Abstract
Objective
Tissue engineered vascular grafts (TEVGs) containing adipose derived mesenchymal stem cells (ADMSCs) offer an alternative to small diameter vascular grafts currently used in cardiac and lower extremity revascularization procedures. ADMSC infused TEVGs have been shown to promote remodeling and vascular homeostasis in vivo and offer a possible treatment solution for those suffering from cardiovascular disease. Unfortunately, the time needed to cultivate ADMSCs remains a large hurdle for TEVGs as a treatment option. The purpose of this study was to determine if stromal vascular fraction (SVF) (known to contain progenitor cells) seeded TEVGs would remain patent in vivo and remodel allowing for a “same-day” process for TEVG fabrication and implantation.
Methods
SVF, obtained from adult human adipose tissue, was seeded within 4 hours after acquisition from the patient onto poly(ester urethane)urea bilayered scaffolds using a customized rotational vacuum seeding device. Constructs were then surgically implanted as abdominal aortic interposition grafts in Lewis rats.
Results
Findings revealed patency in 5 of 7 implanted scaffolds at 8 weeks, along with neotissue formation and remodeling occurring in patent TEVGs. Patency was documented using angiography and gross inspection, while remodeling and vascular components were detected using immunofluorescent chemistry.
Conclusions
A “same-day” cell-seeded TEVG can remain patent after implantation in vivo, with neotissue formation and remodeling occurring by 8 weeks.

Introduction:
Cardiovascular disease remains the leading cause of death in the United States, significantly contributing to morbidity and mortality 1–3. Treatment costs are expected to reach over $918 billion per year by 2020 making it the highest total healthcare expenditure in the United States 4. Commonly performed procedures in the treatment of cardiovascular disease include bypass graft replacement using native autologous vessels comprising the mammary and radial arteries and saphenous vein 2, 5, 6, with coronary artery bypass accounting for approximately 400,000 operations performed annually in the United States as of 2016 7. However, use of autologous vessels comes with inherent drawbacks such as size and mechanical mismatch, availability, and complications associated with morbidity from surgical harvest 8–11. Additionally, synthetic grafts fail due to acute thrombosis at diameters less than 6mm 12-14.
To overcome these limitations, small diameter (<6mm) tissue engineered vascular grafts (TEVGs) have been developed that resist thrombosis and intimal hyperplasia 15–21. Much of this work uses cell-based TEVGs. However, such cell-based TEVGs come with the limitations that cells often require time to expand prior to TEVG fabrication and cell-seeding on the TEVG scaffold often requires lengthy bioreactor culture prior to implantation. Furthermore, cell culture and expansion limits clinical applicability due to regulatory concerns set by the Food and Drug Administration, which recommends the use of fresh cells with minimized time between isolation and implantation 22.
A non-cultured, progenitor cell-rich population known as the stromal vascular fraction (SVF) could circumvent the above concerns about cell-based TEVGs. In a recent study by our group, scaffolds seeded with SVF and exposed to a 48 hour dynamic culture period produced TEVGs in vivo that were comparable to TEVGs produced using cultured adipose-derived mesenchymal stem cells (ADMSCs) 6. However, although the culture period and time of fabrication were greatly reduced in this study, it still employed an in vitro culture step.
We hypothesized that the removal of any and all culture steps, including the 48-hr dynamic bioreactor culture period in our previously reported SVF seeded TEVG model, would not affect productive in vivo remodeling, allowing for a “same-day” process for TEVG fabrication and implantation. To test this hypothesis, a small scale proof of concept study was conducted to demonstrate the ability for a completely non-cultured cell population seeded onto an elastomeric scaffold and immediately placed as an interpositional graft in the abdominal aorta to remodel into a functional TEVG. Additionally, in order to relate this study to earlier work in our lab, we compared the phenotypic diversity of cells within SVF seeded directly into our TEVGs for immediate implantation to that within our TEVGs after a 48-hr dynamic culture period, as well as to that of SVF that is culture-expanded prior to seeding onto our TEVGs. Our results demonstrate that TEVGs manufactured in a single day perform similar to cultured TEVGs or TEVGs seeded with culture expanded cells, representing a significant advancement in TEVG clinical translation.
Methods:
Patient selection and SVF isolation
SVF was obtained from the discarded waste adipose tissue of non-diabetic female human adults, 45 years of age or younger, undergoing elective standard of care liposuction, abdominoplasty, or panniculectomy procedures. Tissue was transferred to the research team through an honest broker under University of Pittsburgh IRB exempt protocol #0511186 23, 24. Since this was an exempt protocol, and no identifiable information was available to the research staff, informed consent was not obtained. Patient information was limited to age in years, gender, body mass index and diabetic status.
