Abstract
Primary biliary cirrhosis (PBC) is characterized by loss of tolerance against ubiquitously expressed mitochondrial autoantigens followed by biliary and salivary gland epithelial cell (BEC and SGEC) destruction by autoreactive T cells. It is unclear why BECs and SGECs are targeted. Previous work demonstrated that the reduced form of the major PBC autoantigen predominated in apoptotic BECs and SGECs as opposed to an oxidized form in other apoptotic cells. This led to the hypothesis that presentation of novel self-peptides from phagocytosed apoptotic BECs might contribute to BEC targeting by autoreactive T cells. The effect of autoantigen redox status on self-peptide formation was examined along with the phagocytic ability of BECs. Oxidation of PBC autoantigens first was shown to be due to protein S-glutathionylation of lipoyllysine residues. Absence of protein S-glutathionylation generated novel self-peptides and affected T cell recognition of a lipoyllysine containing peptide. Liver biopsy staining revealed BEC phagocytosis of apoptotic BECs (3.74 ± 2.90% of BEC) was present in PBC (7 of 7 cases) but not in normal livers (0 of 3). BECs have the ability to present novel mitochondrial self-peptides derived from phagocytosed apoptotic BECs. Apoptotic cell phagocytosis by non-professional phagocytes may influence the tissue specificity of autoimmune diseases.
Keywords: pyruvate dehydrogenase, autoantigen, apoptosis, protein S-glutathionylation, primary biliary cirrhosis
Introduction
The hallmarks of primary biliary cirrhosis (PBC) are progressive bile duct and salivary gland epithelial cell damage, elevated alkaline phosphatase levels and loss of tolerance against ubiquitously expressed mitochondrial autoantigens [1]. This loss of self-tolerance to mitochondrial autoantigens precedes biliary and salivary gland epithelial cell damage (BEC and SGEC), often by many years [2-4]. Autoantibodies against the major PBC autoantigen, the E2 subunit of the mitochondrial pyruvate dehydrogenase complex (PDC-E2), are present in 95% of PBC cases and are highly specific for PBC. The autoantibodies recognize the inner lipoyl domain of PDC-E2 as well as other mitochondrial proteins that contain lipoyllysine residues. The PDC-E2 self-peptide recognized by autoreactive T cells in PBC also includes the unique lipoyllysine residue [5]. The destruction of bile duct and salivary gland epithelial cells characteristic of PBC appears to be mediated by autoreactive T cells [6-9]. Why these cell types are specifically targeted is uncertain.
Similar to other epithelial cells, BECs and SGECs potentially act as antigen presenting cells. Extra-mitochondrial staining by some anti-PDC-E2 antibodies of PBC patient BECs and SGECs suggest a molecular mimic of PDC-E2 may be present in these cell types [10, 11]. This extra-mitochondrial “PDC-E2” may be a source of unique PDC-E2 self-antigens presented by PBC patient BECs and SGECs. T cell mediated destruction of these cell types may also in part be due to increased basolateral expression of MHC class I and II molecules [12, 13], which enhance peptide presentation. BECs in PBC do not have the capacity to activate primary (or naïve) autoreactive T cells, but are merely the targets of destruction [14, 15]. Identification of potential sources of extra-mitochondrial “PDC-E2” may aid both in understanding the pathogenesis of PBC and in its treatment.
Apoptotic cells phagocytosed by BECs and SGECs are an obvious potential exogenous source of extra-mitochondrial PDC-E2 as well as other autoantigens. Other epithelial cell types are known to phagocytose neighboring apoptotic cells [16-18]. During apoptosis, many autoantigens associated with systemic autoimmune diseases cluster at the cell surface and are known to undergo either proteolytic or non-proteolytic modification, which may lead to generation of unique self-peptides [19-22]. These findings have led to a number of preliminary studies examining the effect of apoptosis on PBC autoantigens. For example, MacDonald et al have reported PDC-E2 is present on the cell surface of cultured apoptotic BECs [23]. Apoptosis specific proteases cleave purified PBC autoantigens [24], however, only oxidative modification of PDC-E2 has been detected in apoptotic cells to date [25]. Interestingly, oxidative modification appears to be cell type specific in that PDC-E2 is spared in apoptotic BECs and SGECs. Lack of oxidative modification may alter subsequent PDC-E2 self-peptide formation. Additionally, bile-induced apoptosis is unique with regard to its activation of the cathepsin B protease [26], which may also generate novel self-peptides. In the current study, BEC and SGEC apoptosis and phagocytosis are examined in order to define their role in the tissue specificity of autoreactive T cell targeting in PBC.
Materials and Methods
Sera and Antibodies
Sera were obtained from patients diagnosed with PBC. The diagnosis of PBC was confirmed by biochemical, serologic, and histological criteria in all cases. The specificity of the sera autoantibodies was confirmed by western blotting and ELISA as previously described [27]. Informed consent in writing was obtained from each participant. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board.
Rabbit polyclonal antibody against lipoic acid (LA) was obtained from Calbiochem, Inc. (San Diego, CA). Anti-PARP p85 rabbit polyclonal antibody was obtained from Promega, Co. (Madison, WI). HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA) and FITC-conjugated secondary antibodies were obtained from Molecular Probes, Inc (Eugene, OR). APC-conjugated anti-murine CD4 antibody was obtained from Becton Dickenson, Inc. (Franklin Lakes, NJ).
Animals
Female SJL/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and all procedures conducted with the approval of IACUC and under National Institutes of Health guidelines. A lipoated (K173), huPDC-E2 peptide (p163) spanning amino acids 163-176 (GDLLAEIETDKATI), corresponding to the major PBC CD4+ T cell epitope [5], was purchased from Alpha Diagnostic International (San Antonio, TX). Two groups of five mice and were immunized at 8 weeks of age with a 200 μl intraperitoneal injection of the following: 50 μg of 5 mM DTT-treated p163 in incomplete Freund's adjuvant (Sigma Inc.); or 50 μg of 10 mM GSSG-treated p163 in incomplete Freund's adjuvant. Mice were sacrificed at 24 weeks of age.
