions.
In our study, increased pERK and pJNK levels appeared after approximately two weeks in ASCs induced with either dexamethasone or VD3 and remained elevated over the entire induction period. In contrast, no change in p38MAPK activation was observed. This increase in ERK/JNK activation was not an artifact of dexamethasone induction as no link could be found between MAPK signaling and mineralization when induced with another glucocorticoid, hydroxycortisol. This mineralization of ASC cultures with hydroxycortisol was surprising as was the mineralization observed with OM containing only ascorbic acid/AA and β-glycerophosphate/BGP (OM/NoDexa). To our knowledge, our study is the first to suggest that ascorbic acid/AA and BGP alone can induce ASC mineralization. However, osteogenic-like mineralization has been found in suspension mononuclear cells exposed to these agents [57] and inorganic phosphate can increase calcification by smooth muscle cells [58, 59]. The mechanism of ASC mineralization in OM/NoDexa medium is unclear but may require some degree of MAPK signaling, as these populations had increased JNK1/2 signaling compared with controls. In support of this, 3D scaffolds of calcium phosphate increase JNK1/2 activation in osteoblast precursor cells [60] and inhibition of JNK1/2 activation can block Pi transport through type III transporters and decrease mineralization [61]. Therefore, it is not unreasonable to propose that AA and βGP may promote JNK1/2 signaling in mineralizing ASCs.
Correlations between elevated ERK activation and mineralization have been found in MSCs [31], in calvarial osteoblasts [62], in several in vivo bone models [62, 63] and, most recently, in ASCs [51]. Moreover, increased JNK activity has been measured in MSCs [64] and in periodontal stem cells [65]. Therefore, our results in osteogenic ASCs were not unexpected. However, when ERK and JNK activation were considered together with ASC mineralizationcapacity, three categories emerged. Category 3 populations with their high osteogenic potential demonstrated increased JNK1/2 and ERK1/2 activation versus controls, whereas Category 1 cultures with negligible mineralization exhibited both basal pERK and pJNK levels. Although cooperativity between ERK and JNK pathways in MSC osteogenesis is well known [31, 66, 67], we believe our study is the first to identify possible cooperation between ERK and JNK signaling and ASC osteogenesis. However, the nature of this signaling will require further study as the relationship between ERK and JNK is not likely to be straightforward, as evidenced in the Category 2 ASC populations with their moderate mineralization levels correlating to the activation of either ERK1/2 or JNK1/2. Therefore, how each kinase contributes to mineralization will need to be examined further. Moreover, how kinase activation and mineralization may be affected by ASC source should also be considered. The segregation of ASC populations into three distinct osteogenic categories may be a non-specific effect attributable to differences in donor age or the depot site used for ASC isolation. Age-related differences in proliferation, senescence, and mineralization potential have been noted in ASCs [68–70]. However, a correlation between adipose depot site and ASC behavior is less obvious [71–73]. Because our study used cosmetic lipoaspirates obtained from anonymous donors, the exact age and adipose depot for each donor were not known and conclusions about ASC source and the observed results cannot be made. Future studies will need to correlate these parameters to levels of mineralization and MAPK activation to increase the chances of successful bone regeneration using ASCs.
ASC osteogenesis is also mediated through the action of MAPK phosphatases
Previous work in HeLa cells [74] links dexamethasone action to increases in the expression and activity of MAPK phosphatase 1/MKP-1, a nuclear phosphatase that dephosphorylates members of the MAPK family [46, 75]. Because ERK and JNK phosphorylation are affected by phosphatases [76, 77], ASC mineralization, kinase activation, and the expression of the MKP-1 were examined in this study. Not surprisingly, we found that Category 3 ASCs with their increased kinase phosphorylation levels and significant mineralization were also associated with decreased MKP-1 expression. Category 1 ASC populations showed the opposite; increased MKP-1 expression, basal phosphorylation and negligible mineralization. The inhibition of MKP-1 expression (and activity) using sodium orthovanadate provided additional support for this relationship, augmenting ERK1/2 phosphorylation levels and promoting mineralization in low capacity Category 1 ASC samples, while having no effect on high capacity Category 3 samples. The mechanism of MKPs in ASC osteogenesis currently remains unknown. However, dexamethasone-mediated mineralization in dermal fibroblasts is associated with MKP1-dependent de-phosphorylation of a key serine residue in Runx-2 [78], while MAPK-driven phosphorylation of additional residues on this transcription factor has been linked to osteogenesis in C3H10T1/2 cells [79] Although no study to date has examined this in ASCs, the observed correlation between MKP-1 expression, ERK1/2 and JNK1/2 activation, and mineralization levelsin ASCs may be due to a similar Runx-2-mediated mechanism. Future studies, perhaps using siRNA directed to MKP-1 and an examination of Runx-2 phosphorylation will help uncover this mechanism.