SVF was freshly isolated using previously described methods 25, 26. Briefly, adipose tissue (~250 cc per patient) was cut into ~10 cc portions which were each placed into separate 50 cc conical tubes. Each piece was minced and added to a collagenase solution (Hanks’ Balanced Salt Solution ((HBSS, Invitrogen, Carlsbad, CA) containing 3.5% bovine serum albumin (BSA, Millipore, Charlottesville, VA) and 1 mg/mL collagenase type II (Worthington, Lakewood, NJ)) 26. Tubes were then incubated at 37 °C with agitation for approximately 1 hour. Digested tissue was filtered through successive 425 and 180 μm sieves (W.S. Tyler, Mentor, OH) to remove undigested pieces and then centrifuged at 1000 RPM for 10 min at ambient temperature. After centrifugation, the cell pellet was resuspended in an NH4Cl-based buffer (Beckman Coulter, Miami, FL, Cat No. IM3630d) to lyse red blood cells. The resulting cell suspension was filtered once more through a 180 μm sieve and centrifuged (1000 RPM, 10 min, ambient temperature). The resulting pellet, termed SVF, was resuspended in HBSS and maintained on ice for up to 2 hours until scaffold seeding or cell culture. The process typically yielded 30 to 40 million fresh SVF cells when using 200 cc of adipose tissue. Freshly isolated SVF was either used for culture or immediately seeded onto our TEVG scaffolds for either analysis or immediate implantation.
Scaffold fabrication
Scaffolds used in this study were fabricated out of poly(ester urethane)urea (PEUU), which is biodegradable and elastomeric. Scaffolds were bilayered, tubular, approximately 1 cm long, and had a ~1.3 mm inner diameter, to approximate the shape and size of a rat infrarenal abdominal aorta. Scaffolds were manufactured and used as described previously 16, 17, 27, 28. Briefly, PEUU polymer was used to fabricate both layers of the scaffold. The porous inner layer of the scaffold was created using thermally induced phase separation (TIPS) in a tubular mold. The TIPS layer was then coated by electrospinning an additional layer of PEUU for mechanical stability. This scaffold has already shown efficacy in the TEVG context using multiple cell types 6, 17, 19, 27–29.
Cell culture and SVF expansion for phenotypic analysis
A fraction of SVF was expanded in cell culture using defined culture media ([1:1 Dulbecco’s modified Eagle’s medium (DMEM, #11965; Gibco) to DMEM/F12 (#113300; Gibco) with 10% fetal bovine serum (#S11550; Atlanta Biologics), antibiotics (1% penicillin/streptomycin, 0.5% Fungizone, 0.1% gentamycin), and 10μl/l dexamethasone]) mixed with 25% Preadipocyte Growth Medium (#C27410, #C-39425; PromoCell). Upon reaching 80% confluency, the cells were removed from the plate by trypsinization and termed “passage 0 (P0) ADMSC”. These cells were then plated for additional expansion up to passage 4 using the same protocol, or used directly for seeding experiments.
Scaffold seeding and incubation
Scaffolds were seeded with SVF or P0-P4 ADMSC using a customized rotational vacuum seeding device as described and used previously 19, 29–31. Briefly, the scaffolds were mounted within the device, and the cell suspension was infused with vacuum and rotation (flow rate of 1 ml/min, vacuum pressure of −120 mmHg, and rotation speed of 15 rpm). Each scaffold seeded with approximately 3 million SVF cells.
After seeding, the constructs were placed in defined culture media (see Cell culture and SVF expansion for phenotypic analysis) for transport and immediate implantation (see Aortic implantation of seeded scaffolds) or given 4 hours incubation for phenotypic analysis, allowing the cells to adhere to the scaffold under static conditions. At this point, constructs were either fixed or dynamically cultured for an additional 48 hours. As previously described, dynamic culture entailed suspension in a 500-mL spinner flask (Kontes #Cytostir 882911–0250) (100 ml of defined culture media, rotation speed 15 rpm) 6, 16, 25.
Phenotypic analysis of seeded scaffolds
Seeded constructs used for phenotypic analysis were fixed in 4% paraformaldehyde, washed, placed in a 30% sucrose solution for 30 min, and frozen at −20 C. Frozen seeded constructs were then sectioned using a cryostat at a thickness of 10 microns onto gelatin coated slides. In order to determine the SVF cell populations adhering to the PEUU scaffolds, immunofluorescent chemistry (IFC) was performed. Sections of the seeded constructs were permeablized using a 0.1% Triton solution, and 5% goat serum was used for blocking. Sections were incubated with 1:100 dilutions of either rabbit antihuman CD90 (ab133350, Abcam, Cambridge, UK), rabbit anti-human CD31 (ab28364, Abcam, Cambridge, UK), or rabbit anti-human CD34 (ab81289, Abcam, Cambridge, UK), to evaluate the presence of mesenchymal stem cell, endothelial cell, or endothelial progenitor cell populations respectively. Additional IFC for SVF markers CD14, CD45, and CD105 was performed (see Supplemental Material). All IFC staining included a primary delete for a negative control and an additional isotype control (ab172730, Abcam, Cambridge, UK). Seeded constructs were then incubated with 1:1000 dilutions of FITC-conjugated goat anti-rabbit IgG secondary antibody (611–1202, Rocklandinc, Pottstown, PA) and then counterstained with DAPI (B2883, Sigma-Aldrich, St. Louis, MO) to mark cell nuclei. Imaging was conducted on a Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan).