Cell Culture and Apoptosis Induction
HeLa cells, McNtcp.24 hepatoma cells, normal rat biliary epithelial cells (NRC) and human salivary gland epithelial (HSG) cell lines were passaged in defined media supplemented with heat-inactivated bovine serum as previously described [25, 26]. Cells were cultured at 37 °C in a humidified 5% CO2 incubator. Freshly isolated rat BECs were prepared as described [28]. Addition of a hydrophobic bile acid, glycochenodeoxycholate (GCDC) (Sigma), to McNtcp.24 hepatoma cells was used to induce cathepsin B dependent apoptosis as described previously [26]. Cells incubated with the hydrophilic bile acid, ursodeoxycholate (UDCA) (Sigma), were used as a negative control. Lysates of GCDC and UDCA treated cells were subjected to SDS-PAGE and then immunoblotted with anti-PARP p85 antibody to confirm apoptosis induction and with PBC patient sera to detect any PDC-E2 cleavage fragments. In other experiments, cells were incubated overnight in serum-free DMEM followed by complete media containing cycloheximide (CHX) (1 ug/mL) (Sigma, St. Louis, MO) and tumor necrosis factor (TNF-α) (10 ng/mL) (Sigma) to induce apoptosis [29].
Western Blotting of Cell Lysates and Purified Autoantigens
HeLa cell lysates were routinely prepared as previously described [30] with addition of a sulfhydryl reducing agent, DTT (5 mM) (Sigma), or oxidizing agent, glutathione disulfide (GSSG) (10 mM) (Sigma). Purified, recombinant PDC-E2 (500 ng) or complexes of PDC-E2/PDC-E3BP (750 ng) purified from human liver (gifts of M.S. Patel) were incubated for 60 min at 37 °C with cathepsin B (300 nM) ± CA074 (10 μM) (a cathepsin B inhibitor) (Sigma), subjected to SDS-PAGE, and immunoblotted with PBC patient sera. After SDS-PAGE, proteins were then transferred to nitrocellulose and transiently stained with 0.1% ponceau S (Sigma) in 5% acetic acid to confirm equivalent transfer of proteins. Immunoblotting was performed with PBC patient sera (diluted 1:2000) or polyclonal rabbit antibody (diluted according to manufacturer's recommendation), followed by an HRP-conjugated secondary antibody, and developed by ECL (Pierce, Co., Rockford, IL) as described [30].
Immunofluorescent Staining
Cells grown on #1 glass coverslips were washed twice in ice-cold PBS without calcium or magnesium, fixed in 4% paraformaldehyde (5 min at 4 °C), and permeabilized in acetone (20 s at 4 °C). Immunofluorescent staining was performed with patient sera diluted 1:200 (20 min at 4 °C) followed by FITC-conjugated goat anti-human polyclonal antibody (1:100) [25]. Cells were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI) (Molecular Probes, Inc.) to detect chromatin condensation and nuclear fragmentation and propidium iodide (PI) (Molecular Probes, Inc.) to detect cell membrane blebbing characteristic of apoptotic cells (12). All apoptotic cells in 5 high power fields (typically 50-100 apoptotic cells in total) were scored for the presence or absence of staining of the E2 subunit of the branched chain 2-oxoacid dehydrogenase complex (BCOADC-E2). Imaging was performed on a Leica TCS-SP (UV) scanning confocal microscope system or a Zeiss Axiophot 2 fluorescent microscope system.
A modification of previously described methods [31-33] was used to identify phagocytosed apoptotic cells in 4 μm thick, formalin-fixed liver sections. Apoptotic cells were identified by their high intensity staining by DAPI, as opposed to weaker staining of normal and necrotic cells, due to their condensed chromatin. BECs were identified by counterstaining with eosin. Phagocytosis of an apoptotic body was defined by the presence of a circumferential, luminescent “halo” surrounding the apoptotic cell. The “halo” is caused by the apposition of the cell membrane of the apoptotic body and the lipid bilayer of a phagocytic vacuole as confirmed previously by electron microscopy. Staining of normal control specimens (3) were compared to pre-cirrhotic PBC specimens (7) and specimens from individuals with other liver diseases: chronic HCV (8); primary sclerosing cholangitis (PSC) (3); and mild acute cellular rejection (AR) (3). Aside from the normal controls, biopsy specimens reported to have portal inflammation and/or bile duct damage were selected for staining. For each specimen, five portal tracts, each containing at least one bile duct, were evaluated. Sections with less than 100 total BECs were excluded. Imaging was performed a Zeiss Axiophot 2 fluorescent microscope.
Detection of Protein S-glutathionylation Using BioGEE
Glutathione ethyl ester was biotinylated as previously described to form BioGEE [29, 34]. BioGEE readily enters cells and binds to oxidized protein sulfhydryl groups. The BioGEE mixture was added to the cell culture medium at a final concentration of 250 μM free-SH concurrently with induction of apoptosis using TNF-α/CHX treatment. At designated time points, cell lysates were prepared, pre-cleared with agarose beads and then incubated with Streptavidin conjugated agarose beads (100 μL/mg of protein) for 30 min at 4 °C to specifically bind protein-BioGEE complexes. After extensive washing, the beads were incubated for 30 min with 10 mM DTT in PBS/EDTA/SDS to elute proteins. Proteins in the eluent were resolved by SDS-PAGE and specific proteins were detected by western blotting.
Protease Digestion of PDC-E2
Purified, recombinant human PDC-E2 (4 μg) was incubated in 100 μl of 1 mM EDTA, 10mM sodium phosphate buffer, pH 6.5 containing either 5 mM DTT or 10 mM GSSG for 5 min at room temperature. Each sample was then dialyzed against 1 mM EDTA, 10 mM sodium phosphate buffer, pH 6.5 for 4 h at 4 °C to remove DTT or GSSG. Overnight tryptic digestion was carried out on 10 μl of each sample solution by adding 0.1 μg trypsin (Roche Applied Science, Indianapolis, IN) in 10 μl of 100mM Tris-HCl, pH 6.8 at room temperature. For mass spectrometric analysis, 5 μl of each digested sample solution was desalted using a C18 ZipTip (Millipore Corporation, Billerica, MA) and peptides were eluted with 50% acetonitrile/0.1% TFA. The eluted peptides were dried and redissolved in 3 μl of matrix solution (10 mg/mL 4-hydroxy-α-cyanocinnamic acid in 50% acetonitrile/0.1% TFA) and 0.7 μl of each sample was spotted on the target. MALDI mass spectrometric analysis was performed using a PerSeptive Voyager DE-RP mass spectrometer in the linear mode. All possible peptide fragments and their masses were calculated using the program MS-Fit (http://prospector.ucsf.edu).