Functional assessment of MAPK signaling in ASCs identifies possible stage-specific roles in promoting osteogenesis
Decreased expression of Runx-2 and other osteogenic markers has been reported in PD98059-treated MSCs grown on chemically modified surfaces [66, 80] and in periodontal ligament stem cells treated with SB203580 [81]. A link between PD98059, U0126, or SB203580 addition and decreased osteogenic capacity has also been reported in MC3T3 pre-osteoblasts, C2C12 cells, and MSCs [29, 32, 49] and human ASCs treated with PD98059 show a substantial decrease in mineralization [39]. Consistent with this, inhibiting ERK1/2, p38MAPK, or JNK1/2 activation in our study significantly decreased the expression of several key osteogenic genes, including Runx-2 and osteocalcin, along with extracellular mineralization (and AP activity), supporting a role for MAPK signaling in ASC osteogenesis. The level of the kinase affected appeared to be important, as the inhibition of upstream kinases, MEK1/2 or raf-1, did not significantly and consistently impact osteogenic gene expression, ERK1/2 phosphorylation, or ASC mineralization, suggesting that ERK1/2 activity in ASCs might be affected by MEK-independent pathways. Additional pathways such as the phosphoinositide-3-kinase (PI3K) and protein kinase C (PKC) paths are thought to intersect with ERK1/2 [82] and mediate its activation outside of MEK. MEK-independent ERK activation has also been reported in human neutrophils [83] and breast cancer cells [84]. Whether ERK1/2 signaling in ASC osteogenesis is under MEK-independent control will need to be examined in the future.
Three critical stages have been identified in osteogenesis: commitment, matrix synthesis, and matrix calcification/terminal differentiation [85]. Previous work has suggested similar stages in ASC osteogenesis [10]. Although the continuous addition of inhibitors to differentiating ASC cultures and the resulting decreases in mineralization suggests MAPK signaling is involved in the overall process of osteogenesis, it cannot provide information about stage-specific function. Therefore, inhibitors were also added at specific time points during differentiation to affect commitment, matrix synthesis, and the onset of mineralization. Overall, osteogenic commitment in ASCs may involve JNK1/2 signaling, with p38MAPK playing a role in matrix synthesis and mineralization. In support of this, mineralization levels decreased when p38MAPK activation was inhibited during the matrix synthesis stage (7–14 days) and during the onset of mineralization (14–21 days), whereas mineralization was impacted only when JNK1/2 inhibitors were added during the first 24 hours of induction. Because ERK1/2 inhibitors, with the exception of PD98059, had a more pronounced effect on ASC osteogenesis when added continuously and not during individual stages, this signaling pathway may mediate several events in this process. Our findings are consistent with those of others. A role for JNK in osteogenic commitment has been proposed in C3H10T1/2 cells [86] and periodontal ligament stem cells [65] while inhibiting p38MAPK activity decreases mineralization in bone marrow osteogenic precursors and AP activity in MC3T3 cells [32]. ERK-mediated mineralization has been proposed in human osteoblasts [62, 87] and osteosarcoma cells [88]. However, one should be cautious when making conclusions based solely on exogenous inhibitors. U0126 can target both MEK1/2 and ERK1/2. As a tyrosine phosphorylation inhibitor, Tyrphostin can also inhibit several growth factor receptor kinases and attenuate signaling though NF-κB [89]. Furthermore, PD98059 is thought to have an estrogenic effect [90]. Therefore, the reduction in ASC mineralization may be due to these alternative kinase functions.