Cell populations were quantified by calculating the ratio between cells exhibiting each specific cell marker (CD90, CD31, and CD34) and the total cell count as established by evaluating the DAPI stained nuclei; a custom ImageJ macro was built for this purpose (for greater detail, please see Supplemental Material: ImageJ Macro Analysis).
Aortic implantation of seeded scaffolds
All animal procedures were performed under a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Seeded scaffolds were surgically implanted as abdominal aortic interpositional grafts in Lewis rats as described previously 17, 27, 28. Briefly, rats were anesthetized using ketamine (30 mg/kg) and placed in a supine position and kept under sedation for the duration of the surgery using nose cone administration of isofluorane (Induction (2–5%) and maintenance (0.25–4%) 1.5 L/min in oxygen). An incision was made into the abdominal wall and the infrarenal abdominal aorta was exposed through blunt dissection. Microclamps were applied to the aorta, which was then bisected, upon which a 1 cm seeded construct was placed interpositionally and sutured with 10–0 Prolene (Ethicon, Somerville, NJ) (Supplemental Figure 1). After the graft was secured, the clamps were released and the patency verified by observation of the distal pulse pressure. The animals were then closed with 3–0 polyglactin sutures (McKesson, Richmond, VA). Buprenorphine hydrochloride was administered postoperatively every 12 hours for the first 72 hour, and animals were maintained on an anticoagulant (dipyridamole, aspirin) schedule for 4 weeks, as previously described 16. Implanted scaffolds were allowed to remodel for 8 weeks in order to allow enough time for remodeling and neotissue formation to occur as previously observed 6, 16, 29, 30. Rats were then sacrificed using isofluorane (Induction (2–5%) 1.5 L/min oxygen) and a single intracardiac injection of heparin/KCl. The descending aorta proximal to the TEVG was catheterized and injected with X-ray contrast agent for imaging and an angiography was performed to determine in vivo patency. The TEVG and adjacent aortic tissue were excised for gross inspection and further analysis.
Immunofluorescent and histologic evaluation
Explanted TEVGs were fixed in 4% paraformaldehyde prior to frozen sectioning or paraffin embedding. Frozen sectioning was performed as described above using FITC conjugated mouse anti-von Willebrand Factor (vWF – 1:250; V2700–07, US Biological, Salem, MA) and mouse anti-smooth muscle α-actin (αSMA) (A5228, Sigma-Aldrich, St. Louis, MO) along with a TRITC conjugated anti-mouse IgG secondary antibody (ab6786, Abcam, Cambridge, UK). The presence of any remaining implanted cells was also determined using IFC for rabbit anti-human CD90 (ab133350, Abcam, Cambridge, UK) and human nuclear antigen (MAB1281, MEMD Millipore, USA) with FITC-conjugated goat anti-rabbit IgG secondary antibody (611–1202, Rockland-inc, Pottstown, PA) and counterstained with DAPI (B2883, Sigma-Aldrich, St. Louis, MO) to mark cell nuclei.
Histology was performed on paraffin embedded sections of both explanted TEVGs and native rat aorta proximal to the anastomosis using the Histology Core at the McGowan Institute for Regenerative Medicine. Sections were stained for Verhoff van Gieson staining to indicate elastin fibers and Masson’s Trichrome to indicate collagen and other vascular components.
Statistical analysis
A 2-way ANOVA was performed on the phenotypic analysis data using SPSS software (IBM, Armonk, New York) between the different cell markers and each passage of cells. A Bonferroni confidence interval adjustment was made, and pairwise comparisons were made post hoc. Additionally, a paired student’s t-test was used to evaluate statistical differences in cell markers between immediate and 48 hour cultured constructs.