Murine T cell Proliferative Responses to PDC-E2 Peptide
Peripheral blood mononuclear cells (PBMC) were isolated from blood and loaded with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (Molecular Probes, Inc.) according to the manufacturer's protocol prior to exposure to DTT- versus GSSG-treated p163 (10μg/ml) in RPMI media. After seven days in culture, non-adherent cells were collected, stained with APC-conjugated anti-CD4 antibody in PBS (30 min at 4 °C), and analyzed by flow cytometry to determine the percentage of CD4+ T cells that had undergone two or more cell divisions as previously described [35]. Proliferative responses to PDC-E2 peptides were compared to responses to negative control peptides. A mean response three fold greater than the negative control mean was considered positive.
Results
Purified PDC-E2 is Cleaved by Cathepsin B in vitro, but not During Cathepsin B-dependent Apoptosis
Unlike many other autoantigens, cleavage of PBC autoantigens has not been detected during apoptosis [25]. However, Roberts et al. have shown that apoptosis induced by toxic bile salts (e.g. GCDC) occurs via a novel pathway involving release of the lysosomal protease cathepsin B [26]. In cholestatic diseases such as PBC, this novel pathway is particularly relevant.
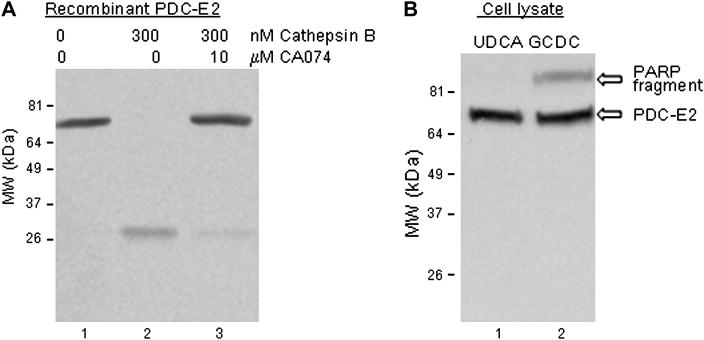
Purified PDC-E2 (500 ng) was first incubated with purified cathepsin B (300 nM) in vitro to determine if it is a potential substrate (Fig. 1A). A 26 kDa cleavage fragment of PDC-E2 (lane 2) was detected following SDS-PAGE by immunoblotting with PBC patient antisera specific for PDC-E2. A second, larger cleavage fragment was detectable by CoomassieR brilliant blue staining, not by immunoblotting. The site of cleavage by cathepsin B as determined by N-terminal sequencing of this larger cleavage fragment was just prior to A242 (i.e. distal to the inner lipoyl domain of PDC-E2), which explains why it was not detected by immunoblotting with PBC patient antisera. Cleavage was blocked by inclusion of the cathepsin B inhibitor CA 074 (10 μM) (lane 3). Of note, granzyme B cleavage of PDC-E2 was also unexpectedly inhibited CA 074, which was not known to affect granzyme B (data not shown). Though PDC-E2 is a substrate for cathepsin B, GCDC-induced apoptosis did not induce cleavage of PDC-E2 (Fig. 1B). Cleavage of PARP confirmed induction of apoptosis. It seems increasingly unlikely that cleavage by apoptosis specific proteases of PBC autoantigens yields novel self-peptides as suggested for other autoantigens.
Fig. 1.
Analysis of PDC-E2 in response to cathepsin B and bile acid induced apoptosis. (A) Cathepsin B treatment of PDC-E2 (MW 74 kDa) generated a 27 kDa cleavage product recognized by PBC patient serum (lane 2 versus lane 1). Cleavage was inhibited by the cathepsin B inhibitor CA074 (lane 3). (B) Western blot of UDCA- and GCDC-treated cells. UDCA treatment did not induce apoptosis (i.e. no cleavage of PARP). The typical caspase cleavage fragment of PARP was detectable after GCDC treatment (upper panel of lane 2, indicative of apoptosis), but no PDC-E2 cleavage product was detected (lower panel of lane 2). Experiments were done at least three times. Representative images are shown.
Cell Type-Specific Oxidation During Apoptosis is a Common Feature of PBC Autoantigens
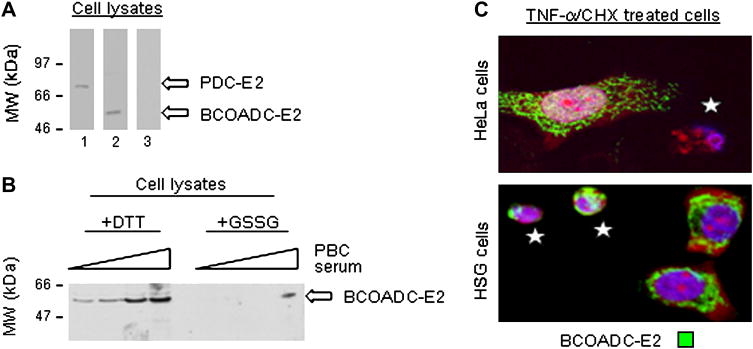
Only oxidative modification of PDC-E2 during apoptosis has been detected and it was cell type specific [25]. If this apoptosis related modification has any significance, one should expect cell type specific oxidative modification to be a feature of other PBC autoantigens during apoptosis. As before, PBC patient serum was utilized to detect oxidative changes. PBC patient sera specific for PDC-E2 preferentially recognizes reduced PDC-E2 and was instrumental in examining the effect of apoptosis on PDC-2. Though 95% of PBC patient sera contain anti-PDC-E2 autoantibodies, a PBC patient serum mono-specific for the E2 subunit of the branched chain 2-oxoacid dehydrogenase complex (BCOADC-E2) was identified by screening sera against cell lysates (Fig. 2A) and its specificity confirmed by ELISA (data not shown). Compared to addition of DTT, addition of glutathione disulfide (GSSG) to HSG cell lysates decreased autoantibody immunoblotting of BCOADC-E2 (Fig. 2B). As with PBC patient sera specific for PDC-E2 [25], the BCOADC-E2 specific serum preferentially recognized reduced versus oxidized autoantigen.
Fig. 2.
Effect of redox status and apoptosis in different cell types on PBC autoantigen BCOADC-E2. (A) Western blot of DTT-treated whole HeLa cell lysate demonstrates the mono-specificity of a PBC patient serum for PDC-E2 (lane 1) and of a PBC patient serum for BCOADC-E2 (lane 2). Normal control serum did not recognize either autoantigen (lane 3). (B) Western blot showing that treatment of HSG cell lysates with GSSG inhibits recognition of BCOADC-E2 by PBC patient sera. Experiments were performed at least three times. (C) Immunofluorescent staining by PBC patient sera mono-specific for BCOADC-E2 is absent in the apoptotic HeLa cell labeled with a white star (top panel), but is preserved in the apoptotic HSG cells (lower panel). 100× magnification. Representative images and blots are shown.