Viral manipulation of ERK1/2 supports a role for ERK signaling in ASC osteogenesis
Because non-specificity is a problem with ERK1/2 inhibitors, targeted inhibition of ERK1/2 activation was achieved using shRNA lentiviruses and their production of cytoplasmic siRNA [91, 92]. siRNA in the study of ASC osteogenesis is relatively new. siRNA-dependent knockdown of PPARγ dramatically enhances ASC osteogenesis [93], while knockdown of osterix has been used to study the role of p38MAPK signaling in this process [94]. Due to homology in the region targeted by the shRNA oligos used in our study, the activation of both ERK isoforms was inhibited in ASCs, mimicking the effect of ERK1/2 exogenous inhibitors, such as PD98059 and Tyrphostin, but without their non-specific effects. The significant decreases in mineralization produced by ERK1/2 shRNA-transduced cells support our inhibitor studies and suggest that ERK1/2 signaling is involved in ASC osteogenesis. However, because ERK1/2 shRNA decreased pERK1/2 levels as far out as 21 days, mimicking that of continuous inhibitor administration, we cannot identify the specific stage at which ERK1/2 mediates osteogenesis. Future studies where ASCs are transduced with ERK1/2 shRNA at specific stages of osteogenesis could resolve this issue.
As an extension of our siRNA studies, the effect of ERK1/2 overexpression on ASC osteogenesis was also examined using recombinant ERK1 or ERK2 adenoviruses. Both ERK1 and ERK2 adenoviruses significantly increased kinase expression and mineralization levels in ASC monolayers as compared with mock-transduced controls. This pro-osteogenic effect was also observed on hydroxyapatite-coated PLGA scaffolds, with scaffolds overexpressing ERK1/2 demonstrating qualitatively more mineral deposition compared with controls expressing basal levels of these isoforms. Increased ERK1/2 activity on 3D scaffolds has translated to augmented mineralization and bone formation in vivo [95–97], making our results promising for future animal studies. Interestingly, when the ERK1 and ERK2 expressing scaffolds were compared with one another, it appeared that more mineral was deposited on the ERK2-expressing scaffolds. A few studies have proposed isoform-specific actions for ERK1/2. In vascular smooth muscle cells, increased ERK2 activation is measured after exposure to homocysteine, a risk factor for atherosclerosis [98], thereby suggesting a link between calcification and ERK2. Similarly, a link between ERK2, enhanced AP activity and taurine-induced osteogenesis has also been suggested in osteoblast-like cells [99]. Therefore, although the two ERK isoforms may appear to work cooperatively to promote osteogenesis in ASCs, the possibility of isoform-specific roles cannot be discounted and will need to be studied in the future.
In the current study, we propose a link between MAPK signaling and ASC osteogenesis. Direct manipulation of activation levels using inhibitors or viral technology supports this link, with osteogenic commitment mediated by JNK1/2, with matrix synthesis/mineralization mediated by p38MAPK and with ERK1/2 activity playing a more general role in the overall process of osteogenesis. Although the precise mechanism of MAPK signaling during these osteogenic stages remains unclear, future studies may clarify the matter and allow us to mimic the effects of powerful osteogenic agents, such as BMP2, by directly manipulating their downstream pathways and their osteogenic targets. Such an approach might open up a new cohort of patients available for regenerative medicine strategies as it would eliminate the need for growth factors and their potentially undesirable systemic effects.
Supplementary Material
Acknowledgements
This study was supported in part from a National Institutes of Health grant (NIDCR R01DE018931; PI: Zuk, P) and a Thrasher Family Foundation grant (PI: Zuk, P). The authors thank the following UCLA core facilities: the UCLA Genotyping and Sequencing Core for their sequencing of our MAPK constructs and the UCLA Vectorcore for the cloning, amplification, and titration of the recombinant adenoviruses used in this study. The UCLA Vectorcore is supported by the UCLA Jonsson Comprehensive Cancer Center (JCCC), under a P30 grant (CA016042) and the UCLA CURE: Digestive Diseases Research Center under P30 grant (DK041301).