Results:
Phenotypic analysis of SVF and passaged ADMSC
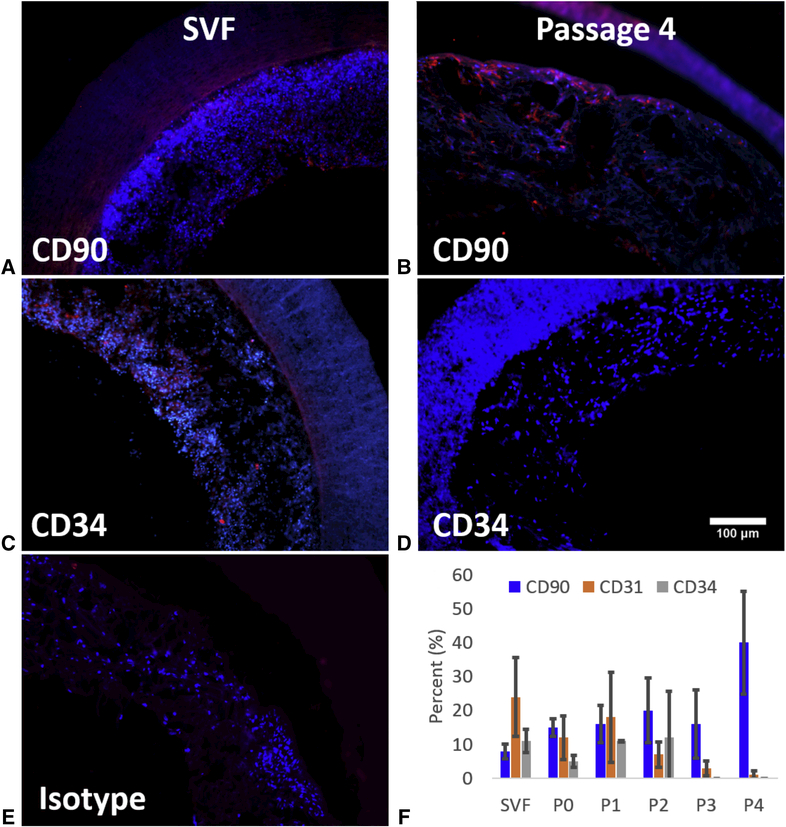
SVF was gathered from 6 different subjects at the Adipose Stem Cell Research Laboratory – University of Pittsburgh (available patient information provided in Table 1). IFC analysis of the seeded SVF/ADMSC phenotypes showed a distinct shift with increasing passage (Figure 1). Specifically, scaffolds seeded with culture expanded cells were found to have a nearly 4-fold increase in CD90 positive staining, while the percentage of cells positive for CD31 and CD34 became undetectable, by passage 4 (P=0.004).
Table 1).
SVF donor information, along with collection and procedure information. Use refers to use in either cellular culture and IFC comparisons to uncultured seeded scaffolds, or use in “same-day” implant procedures and explant results.
| DONOR ID NO. | GENDER | AGE, YEARS | PROCEDURE | USE | RESULT OF IMPLANTATION |
|---|---|---|---|---|---|
| HS 1 | Female | 45 | Panniculectomy | IFC and Culture | not implanted |
| HS 2 | Female | 27 | Panniculectomy | IFC and Culture | not implanted |
| HS 3 | Female | 20 | Panniculectomy | IFC and Culture | not implanted |
| HS 4 | Female | 35 | Liposuction | IFC and Culture | not implanted |
| HS 5 | Female | 41 | Abdominoplasty | IFC and Culture | not implanted |
| HS 6 | Female | 44 | Panniculectomy | IFC and Culture | not implanted |
| HS 7 | Female | 28 | Panniculectomy | Implant | 1 occluded (SVF-1), 1 fully remodeled (SVF-2) |
| HS 8 | Female | 42 | Liposuction | Implant | 1 occluded (SVF-3) |
| HS 9 | Female | 33 | Panniculectomy | Implant | 2 partially remodeled (SVF–4 & –5) |
| HS 10 | Female | 25 | Panniculectomy | Implant | 2 fully remodeled (SVF–6 & –7) |
Figure 1:
Representative IFC images depicting positive staining of cells for CD90 (A and B) and CD34 (C and D). Initial SVF cells stained positively for both CD90 (A) and CD34 (C), while positive staining for CD90 (B) increased and positive staining for CD34 (D) decreased at passage 4. E) Isotype control demonstrating no non-specific immunopositive cells. F) IFC analysis of percentage of adherent cells positive for cell markers CD90 (mesenchymal stem cell marker), CD31 (endothelial marker), and CD34 (endothelial progenitor marker) seeded onto PEUU scaffolds with cells from fresh SVF out to passage 0 through 4 and given a 48 hour dynamic culture period.
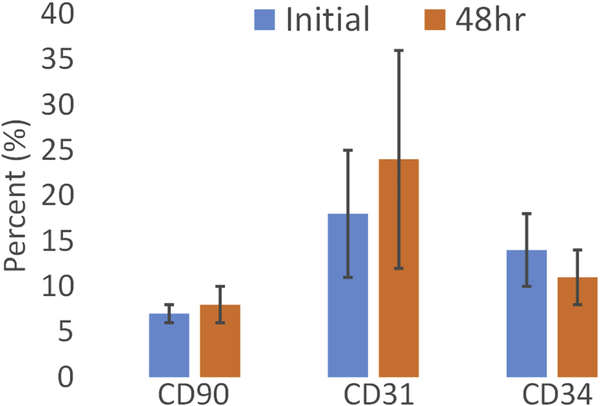
Additional evaluations were made comparing scaffolds seeded with fresh, uncultured SVF that were fixed immediately after seeding vs. scaffolds that had 48 hours of dynamic culture before being fixed. Adherent cells in constructs seeded with fresh, uncultured SVF displayed markers for CD90, CD31, and CD34 in varying amounts with CD31 positive cells significantly higher than CD90 positive cells in the fresh SVF (P=0.023). Additional IFC analysis comparing the fresh, uncultured SVF found no difference in CD marker expression between constructs given only a brief time for cells to adhere to the scaffold (to compare to constructs used for implantation) and constructs given a 48 hour dynamic culture period (the standard culture used in previous studies 6, 16, 30) (Figure 2).