TNF-α/CHX exposure was used to induce apoptosis in an epithelial cell type commonly affected in PBC (SGEC) and a control epithelial cell type (HeLa). For convenience the SGEC line (HSG) was used routinely as opposed to a BEC line. These cell lines have been adapted to grow well in the same media and are equally sensitive to apoptotic stimuli. In prior studies, the same results have been obtained in both HSG and BEC lines [25]. To evaluate the oxidative status of BCOADC-E2 in individual apoptotic cells, cells were stained with the PBC patient sera specific for BCOADC-E2. Apoptotic cells were identified by characteristic morphologic changes including nuclear condensation and cytoplasmic shrinkage. Loss of BCOADC-E2 staining (green) occurred in 82 ± 7% of apoptotic HeLa cells (Fig. 2C, top panel, an apoptotic cell is labeled with a white star). Conversely, BCOADC-E2 staining was preserved in 81±9% of apoptotic HSG cells (p < 0.05) (Fig. 2C, bottom panel), similar to prior results for PDC-E2 staining in apoptotic cells [25]. Loss of staining by poly-specific PBC patient sera was also increased in apoptotic HeLa cells compared to apoptotic HSG and BEC cells (data not shown). Thus, selective preservation of redox sensitive autoantigen epitopes following apoptosis is a common feature of PBC autoantigens.
GSH Binding to PDC-E2 Follows Oxidation of PDC-E2 During Apoptosis
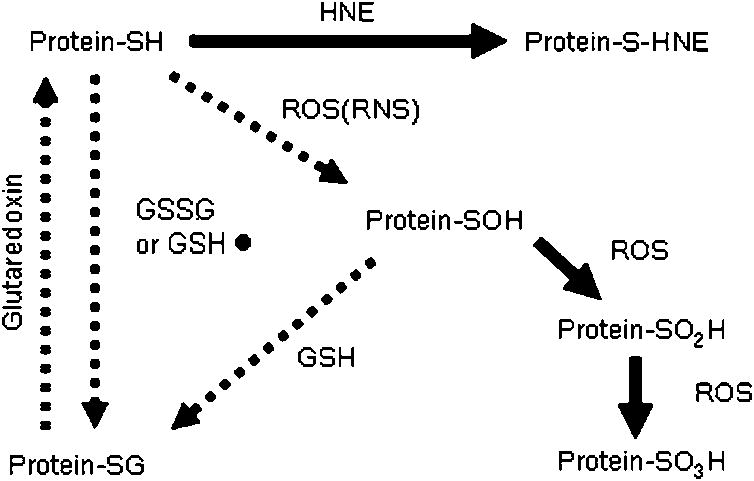
Unlike BECs and SGECs, oxidation of PBC autoantigens sulfhydryl groups occurs during apoptosis of other cell types. Several pathways of protein sulfhydryl oxidation are possible that are either reversible or irreversible (Fig. 3). Treatment of HeLa cell apoptotic lysates with DTT prior to immunoblotting completely restores recognition of PBC autoantigens [25], which suggests reversible protein S-glutathionylation. Protein S-glutathionylation is the covalent binding of glutathione to protein sulfhydryl groups either directly or indirectly [29, 34]. The latter pathway was evaluated using BioGEE, a cell-permeable, biotinylated GSH analog that binds oxidized protein sulfhydryl groups [29].
Fig. 3.
Reversible and Irreversible Protein Sulfoxidation Pathways. Two possible pathways are shown for protein S-glutathionylation: a direct oxidation pathway by GSSG or GS• and one requiring a protein sulphene (protein-SOH) intermediate. Further oxidation by ROS is irreversible. Protein S-glutathionylation can be reversed by glutaredoxins. Irreversible oxidation reactions are indicated by solid arrows. ROS, reactive oxygen species; GSH, reduced glutathione; GSSG, oxidized glutathione; GS•, glutathione radical; HNE, 4-hydroxy-2-nonenal; RNS, reactive nitrogen species.
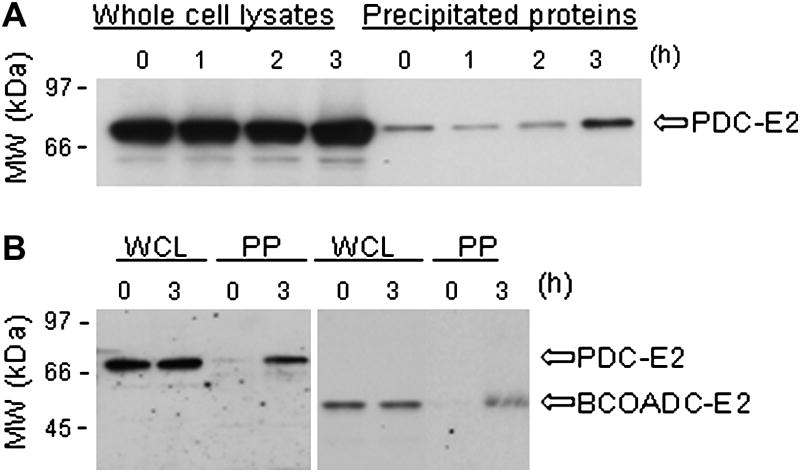
The biotin end of BioGEE allows for precipitation of proteins that have formed mixed disulfides with the glutathione portion of BioGEE. Within 3h, the amount of PDC-E2 precipitated was significantly increased following TNF-α/CHX treatment (6.2 ± 3.0 fold, p < 0.05) (Fig. 4A). Precipitation of BCOADC-E2 increased also following TNF-α/CHX treatment (Fig. 4B) as well as other PBC autoantigens (data not shown). GSH binding to PBC autoantigens increases during apoptosis of HeLa cells, apparently following autoantigen sulfhydryl group oxidation. Direct binding by GSSG is unlikely given the low concentrations of GSSG in cells.
Fig. 4.
Precipitation of protein S-glutathionylated PBC autoantigens from apoptotic cells. (A) Western blot with PBC patient sera of whole cell lysates (left lanes) and of proteins precipitated using Streptavidin-coated beads (right lanes) at various time points following treatment of BioGEE loaded HeLa cells with TNF-α/CHX to induce apoptosis. The addition of DTT to the precipitates prior to SDS-PAGE released PDC-E2 from BioGEE. Following the addition of TNF-α/CHX, the amount of BioGEE conjugated PDC-E2 increased (i.e. protein S-glutathionylation of PDC-E2 increased) (B) Western blot comparing precipitation of BioGEE coupled PDC-E2 and BCOADC-E2 from the same preparation. Treatment with TNF-α/CHX increased BioGEE conjugation with both PDC-E2 and BCOADC-E2. WC-whole cell proteins. IP-BioGEE coupled proteins. Experiments were repeated at least three times. Representative blots are shown.