REFERENCES
- [1].Zuk PA, Zhu M, Mizuno H, Huang JI, Futrell WJ, Katz AJ, Benhaim P, Lorenz HP, and Hedrick MH. Multi-lineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7:211–26. [DOI] [PubMed] [Google Scholar]
- [2].Dudas JR, Marra KG, Cooper GM, Penascino VM, Mooney MP, Jiang S, Rubin JP, Losee JE. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg. 2006;56:543–8. [DOI] [PubMed] [Google Scholar]
- [3].Hicok KC, Du Laney TV, Zhou YS, Halvorsen YD, Hitt DC, Cooper LF, Gimble JM. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–80. [DOI] [PubMed] [Google Scholar]
- [4].Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–9. [DOI] [PubMed] [Google Scholar]
- [5].Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–27. [DOI] [PubMed] [Google Scholar]
- [6].Ahn HH, Kim KS, Lee JH, Lee JY, Kim BS, Lee IW, Chun HJ, Kim JH, Lee HB, Kim MS. In Vivo Osteogenic Differentiation of Human Adipose-Derived Stem Cells in an Injectable In Situ-Forming Gel Scaffold. Tissue Eng Part A. 2009. [DOI] [PubMed] [Google Scholar]
- [7].Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–7. [DOI] [PubMed] [Google Scholar]
- [8].Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 2007;28:5477–86. [DOI] [PubMed] [Google Scholar]
- [9].Conejero JA, Lee JA, Parrett BM, Terry M, Wear-Maggitti K, Grant RT, Breitbart AS. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast Reconstr Surg. 2006;117:857–63. [DOI] [PubMed] [Google Scholar]
- [10].Zuk PA, Zhu M., Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knippenberg M, Helder MN, Doulabi BZ, Semeins CM, Wuisman PI, Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–8. [DOI] [PubMed] [Google Scholar]
- [12].Duque G, Rivas D. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. J Bone Miner Res. 2007;22:1603–11. [DOI] [PubMed] [Google Scholar]
- [13].Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–46. [DOI] [PubMed] [Google Scholar]
- [14].Cowan CM, Aalami OO, Shi YY, Chou YF, Mari C, Thomas R, Quarto N, Nacamuli RP, Contag CH, Wu B, Longaker MT. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645–58. [DOI] [PubMed] [Google Scholar]
- [15].Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–9. [DOI] [PubMed] [Google Scholar]
- [16].Daniels O Guidelines for training in paediatric cardiology. Cardiol Young. 2000;10:76–9. [DOI] [PubMed] [Google Scholar]
- [17].Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel GE. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop Relat Res. 2002:110–20. [DOI] [PubMed] [Google Scholar]
- [18].Woo EJ. Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg. 2012;70:765–7. [DOI] [PubMed] [Google Scholar]
- [19].Chou Y-F, Zuk PA, Chang T-L, Benhaim P, Wu BM. Adipose-Derived Stem Cells and BMP2: Part 1 BMP2-Treated Adipose-Derived Stem Cells Do Not Improve Repair of Segmental Femoral Defects. Conn Tiss Res. 2010;in press. [DOI] [PubMed] [Google Scholar]
- [20].Zuk P, Chou YF, Mussano F, Benhaim P, Wu BM. Adipose-derived stem cells and BMP2: part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connect Tissue Res. 2011;52:119–32. [DOI] [PubMed] [Google Scholar]
- [21].Gomez N, Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353:170–3. [DOI] [PubMed] [Google Scholar]
- [22].Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–8. [DOI] [PubMed] [Google Scholar]
- [23].Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–64. [DOI] [PubMed] [Google Scholar]
- [24].Lou J, Tu Y, Li S, Manske PR. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun. 2000;268:757–62. [DOI] [PubMed] [Google Scholar]
- [25].Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–8. [DOI] [PubMed] [Google Scholar]
- [26].Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. [DOI] [PubMed] [Google Scholar]
- [27].Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–20. [DOI] [PubMed] [Google Scholar]
- [28].Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17:1785–94. [DOI] [PubMed] [Google Scholar]
- [29].Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–74. [DOI] [PubMed] [Google Scholar]
- [30].Huang Z, Cheng SL, Slatopolsky E. Sustained activation of the extracellular signal-regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J Biol Chem. 2001;276:21351–8. [DOI] [PubMed] [Google Scholar]