Figure 2:
Average cell populations for SVF constructs fixed after initial incubation and 48-hours of dynamic culture (n = 6). A paired t-test indicated no significant statistical difference between initial and 48-hour constructs for each of the CD90, CD31, and CD34 markers (P = 0.63, 0.68, and 0.46 respectively).
“Same-day” TEVG implantation and remodeling
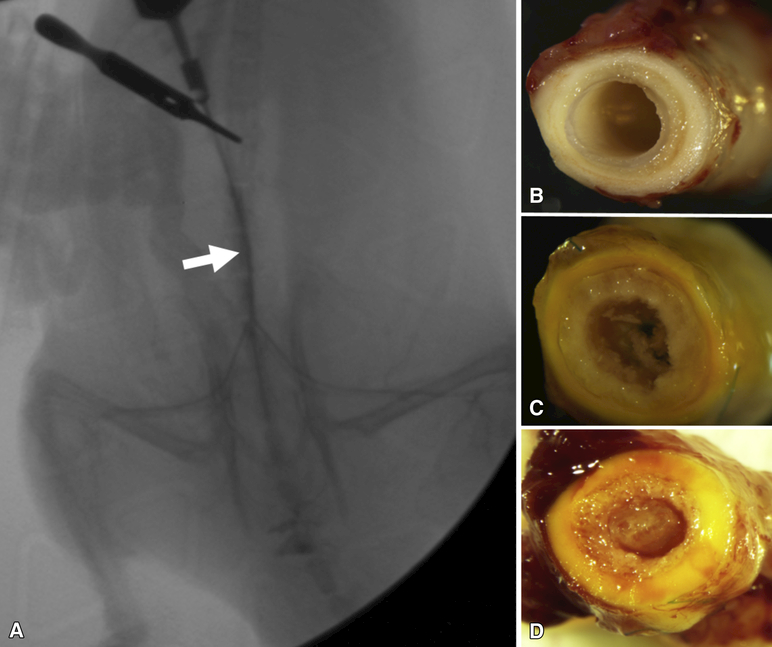
A total of 7 “same-day” implantation procedures, from 4 different SVF collections, were performed for this study, with most SVF harvest procedures resulting in 2 implanted scaffolds each (see Table 1). Patency was observed in 5 of the 7 TEVG explants through angiography (Figure 3A) and gross inspection which classified explants as fully remodeled (Figure 3B), partially remodeled (Figure 3C), or occluded (Figure 3D) (see Supplementary Materials: Remodeling Classifications). Patent explanted “same-day” TEVGs indicated remodeling into a vascular-like tissue after 8 weeks of implantation in vivo, though two only showed partial remodeling (Table 1). Of the two TEVGs that were occluded, intimal hyperplasia was observed at both the proximal and distal anastomoses. No thrombus was found within either of the partially remodeled grafts, nor the occluded grafts, suggesting that blood was able to pass freely though the conduit before the anastomostic hyperplasia emerged.
Figure 3:
A) Angiogram confirming flow through a representative (SVF-2 in Table 1) fully-remodeled, SVF-seeded TEVG (arrow) 8 weeks after implantation. B-D) Gross inspection of explanted TEVGs revealing patency and neotissue formation for a fully remodeled TEVG (B, SVF-6), partially remodeled patent TEVG (C, SVF-5), and an occluded TEVG (D, SVF-3).
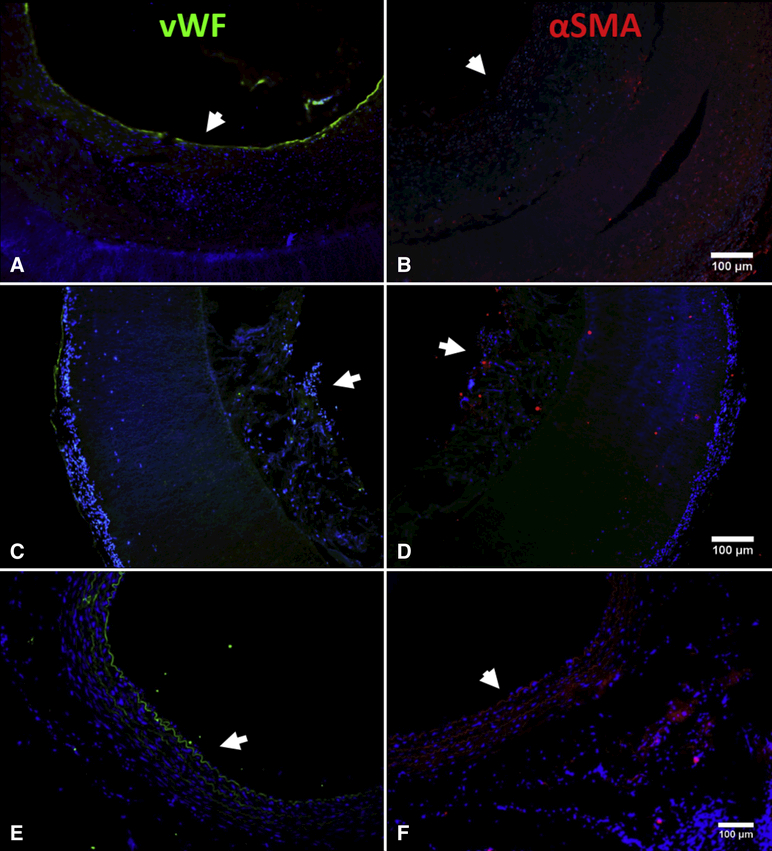
Broadly, patency corresponded with a layer of neotissue that formed on top of the TIPS layer, surrounding an open lumen. Further IFC analysis of the patent grafts revealed extensive vWF staining along the luminal edge in a continuous manner indicating the presence of an endothelial lining and seemingly organized αSMA staining within the neotissue in 3 of the fully remodeled patent grafts (Figure 4A and B). The other 2 partially remodeled grafts lacked an organized luminal lining or vascular-like tissue formation (Figure 4C and D), compared to a native rat aorta (Figure 4E and F). Further histological analysis of the fully remodeled TEVGs using Verhoeff van Gieson (VVG) staining also revealed elastin fibers within the neotissue especially towards the luminal edge and Masson’s Trichrome staining revealed the presence of newly formed collagen (Figure 5).