Protein S-glutathionylation of PDC-E2 Alters Its Degradation by Proteases
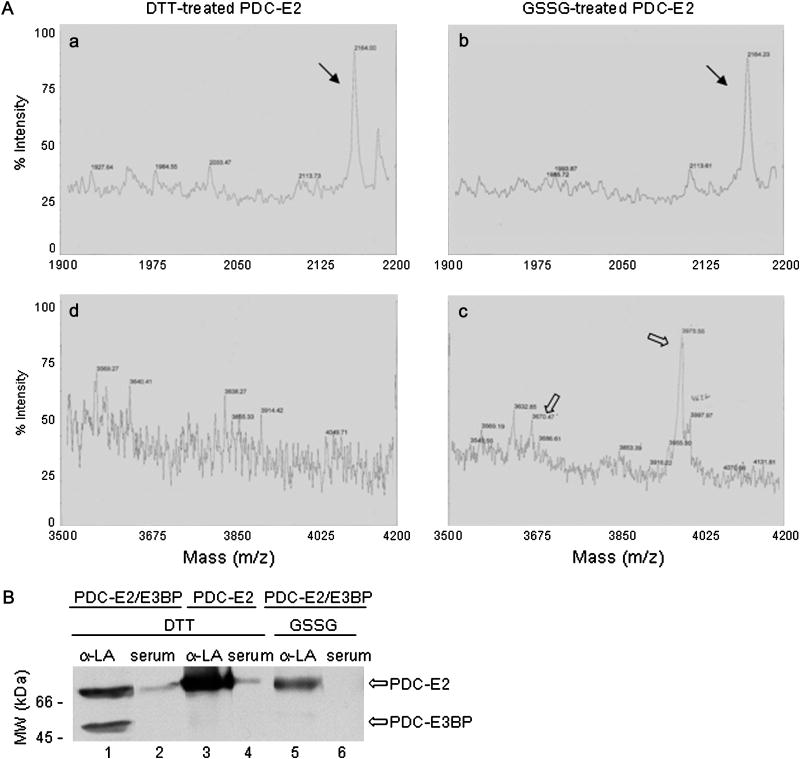
It has been proposed that protease cleavage of autoantigens during apoptosis yields unique self-peptides. The sulfhydryl group redox state of some other proteins is known to significantly alter their cleavage by proteases [36-38]. Though PBC autoantigens are not cleaved during apoptosis, we considered that protein S-glutathionylation of PBC autoantigens may yield unique self-peptides during phagocytosis of apoptotic cells. To examine this possibility, the peptide products of limited trypsin digestion of DTT- versus GSSG-treated purified PDC-E2 were compared by mass spectrometry. Many identical PDC-E2 self-peptides were formed following trypsin digestion regardless of whether PDC-E2 was treated with DTT or GSSG (Fig. 5A, solid arrows in panels a and b), but different PDC-E2 self-peptides were derived from the inner lipoyl domain, which is the site of both autoantibody binding and of PDC-E2 self-peptides recognized by autoreactive PBC patient T cells (Fig. 5A, open arrows in panels c and d). Similarly, differences in self-peptide formation were noted following treatment of DTT- and GSSG-treated PDC-E2 with chymotrypsin (data not shown). Evaluation of the masses of the self-peptides (Fig. 5A, panel d, open arrows) formed following trypsin digestion of GSSG-treated PDC-E2 suggested one or two molecules of glutathione may bind within the inner lipoyl domain, specifically between amino acids L160 and K188. Two sulfhydryl groups are present between amino acids L160 and K188 and both belong to the lipoic acid covalently bound to amino acid K173, one of two lipoyllysine residue in PDC-E2.
Fig. 5.
Tryptic digestion of reduced versus oxidized PDC-E2 and glutathionylation of its lipoyllysine residue. (A) Mass spectrometry indicates that some of the peptide products formed following trypsin digestion of DTT- and GSSG-treated PDC-E2 were identical (panels a and b, solid arrows). Other peptide products were distinct (panels c and d, open arrows). The sizes of the peptides labeled by open arrows in panel d are most consistent with single and double S-glutathionylation of a peptide including the lipoyllysine residue of the inner lipoyl domain. (B) Western blotting of PDC-E2/PDC-E3BP complex purified from human liver (which dissociates during SDS-PAGE) and of recombinant PDC-E2 with either rabbit anti-lipoic acid antibody (α-LA) or PBC patient serum mono-specific for PDC-E2. α-LA recognized both DTT treated PDC-E2 and PDC-E3BP (lane 1). α-LA recognition of PDC-E3BP was more sensitive to GSSG treatment than was its recognition of PDC-E2. In contrast, PBC patient serum recognition of PDC-E2 was completely inhibited by GSSG treatment (lane 6). No bands appeared in lane 6 on even longer exposures. Since the serum, unlike α-LA, is specific for the inner lipoyl domain of PDC-E2, GSSG-treatment likely affects only the inner lipoyl domain of PDC-E2. PDC-E3BP has only one lipoyl domain. Experiments were done at least three times. Representative blots are shown.
Attachment of glutathione residues specifically to the lipoyllysine residue of the inner lipoyl domain would be expected to also affect binding by anti-lipoic acid antibody (anti-LA). Anti-LA immunoblotting of PDC-E3BP, a PBC autoantigen with only one lipoyllysine residue, was completely inhibited by GSSG treatment, but binding to PDC-E2 was inhibited only 42±6% as measured by densitometry (Fig. 5B, lane 1 versus lane 5). This suggests that only one of the two lipoyllysine residues in PDC-E2 is susceptible to protein S-glutathionylation. Immunoblotting of recombinant PDC-E2 confirmed the identity of the upper band as PDC-E2 (lanes 3 and 4). As expected, binding of serum mono-specific for PDC-E2, specifically the inner lipoyl domain was completely inhibited by GSSG treatment (Fig. 5B. lane 2 versus lane 6). Longer exposures failed to demonstrate any other bands in lane 6. These results along with the peptide digestion results indicate that protein S-glutathionylation of PDC-E2 is restricted to the lipoyllysine residue of the inner lipoyl domain and alters protease digestion of PDC-E2. Thus, the absence of lipoyllysine oxidation in PBC autoantigens may yield unique self-peptides following phagocytosis of the apoptotic BECs or SGECs.