- [31].Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–52. [DOI] [PubMed] [Google Scholar]
- [32].Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, Caverzasio J. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002;30:91–8. [DOI] [PubMed] [Google Scholar]
- [33].Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–48. [DOI] [PubMed] [Google Scholar]
- [34].Rodriguez JP, Rios S, Fernandez M, Santibanez JF. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Cell Biochem. 2004;92:745–54. [DOI] [PubMed] [Google Scholar]
- [35].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- [36].Catalano MG, Marano F, Rinella L, de Girolamo L, Bosco O, Fortunati N, Berta L, Frairia R. Extracorporeal shockwaves (ESWs) enhance the osteogenic medium-induced differentiation of adipose-derived stem cells into osteoblast-like cells. J Tissue Eng Regen Med. 2014. [DOI] [PubMed] [Google Scholar]
- [37].Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY, Davies B, Zreiqat H. Activation and promotion of adipose stem cells by tumour necrosis factor-alpha preconditioning for bone regeneration. J Cell Physiol. 2013;228:1737–44. [DOI] [PubMed] [Google Scholar]
- [38].Monaco E, Bionaz M, Rodriguez-Zas S, Hurley WL, Wheeler MB. Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PLoS One. 2012;7:e32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Q, Cen L, Zhou H, Yin S, Liu G, Liu W, Cao Y, Cui L. The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng Part A. 2009;15:3487–97. [DOI] [PubMed] [Google Scholar]
- [40].Pilgaard L, Lund P, Duroux M, Lockstone H, Taylor J, Emmersen J, Fink T, Ragoussis J, Zachar V. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res. 2009;315:1937–52. [DOI] [PubMed] [Google Scholar]
- [41].Charoenpanich A, Wall ME, Tucker CJ, Andrews DM, Lalush DS, Loboa EG . Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng Part A. 2011;17:2615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2015:e50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75:81–90. [DOI] [PubMed] [Google Scholar]
- [44].Reffas S, Schlegel W. Compartment-specific regulation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) by ERK-dependent and non-ERK-dependent inductions of MAPK phosphatase (MKP)-3 and MKP-1 in differentiating P19 cells. Biochem J. 2000;352 Pt 3:701–8. [PMC free article] [PubMed] [Google Scholar]
- [45].Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–93. [DOI] [PubMed] [Google Scholar]
- [46].Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Johnson RB, Henderson JS. Enhancement by sodium orthovanadate of the formation and mineralization of bone nodules by chick osteoblasts in vitro. Arch Oral Biol. 1997;42:271–6. [DOI] [PubMed] [Google Scholar]
- [48].Chou YF, Chiou WA, Xu Y, Dunn JC, Wu BM. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials. 2004;25:5323–31. [DOI] [PubMed] [Google Scholar]
- [49].Schindeler A, Little DG. Ras-MAPK signaling in osteogenic differentiation: friend or foe? J Bone Miner Res. 2006;21:1331–8. [DOI] [PubMed] [Google Scholar]
- [50].Greenblatt MB, Shim JH, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol. 2013;29:63–79. [DOI] [PubMed] [Google Scholar]
- [51].Liu Y, Zheng WK, Gao WS, Shen Y, Ding WY. Function of TGF-beta and p38 MAKP signaling pathway in osteoblast differentiation from rat adipose-derived stem cells. Eur Rev Med Pharmacol Sci. 2013;17:1611–9. [PubMed] [Google Scholar]
- [52].Gu H, Huang Z, Yin X, Zhang J, Gong L, Chen J, Rong K, Xu J, Lu L, Cui L. Role of c-Jun N-terminal kinase in the osteogenic and adipogenic differentiation of human adipose-derived mesenchymal stem cells. Exp Cell Res. 2015;339:112–21. [DOI] [PubMed] [Google Scholar]
- [53].MacDonald DE, Betts F, Stranick M, Doty S, Boskey AL. Physicochemical study of plasma-sprayed hydroxyapatite-coated implants in humans. J Biomed Mater Res. 2001;54:480–90. [DOI] [PubMed] [Google Scholar]
- [54].Bonar LC, Shimizu M, Roberts JE, Griffin RG, Glimcher MJ. Structural and composition studies on the mineral of newly formed dental enamel: a chemical, x-ray diffraction, and 31P and proton nuclear magnetic resonance study. J Bone Miner Res. 1991;6:1167–76. [DOI] [PubMed] [Google Scholar]