Figure 4:
A and B) Staining for vWF (EC marker) (left) and for αSMA (SMC marker) (right) for a fully remodeled TEVG (SVF-2) indicating a complete endothelial lining and the presence of SMCs. C and D) Staining for vWF (left) and for αSMA (right) for a patent but not fully remodeled TEVG (SVF-5) indicating presence of some endothelial like cells and some cells expressing αSMA but not a full endothelial lining nor vascular like structures within the neotissue that was found in the fully remodeled TEVGs. E and F) Staining for vWF (left) and for αSMA (right) for a native rat aorta for comparison to native tissue. Arrows indicate the lumen. Counter stained with DAPI (blue – cell nuclei).
Figure 5:
Histologic staining of fully a remodeled TEVG for VVG (Top) indicating elastin (black) and Masson’s Trichrome (Bottom) indicating collagen (blue) deposition within the TEVGs (left) compared to native rat aorta (right).
Discussion:
This study showed that adherent cells in the SVF shifted their surface markers away from endothelial and endothelial progenitor markers (CD31 and CD34) and towards expression of the ADMSC marker CD90 by passage 4. Furthermore, the adherent cells in the SVF had the same phenotypic distribution with or without the previously described 48 hour dynamic culture period 6. Most importantly, removal of the 48 hour dynamic culture period (or any culture other than a brief period to allow cells to adhere to the scaffolds), and the implementation of the collection, isolation, seeding, and implantation steps within a 24 hour, ‘same-day’ procedure, generated a functioning TEVG. With streamlining, the entire procedure was able to be accomplished within a 12 hour period by the end of the study.
The focus of work in our lab, and the motivation for this study, is the generation of a TEVG within a clinically relevant time scale. Previous work by Krawiec at al. made use of cultured ADMSCs that took up to a month or more from collection to implantation 16, 25. This waiting time between collection and implantation was able to be drastically reduced when using a non-cultured cell source, but still included dynamic culture, thus limiting its clinical feasibility. When comparing the ‘same-day’ procedure to that of SVF seeded constructs given 48 hours of dynamic culture, patency rates were similar 6. Notably, none of the ‘same-day’ TEVGs failed due to thrombosis. A recent review suggests that TEVG failure due to thrombosis is caused by the graft having poor blood interaction, while in contrast failure due to hyperplasia can often be attributed to conduit mismatch, surgical error, or damage to the endothelium that creates hyperplastic conditions 2. Previously, bare PEUU scaffolds have been used with patency rates of less than 40% 19, while other work in our lab has shown patent TEVGs can be generated using bone marrow-derived MSCs 17, muscle-derived MSCs 27 and pericytes 28 with varying patency rates, while patency rates of 100% have been achieved with ADMSCs from young, non-diabetic donors 16. Thus it is important to note that even though only 5 of the 7 TEVGs were patent at 8 weeks, a microsurgeon highly experienced with the model would likely experience higher patency rates.
Several other groups have moved a TEVG towards clinical implementation. Shinoka and Breuer have tested their technology as low pressure conduits in large animal models and human trials 32,33,34, while L’Heureux and colleagues at Cytograft developed a TEVG constructed using a cell sheet technology as an arteriovenous (AV) shunt for hemodialysis under the product name Lifeline® 35. Of note, the Lifeline technology used a patient’s own fibroblasts and endothelial cells in two rounds of culture that took up to 7 months to complete, though newer methods have moved into human trials with devitalized scaffolds lacking an endothelium 21. Niklason and colleagues at Humacyte have established the “human acellular vessel” line of TEVG products 36. This TEVG is fabricated using bioreactor culture and can take a month to fabricate 33. Although these TEVGs have already made it to clinical trials, the time needed for bioreactor incubation, with potential cellular transformation or contamination, and an indeterminate shelf life of both the Cytograft® and Humacyte® TEVGs may raise some concern and possibly limit their clinical utility 21, 36. Thus the impetus for a scaffold that can be safely stored long term, then seeded and implanted as a TEVG within a single operating theater remains.