The Redox State of the Lipoyllysine Residue of PDC-E2 Affects T cell Recognition
To determine if S-glutathionylation of the inner lipoyllysine residue of PDC-E2 affects T cell recognition, murine CD4+ T cell proliferation was examined after immunization with a reduced or GSSG-treated human PDC-E2 peptide spanning the lipoyllysine residue of the inner lipoyl domain. In female SJL/J mice immunized with the reduced peptide, 3 out of 5 had significant CD4+ T cell proliferation following incubation of isolated PBMC with the reduced peptide, but none had a response to incubation with the oxidized peptide. Immunization of mice with a single peptide injection may have limited T cell responses to the reduced peptide; however, T cell recognition was clearly affected by the redox state of the lipoyllysine containing peptide. None of the mice immunized with the GSSG-treated peptide had a positive response to the reduced peptide and only one had a positive response to the oxidized peptide. The GSSG-treated peptide may be less immunogenic than its reduced counterpart, which is consistent with reported impaired T cell responses to peptides containing a disulfide bond [36].
Bile Duct Epithelial Cells Phagocytose Apoptotic Cells in vitro and in vivo
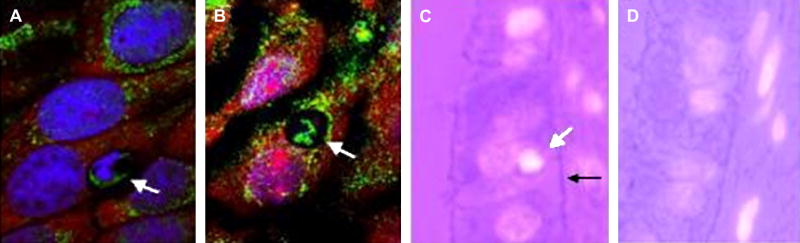
Given the above results, the fate of apoptotic BECs and SGECs may influence autoreactive T cell targeting. Some epithelial cell types are known to phagocytose apoptotic cells. Thus, rather than being cleared by professional phagocytes, some apoptotic BECs might be phagocytosed by neighboring, non-apoptotic BECs. In cultures of a BEC line, microscopic examination revealed that non-apoptotic BECs are able to phagocytose apoptotic BECs (Fig 6, panel A). The antigenic epitope of PDC-E2 persists after phagocytosis as evidenced by PBC autoantibody staining within the phagosome even after degradation of the apoptotic body's DNA (Fig 6, panel B). Similar phagocytosis of apoptotic bodies was noted in cultures of freshly isolated rat BECs (data not shown).
Fig. 6.
Biliary epithelial cell phagocytosis of apoptotic cells. (A & B) Cultured normal rat BEC phagocytose apoptotic BEC. In panel A, a recently phagocytosed apoptotic cell is marked by an arrow. RNA staining by propidium iodine (red) within the apoptotic cell in panel A is absent likely due to its rapid degradation following phagocytosis, but DNA (blue) and PDC-E2 (green) staining persist. In panel B, both RNA and DNA have been degraded in the phagocytosed apoptotic cell in panel B, but PDC-E2 staining persists. 63× magnification. (C) Biopsy specimen from a PBC patient stained with DAPI (white) and eosin. An apoptotic BEC (white arrow) has detached from an intact basement membrane (black arrow). The luminescent “halo” surrounding the apoptotic BEC indicates it has been phagocytosed by the neighboring healthy cell. 100× magnification. Representative images are shown.
Human liver biopsy serial sections were similarly examined for evidence of BEC phagocytosis of apoptotic cells. Apoptotic cells phagocytosed by healthy BECs were noted in liver sections from patients with PBC, but BEC phagocytosis of apoptotic cells was not observed in control normal liver sections (Fig. 6, panel C and D, respectively). DAPI staining (white) was used to identify the condensed chromatin characteristic of apoptotic cells, which appears much brighter than normal or necrotic cells. Phagocytosed apoptotic cells were surrounded by a luminescent “halo” due to apposition of the lipid bilayer of the phagocytic vacuole and the cell membrane of the apoptotic cell as previously described [31-33]. BEC phagocytosis of apoptotic cells was also observed in sections from individuals with other liver diseases (Table 1). It was not possible to determine conclusively whether BECs were the source of the apoptotic cells. However, in many cases the basement membrane was intact as in Fig. 6, panel C suggesting that the phagocytosed apoptotic cell was an apoptotic BEC. A statistical comparison of the frequency of BEC phagocytosis of apoptotic cells among different disease etiologies would not be meaningful since biopsies known to have portal inflammation and bile duct injury were chosen for staining. Since other antigen presenting cells are known to present self-peptides derived from phagocytosed apoptotic cell proteins, unique self-peptides derived from autoantigens within apoptotic BECs may be presented by nearby healthy BECs.
Table 1. Biliary Epithelial Cell Phagocytosis of Apoptotic Cells.
| Disease/Condition (# of biopsies) | % of cholangiocytes with apoptotic bodies (mean ± S.E.M.) |
|---|---|
| None (3) | 0.00 ± 0.00 |
| Primary biliary cirrhosis (7) | 3.74 ± 2.90 |
| Chronic hepatitis C (8) | 2.91 ± 3.53 |
| Primary sclerosing cholangitis (3) | 4.07 ± 3.50 |
| Acute rejection (3) | 0.83 ± 0.85 |
S.E.M., standard error of the mean.
Discussion
These results demonstrate for the first time that apoptotic cells are phagocytosed by BECs and consequently are an exogenous source of autoantigens in BECs. Protease digestion of the reduced form of PDC-E2, which predominates in apoptotic BECs and SGECs, unlike other cell types, yields distinctive self-peptides. These findings support the paradigm that tissue specific damage in some autoimmune diseases, such as PBC, is due to cell type specific differences in apoptosis and phagocytosis of apoptotic cells. In autoimmune disease, apoptotic cells may have significant roles other than induction of loss of self-tolerance. In PBC, presentation by BECs of distinctive self-peptides derived from phagocytosed apoptotic BECs may inadvertently promote bile duct destruction in those who previously lost tolerance against ubiquitously expressed mitochondrial autoantigens [2-4]. Animal models can be used to test this paradigm of tissue specificity in autoimmune disease.