- [55].Querido W, Abracado LG, Rossi AL, Campos AP, Rossi AM, San Gil RA, Borojevic R, Balduino A, Farina M. Ultrastructural and mineral phase characterization of the bone-like matrix assembled in F-OST osteoblast cultures. Calcif Tissue Int. 2011;89:358–71. [DOI] [PubMed] [Google Scholar]
- [56].Rey C, Kim HM, Gerstenfeld L, Glimcher MJ. Structural and chemical characteristics and maturation of the calcium-phosphate crystals formed during the calcification of the organic matrix synthesized by chicken osteoblasts in cell culture. J Bone Miner Res. 1995;10:1577–88. [DOI] [PubMed] [Google Scholar]
- [57].Hadzir SN, Ibrahim SN, Abdul Wahab RM, Zainol Abidin IZ, Senafi S, Ariffin ZZ, Abdul Razak M, Zainal Ariffin SH. Ascorbic acid induces osteoblast differentiation of human suspension mononuclear cells. Cytotherapy. 2013;16:674–82. [DOI] [PubMed] [Google Scholar]
- [58].Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–7. [DOI] [PubMed] [Google Scholar]
- [59].Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67. [DOI] [PubMed] [Google Scholar]
- [60].Appleford MR, Oh S, Cole JA, Carnes DL, Lee M, Bumgardner JD, Haggard WO, Ong JL. Effects of trabecular calcium phosphate scaffolds on stress signaling in osteoblast precursor cells. Biomaterials. 2007;28:2747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, Ono Y, Miura Y, Oiso Y, Itoh M, Caverzasio J. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21:674–83. [DOI] [PubMed] [Google Scholar]
- [62].Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen YJ, Kuo YR, Yang KD, Wang CJ, Sheen Chen SM, Huang HC, Yang YJ, Yi-Chih S, Wang FS. Activation of extracellular signal-regulated kinase (ERK) and p38 kinase in shock wave-promoted bone formation of segmental defect in rats. Bone. 2004;34:466–77. [DOI] [PubMed] [Google Scholar]
- [64].Fu L, Tang T, Miao Y, Zhang S, Qu Z, Dai K. Stimulation of osteogenic differentiation and inhibition of adipogenic differentiation in bone marrow stromal cells by alendronate via ERK and JNK activation. Bone. 2008;43:40–7. [DOI] [PubMed] [Google Scholar]
- [65].Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, Tang C, Wang Z, Yu J, Zhang G. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 2012;137:513–25. [DOI] [PubMed] [Google Scholar]
- [66].Wang Y, Li J, Song W, Yu J. Mineral trioxide aggregate upregulates odonto/osteogenic capacity of bone marrow stromal cells from craniofacial bones via JNK and ERK MAPK signalling pathways. Cell Prolif. 2014;47:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chiu LH, Yeh TS, Huang HM, Leu SJ, Yang CB, Tsai YH. Diverse effects of type II collagen on osteogenic and adipogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2011;227:2412–20. [DOI] [PubMed] [Google Scholar]
- [68].Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290–301. [DOI] [PubMed] [Google Scholar]
- [69].Kornicka K, Marycz K, Tomaszewski KA, Maredziak M, Smieszek A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid Med Cell Longev. 2015;2015:309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang HJ, Kim KJ, Kim MK, Lee SJ, Ryu YH, Seo BF, Oh DY, Ahn ST, Lee HY, Rhie JW. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs. 2014;199:373–83. [DOI] [PubMed] [Google Scholar]
- [71].Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–22. [DOI] [PubMed] [Google Scholar]
- [72].Levi B, James AW, Glotzbach JP, Wan DC, Commons GW, Longaker MT. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126:822–34. [DOI] [PubMed] [Google Scholar]
- [73].Iwen KA, Priewe AC, Winnefeld M, Rose C, Siemers F, Rohwedel J, Cakiroglu F, Lehnert H, Schepky A, Klein J, Kramer J. Gluteal and abdominal subcutaneous adipose tissue depots as stroma cell source: gluteal cells display increased adipogenic and osteogenic differentiation potentials. Exp Dermatol. 2014;23:395–400. [DOI] [PubMed] [Google Scholar]
- [74].Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22:7802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Horsch K, de Wet H, Schuurmans MM, Allie-Reid F, Cato AC, Cunningham J, Burrin JM, Hough FS, Hulley PA. Mitogen-activated protein kinase phosphatase 1/dual specificity phosphatase 1 mediates glucocorticoid inhibition of osteoblast proliferation. Mol Endocrinol. 2007;21:2929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- [77].Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–13. [DOI] [PubMed] [Google Scholar]