Future work will have to go beyond a preliminary study performed in an immunotolerant rat model implanted with xenogeneic human cells, and move toward a more clinically relevant model. This should include a large animal model in order to accommodate a longer and larger caliber TEVG fabricated with autologous or allogeneic cells. Such large animal models would be able to answer critical questions, such as the robustness of host cell infiltration into a longer graft. Additionally, this study, like nearly all other studies done previously using TEVGs in an animal model 16, 37, made use of only young animals. Going forward, a more clinically relevant recipient population must be employed including older animals and non-immunotolerant hosts. It might also be valuable to examine more closely the mechanisms of remodeling and prevention of acute thrombosis of the implant. Another, limitation of our study was the amount of time and effort required to manually process enough SVF cells for seeding the TEVG. However, devices and methods to more rapidly produce SVF cells in large volumes have been advancing rapidly. Devices such as the Tissuegenesis Icellator®, GID SVF-1™, Puregraft™, and Stem.pras® 38 are already being used clinically and provide a feasible method to accelerate TEVG fabrication with SVF. Lastly, a potential limitation of implanting a construct seeded with SVF is that these cells represent a phenotypically diverse population that could differentiate in an uncontrolled manner. However, the cells are not expected to remain in the construct for long 39 and any manipulation of the SVF (e.g., purification to specific cell types) would defeat the purpose of the “same-day” strategy by restoring regulatory concerns. Moreover, the SVF cell population has recently been shown to have possibly better pro-remodeling effects than cultured ADMSCs 24, 40, and thus is another important facet of this model that is of current interest for further study.
This was a small scale feasibility study demonstrating that a culture-free single-day process could be successful and comparable to our standard ADMSC seeded TEVG model, with phenotypic comparisons between the two cell populations. The motivation behind the study – to create a ‘same-day’ TEVG – moves towards a clinically relevant technology in which an off the shelf scaffold could be seeded and implanted within a practical timeframe. Future directions must also answer what the long term outcomes (beyond 8 weeks) are for such technology and if there is any phenotypic difference between donor populations that might contribute or detract from overall TEVG remodeling and maturation. Even so, the ability to generate and implant a TEVG within a single day that remodels and generates a vessellike conduit marks significant progress. Thus, the overall goal of our work towards creating a functional TEVG by removing any culture period and streamlining the process of cell population collection, fabrication and seeding, and implantation to within a “same-day” period was met. With this and other techniques in hand, novel grafts and methods could someday make TEVGs a clinical reality.
Conclusions:
Patency of our SVF seeded TEVGs after 8 weeks in vivo was achieved using a “same day” process. The seeded SVF cell population had no phenotypic differences between those with or without a 48 hour dynamic culture period, but was significantly different from ADMSCs at passage 4. TEVGs were able to remodel in vivo with gross inspection and histology indicating neotissue formation and immunostaining for vWF and αSMA demonstrating the presence of an endothelium and smooth muscle cells respectively.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Institutes of Health [T32 HL098036 to DGH, R21 EB016138, R21 HL130784, and R01 HL130077 to DAV]; and the RiMED Foundation [0057091 to AD].
Glossary of Abbreviations:
- TEVG
- ADMSC
- SVF
- IFC
- PEUU
- vWF
- αSMA
Footnotes
Conflict of interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mathers CD and Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pashneh-Tala S, MacNeil S and Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng Part B Rev. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoghbi WA, Duncan T, Antman E, Barbosa M, Champagne B, Chen D, et al. Sustainable Development Goals and the future of cardiovascular health. A statement from the Global Cardiovascular Disease Taskforce. Eur Heart J. 2014;35:3238–9. [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitrievska S and Niklason LE. Historical Perspective and Future Direction of Blood Vessel Developments. Cold Spring Harb Perspect Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krawiec JT, Liao HT, Kwan LL, D’Amore A, Weinbaum JS, Rubin JP, et al. Evaluation of the stromal vascular fraction of adipose tissue as the basis for a stem cell-based tissue-engineered vascular graft. J Vasc Surg. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander JH and Smith PK. Coronary-Artery Bypass Grafting. N Engl J Med. 2016;375:e22. [DOI] [PubMed] [Google Scholar]
- 8.Athanasiou T, Saso S, Rao C, Vecht J, Grapsa J, Dunning J, et al. Radial artery versus saphenous vein conduits for coronary artery bypass surgery: forty years of competition--which conduit offers better patency? A systematic review and meta-analysis. Eur J Cardiothorac Surg. 2011;40:208–20. [DOI] [PubMed] [Google Scholar]
- 9.Cho KR, Kim JS, Choi JS and Kim KB. Serial angiographic follow-up of grafts one year and five years after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:511–6. [DOI] [PubMed] [Google Scholar]