Cell type specific preservation of epitopes recognized by PBC patient autoantibodies was demonstrated to be a common feature of PBC autoantigens in apoptotic BECs and SGECs. For practical reasons, apoptotic SGECs rather than BECs were primarily used in some experiments since these cells are more readily cultures. This may limit extrapolation of our findings to the liver. However, our prior study demonstrated similar effects of apoptosis in both cell types on PDC-E2, the major PBC autoantigen, due to the high level expression of bcl-2 in both cell types as opposed to other cell types [25]. Cell surface expression and protease cleavage of PBC autoantigens, unlike other autoantigens, were not observed during apoptosis, even bile acid induced apoptosis. We have not used flow cytometry to rule out the presence of PBC autoantigens on the cell surface of apoptotic cells as suggested by MacDonald et al [23] since immunocytochemistry has been sufficient to detect nuclear autoantigens at the cell surface [39]. Our previous study provided indirect evidence of oxidation of PDC-E2 by protein S-glutathionylation in some cell types during apoptosis. The immunoprecipitation results presented confirm oxidation of PDC-E2 (and other PBC mitochondrial autoantigens) is due to protein S-glutathionylation.
The site of protein S-glutathionylation has now been identified as the lipoyllysine residue in the inner lipoyl domain of PDC-E2, which is the site involved in immune recognition by autoreactive B and T cells. Additionally, protein S-glutathionylation significantly affected subsequent protease digestion of PDC-E2. Protein S-glutathionylation of PDC-E2 inhibited trypsin cleavage within the inner lipoyl domain. Disulfide bonds within a protein frequently alter lysosomal digestion of the protein and peptides must be free from disulfide bonding for efficient stimulation of T cells [36-38]. Taken together, these findings indicate that lack of protein S-glutathionylation of PDC-E2 in apoptotic BECs and SGECs likely leads to formation of unique PDC-E2 peptides following digestion of apoptotic BECs and SGECs in phagocytic cells.
With regard to autoreactive T cell targeting of BEC in PBC, it was therefore important to determine whether glutathione binding to lipoyllysine of the inner lipoyl domain affects T cell recognition and whether BECs phagocytose apoptotic BECs. Analysis of PBMC isolated from mice immunized with a reduced lipoyllysine containing peptide indicate that phagocyte presentation of only the reduced peptide, not its oxidized counterpart, elicits T cell recognition. Phagocytosis of either peptide apparently did not affect its redox status in antigen presenting cells. BEC phagocytosis of apoptotic cells was evident both in cultured BECs and in vivo. DAPI staining, which identifies condensed chromatin characteristic of apoptotic cells, was used to detect apoptotic cells due to its greater specificity compared to TUNEL staining, which identifies fragmented DNA found in both apoptotic and necrotic cells. TUNEL staining may overestimate the number of apoptotic cells [40]. Increased BEC phagocytosis of apoptotic BECs was noted in PBC and in other liver diseases as well. This finding re-enforces studies of PBC patients indicating that loss of self-tolerance is not an epiphenomenon related to bile duct damage, but precedes bile duct injury [2-4]. Additionally, BEC phagocytosis of apoptotic cells in other liver diseases renders it unlikely that PDC-E2 from phagocytosed apoptotic cells is solely responsible for the extra-mitochondrial staining of BEC in PBC with anti-PDC-E2 antibodies [10, 11]. The increased BEC phagocytosis of apoptotic BECs may be secondary to portal inflammation regardless of the underlying liver disease. In the absence of loss of self-tolerance, BEC presentation of unique self-peptides from apoptotic cells would not engender damage by autoreactive T cells.
BEC phagocytosis of apoptotic BEC has not been studied previously. Epithelial cell phagocytosis of apoptotic cells may stimulate TGF-beta production [16]. It is unknown whether phagocytosis of apoptotic cells by BECs normally stimulates TGF-beta production, but BEC production of TGF-beta was important in preventing alloantigen-specific T cell mediated bile duct destruction in an animal model of hepatic allograft rejection [41]. Significantly, pro-inflammatory conditions were able to overwhelm the ability of BECs to produce TGF-beta and down regulate T cell responses [42, 43]. A new animal model of PBC in mice transgenic for directed expression of a dominant-negative form of TGF-beta receptor type II using a CD4+ promoter sequence emphasizes the role of this signaling pathway in the pathogenesis of PBC [44].
The increased BEC phagocytosis of apoptotic BECs in PBC and other causes of liver disease may in part be due to impaired macrophage phagocytosis as detected in PBC [45-47]. A variety of defects in macrophage phagocytosis of apoptotic cells in individuals with other autoimmune disease have been identified [48-50]. Defining defects in PBC macrophage phagocytosis of apoptotic cells may be highly beneficial since several therapeutic agents reportedly improve macrophage phagocytosis of apoptotic cells [51-54], which in turn increases TGF-beta release and decreases pro-inflammatory cytokine production [55, 56]. Several animal models are available with defective macrophage clearance of apoptotic cells [57, 58]. Ursodeoxycholate, currently approved for PBC treatment, reportedly has mixed effects on macrophage/Kupffer cell function in vitro [59-63]. Interestingly, ursodeoxycholate treatment in PBC patients increased Kupffer cell uptake of bacterial endotoxin while accumulation in BEC decreased based on immunohistochemical analysis [64]. Similar macrophage pathways are activated for the uptake of bacterial endotoxin and apoptotic cells [65].
In systemic autoimmune disease, emphasis has been placed on the role of apoptotic cells in maintenance and loss of self-tolerance. In PBC, since loss of self-tolerance precedes cell damage, apoptotic cells likely do not induce loss of self-tolerance. Rather, our current paradigm emphasizes the role of apoptotic cells in the specificity of tissue damage in autoimmune diseases. Our findings suggest the specific tissues targeted by autoreactive T cells in autoimmune diseases may depend in part upon both cell type specific modifications during apoptosis of ubiquitously expressed autoantigens as well as the fate of these cell types following apoptosis. The unusually restricted specificity of anti-PDC-E2 autoantibodies in PBC fortuitously enabled detection of a subtle cell type specific autoantigen modification during apoptosis. In other autoimmune diseases, a similar comparison of autoantigen modification during apoptosis in affected and unaffected cell types may also reveal subtle differences. Additionally, phagocytosis of apoptotic cells by affected cell types in targeted tissues should be evaluated in autoimmune diseases to determine its role in autoreactive T cell targeting of specific tissues.
Acknowledgments
The first two authors made equivalent contributions to the manuscript and are listed in alphabetical order. We are grateful for the assistance of Dr. Mary Ann Gawinowicz for MALDI-MS analysis and the Columbia University Protein Core Facility, New York, NY. Helpful advice and assistance from Drs. Nancy Bach, Toren Finkel and Carmen Stanca was much appreciated. This study was supported by an NIH grants DK59653 (JAO) and DK 39588 (MEG) and the Artzt Family PBC Foundation (JAO). Microscopy was performed at the MSSM-Microscopy Shared Research Facility, supported, in part, with funding from NIH-NCI shared resources grant (1 R24 CA095823-01).