- [78].Phillips JE, Gersbach CA, Wojtowicz AM, Garcia AJ. Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J Cell Sci. 2006;119:581–91. [DOI] [PubMed] [Google Scholar]
- [79].Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284:32533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bai B, He J, Li YS, Wang XM, Ai HJ, Cui FZ. Activation of the ERK1/2 signaling pathway during the osteogenic differentiation of mesenchymal stem cells cultured on substrates modified with various chemical groups. Biomed Res Int. 2013;2013:361906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nie J, Zhang B, Gu B, Liu N. Effects of p38 mitogen-activated protein kinase on osteogenic differentiation of human periodontal ligament stem cells in inflammatory microenvironment. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:1–7. [DOI] [PubMed] [Google Scholar]
- [82].Grammer TC, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–42. [DOI] [PubMed] [Google Scholar]
- [83].Simard FA, Cloutier A, Ear T, Vardhan H, McDonald PP. MEK-independent ERK activation in human neutrophils and its impact on functional responses. J Leukoc Biol. 2015;98:565–73. [DOI] [PubMed] [Google Scholar]
- [84].Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bruder SP, Gazit D, Passi-Even L, Bab I, and Caplan AI. Osteochondral differentiation and the emergence of stage-specific osteogenic cell-surface molecules by bone marrow cells in diffusion chambers. Bone Miner. 1990;11:141–51. [DOI] [PubMed] [Google Scholar]
- [86].Zhao YF, Xu J, Wang WJ, Wang J, He JW, Li L, Dong Q, Xiao Y, Duan XL, Yang X, Liang YW, Song T, Tang M, Zhao D, Luo JY. Activation of JNKs is essential for BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep. 2013;46:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–50. [DOI] [PubMed] [Google Scholar]
- [88].Sciandra M, Marino MT, Manara MC, Guerzoni C, Grano M, Oranger A, Lucarelli E, Lollini PL, Dozza B, Pratelli L, Renzo MF, Colombo MP, Picci P, Scotlandi K. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK 1/2. J Bone Miner Res. 2014;29:1295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–22. [DOI] [PubMed] [Google Scholar]
- [90].Cotrim CZ, Amado FL, Helguero LA. Estrogenic effect of the MEK1 inhibitor PD98059 on endogenous estrogen receptor alpha and beta. J Steroid Biochem Mol Biol. 2011;124:25–30. [DOI] [PubMed] [Google Scholar]
- [91].Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. [DOI] [PubMed] [Google Scholar]
- [92].McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–47. [DOI] [PubMed] [Google Scholar]
- [93].Lee MJ, Chen HT, Ho ML, Chen CH, Chuang SC, Huang SC, Fu YC, Wang GJ, Kang L, Chang JK. PPARgamma silencing enhances osteogenic differentiation of human adipose-derived mesenchymal stem cells. J Cell Mol Med. 2013;17:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li CJ, Madhu V, Balian G, Dighe AS, Cui Q. Cross-Talk Between VEGF and BMP-6 Pathways Accelerates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J Cell Physiol. 2015;230:2671–82. [DOI] [PubMed] [Google Scholar]
- [95].Wang C, Lin K, Chang J, Sun J. Osteogenesis and angiogenesis induced by porous beta-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77. [DOI] [PubMed] [Google Scholar]
- [96].Jin Y, Zhang W, Liu Y, Zhang M, Xu L, Wu Q, Zhang X, Zhu Z, Huang Q, Jiang X. rhPDGF-BB via ERK pathway osteogenesis and adipogenesis balancing in ADSCs for critical-sized calvarial defect repair. Tissue Eng Part A. 2014;20:3303–13. [DOI] [PubMed] [Google Scholar]
- [97].Wei J, Xu M, Zhang X, Meng S, Wang Y, Zhou T, Ma Q, Han B, Wei Y, Deng X. Enhanced Osteogenic Behavior of ADSCs Produced by Deproteinized Antler Cancellous Bone and Evidence for Involvement of ERK Signaling Pathway. Tissue Eng Part A. 2015;21:1810–21. [DOI] [PubMed] [Google Scholar]
- [98].Brown JC, Rosenquist TH, Monaghan DT. ERK2 activation by homocysteine in vascular smooth muscle cells. Biochem Biophys Res Commun. 1998;251:669–76. [DOI] [PubMed] [Google Scholar]
- [99].Park S, Kim H, Kim SJ. Stimulation of ERK2 by taurine with enhanced alkaline phosphatase activity and collagen synthesis in osteoblast-like UMR-106 cells. Biochem Pharmacol. 2001;62:1107–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.