- 10.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. [DOI] [PubMed] [Google Scholar]
- 11.Masden DL, Seruya M and Higgins JP. A systematic review of the outcomes of distal upper extremity bypass surgery with arterial and venous conduits. J Hand Surg Am. 2012;37:2362–7. [DOI] [PubMed] [Google Scholar]
- 12.Herring MB, Dilley R, Jersild RA Jr., Boxer L, Gardner A and Glover J Seeding arterial prostheses with vascular endothelium. The nature of the lining. Ann Surg. 1979;190:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham LM, Burkel WE, Ford JW, Vinter DW, Kahn RH and Stanley JC. Immediate seeding of enzymatically derived endothelium in Dacron vascular grafts. Early experimental studies with autologous canine cells. Arch Surg. 1980;115:1289–94. [DOI] [PubMed] [Google Scholar]
- 14.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–62; discussion 362. [DOI] [PubMed] [Google Scholar]
- 15.Gui L, Boyle MJ, Kamin YM, Huang AH, Starcher BC, Miller CA, et al. Construction of tissueengineered small-diameter vascular grafts in fibrin scaffolds in 30 days. Tissue Eng Part A. 2014;20:1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krawiec JT, Weinbaum JS, Liao HT, Ramaswamy AK, Pezzone DJ, Josowitz AD et al. In Vivo Functional Evaluation of Tissue-Engineered Vascular Grafts Fabricated Using Human Adipose-Derived Stem Cells from High Cardiovascular Risk Populations. Tissue Eng Part A. 2016;22:765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, et al. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A. 2010;16:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prichard HL, Manson RJ, DiBernardo L, Niklason LE, Lawson JH and Dahl SL. An early study on the mechanisms that allow tissue-engineered vascular grafts to resist intimal hyperplasia. J Cardiovasc Transl Res. 2011;4:674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR, et al. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res A. 2011;96:436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syedain ZH, Meier LA, Lahti MT, Johnson SL and Tranquillo RT. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng Part A. 2014;20:1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L and L’Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. 2014;60:1353–7. [DOI] [PubMed] [Google Scholar]
- 22.McAllister TN, Audley D and L’Heureux N. Autologous cell therapies: challenges in US FDA regulation. Regen Med. 2012;7:94–7. [DOI] [PubMed] [Google Scholar]
- 23.Bliley JM, Satish L, McLaughlin MM, Kling RE, Day JR, Grahovac TL, et al. Imaging the Stromal Vascular Fraction during Soft-Tissue Reconstruction. Plast Reconstr Surg. 2015;136:1205–15. [DOI] [PubMed] [Google Scholar]
- 24.Kokai LE, Traktuev DO, Zhang L, Merfeld-Clauss S, DiBernardo G, Lu H, et al. Adipose Stem Cell Function Maintained with Age: An Intra-Subject Study of Long-Term Cryopreserved Cells. Aesthet Surg J. 2017;37:454–463. [DOI] [PubMed] [Google Scholar]
- 25.Krawiec JT, Weinbaum JS, St. Croix CM, Phillippi JA, Watkins SC, Rubin JP et al. A CautionaryTale for Autologous Vascular Tissue Engineering: Impact of Human Demographics on the Ability of Adipose-Derived Mesenchymal Stem Cells to Recruit and Differentiate into Smooth Muscle Cells. Tissue Engineering Part A. 2014;21:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Nieponice A, Hong Y, Wagner W and Vorp D. Rapid Engineered Small Diameter Vascular Grafts from Smooth Muscle Cells. Cardiovasc Eng Tech. 2011;2:149–159. [Google Scholar]
- 28.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, v. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomaterialia. 2010;6:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soletti L Development of a stem cell-based tissue engineered vascular graft 2008;Bioengineering PhD Thesis:404. [Google Scholar]
- 31.Soletti L, Nieponice A, Guan J, Stankus JJ, Wagner WR and Vorp DA. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863–70. [DOI] [PubMed] [Google Scholar]
- 32.Fukunishi T, Best CA, Sugiura T, Opfermann J, Ong CS, Shinoka T, et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg. 2017;153:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinoka T What is the best material for extracardiac Fontan operation? J Thorac Cardiovasc Surg. 2017;153:1551–1552. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura T, Matsumura G, Miyamoto S, Miyachi H, Breuer CK and Shinoka T. Tissue-Engineered Vascular Grafts in Children with Congenital Heart Disease: Intermediate Term Followup. Semin Thorac Cardiovasc Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–6. [DOI] [PubMed] [Google Scholar]
- 36.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissueengineered vascular grafts. Science Translational Medicine. 2011;3:68–69. [DOI] [PubMed] [Google Scholar]
- 37.Hashi CK, Derugin N, Janairo RRR, Lee R, Schultz D, Lotz J, et al. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. Faseb J. 2011;25:4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu J, Genheimer CW, Guthrie KI, Sangha N, Quinlan SF, Bruce AT, et al. Expansion of the human adipose-derived stromal vascular cell fraction yields a population of smooth muscle-like cells with markedly distinct phenotypic and functional properties relative to mesenchymal stem cells. Tissue Eng Part C Methods. 2011;17:843–60. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez J, Pratta AS, Abbassi N, Fabre H, Rodriguez F, Debard C, Adobati J, et al. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017;2017:9289213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.