Abbreviations
- BEC
biliary epithelial cell
- SGEC
salivary gland epithelial cells
- ROS
reactive oxygen species
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- PDC-E2
E2 subunit of pyruvate dehydrogenase complex
- PBC
primary biliary cirrhosis
- HSG
human salivary gland epithelial cells
- DAPI
4′, 6′-diamidino-2-phenylindole
- BioGEE
biotinylated glutathione ethyl ester
- BCOADC-E2
E2 subunit of the branched chain 2-oxoacid dehydrogenase complex
- LA
lipoic acid
- GCDC
glycochenodeoxycholate
- UDCA
ursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865. doi: 10.1136/gut.2003.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishibashi H, Shimoda S, Gershwin ME. The immune response to mitochondrial autoantigens. Semin Liver Dis. 2005;25:337. doi: 10.1055/s-2005-916325. [DOI] [PubMed] [Google Scholar]
- 4.Tsuneyama K, Van De Water J, Yamazaki K, Suzuki K, Sato S, Takeda Y, Ruebner B, Yost BA, Nakanuma Y, Coppel RL, Gershwin ME. Primary biliary cirrhosis an epithelitis: evidence of abnormal salivary gland immunohistochemistry. Autoimmunity. 1997;26:23. doi: 10.3109/08916939709009547. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada K, Ozaki S, Gershwin ME, Nakanuma Y. Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology. 1997;26:1399. doi: 10.1002/hep.510260604. [DOI] [PubMed] [Google Scholar]
- 10.Van de Water J, Turchany J, Leung PS, Lake J, Munoz S, Surh CD, Coppel R, Ansari A, Nakanuma Y, Gershwin ME. Molecular mimicry in primary biliary cirrhosis. Evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J Clin Invest. 1993;91:2653. doi: 10.1172/JCI116504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung PS, Cha S, Joplin RE, Galperin C, Van de Water J, Ansari AA, Coppel RL, Schatz PJ, Cwirla S, Fabris LE, Neuberger JM, Gershwin ME. Inhibition of PDC-E2 human combinatorial autoantibodies by peptide mimotopes. J Autoimmun. 1996;9:785. doi: 10.1006/jaut.1996.0101. [DOI] [PubMed] [Google Scholar]
- 12.Nishimoto H, Yamada G, Mizuno M, Tsuji T. Immunoelectron microscopic localization of MHC class 1 and 2 antigens on bile duct epithelial cells in patients with primary biliary cirrhosis. Acta Med Okayama. 1994;48:317. doi: 10.18926/AMO/31096. [DOI] [PubMed] [Google Scholar]
- 13.Van den Oord JJ, Sciot R, Desmet VJ. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3:310. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 14.Leon MP, Kirby JA, Gibbs P, Burt AD, Bassendine MF. Immunogenicity of biliary epithelial cells: study of the expression of B7 molecules. J Hepatol. 1995;22:591. doi: 10.1016/0168-8278(95)80456-0. [DOI] [PubMed] [Google Scholar]
- 15.Leon MP, Bassendine MF, Wilson JL, Ali S, Thick M, Kirby JA. Immunogenicity of biliary epithelium: investigation of antigen presentation to CD4+ T cells. Hepatology. 1996;24:561. doi: 10.1002/hep.510240317. [DOI] [PubMed] [Google Scholar]
- 16.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12:107. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 17.Travaglione S, Falzano L, Fabbri A, Stringaro A, Fais S, Fiorentini C. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol In Vitro. 2002;16:405. doi: 10.1016/s0887-2333(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 18.Walsh GM, Sexton DW, Blaylock MG. Corticosteroids, eosinophils and bronchial epithelial cells: new insights into the resolution of inflammation in asthma. J Endocrinol. 2003;178:37. doi: 10.1677/joe.0.1780037. [DOI] [PubMed] [Google Scholar]
- 19.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 20.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1996;93:1624. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellone M. Apoptosis, cross-presentation, and the fate of the antigen specific immune response. Apoptosis. 2000;5:307. doi: 10.1023/a:1009671105696. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald P, Palmer J, Kirby JA, Jones DE. Apoptosis as a mechanism for cell surface expression of the autoantigen pyruvate dehydrogenase complex. Clin Exp Immunol. 2004;136:559. doi: 10.1111/j.1365-2249.2004.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumura S, Van De Water J, Kita H, Coppel RL, Tsuji T, Yamamoto K, Ansari AA, Gershwin ME. Contribution to antimitochondrial antibody production: cleavage of pyruvate dehydrogenase complex-E2 by apoptosis-related proteases. Hepatology. 2002;35:14. doi: 10.1053/jhep.2002.30280. [DOI] [PubMed] [Google Scholar]
- 25.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts LR, Kurosawa H, Bronk SF, Fesmier PJ, Agellon LB, Leung WY, Mao F, Gores GJ. Cathepsin B contributes to bile salt-induced apoptosis of rat hepatocytes. Gastroenterology. 1997;113:1714. doi: 10.1053/gast.1997.v113.pm9352877. [DOI] [PubMed] [Google Scholar]
- 27.Moteki S, Leung PS, Coppel RL, Dickson ER, Kaplan MM, Munoz S, Gershwin ME. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 28.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303. [PubMed] [Google Scholar]
- 29.Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 30.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh NP. A simple method for accurate estimation of apoptotic cells. Exp Cell Res. 2000;256:328. doi: 10.1006/excr.2000.4810. [DOI] [PubMed] [Google Scholar]
- 32.Gaffney EF, O'Neill AJ, Staunton MJ. In situ end-labelling, light microscopic assessment and ultrastructure of apoptosis in lung carcinoma. J Clin Pathol. 1995;48:1017. doi: 10.1136/jcp.48.11.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johkura K, Cui L, Asanuma K, Okouchi Y, Ogiwara N, Sasaki K. Cytochemical and ultrastructural characterization of growing colonies of human embryonic stem cells. J Anat. 2004;205:247. doi: 10.1111/j.0021-8782.2004.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan DM, Levine RL, Finkel T. Detection and affinity purification of oxidant-sensitive proteins using biotinylated glutathione ethyl ester. Methods Enzymol. 2002;353:101. doi: 10.1016/s0076-6879(02)53040-8. [DOI] [PubMed] [Google Scholar]
- 35.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 36.Kang HK, Mikszta JA, Deng H, Sercarz EE, Jensen PE, Kim BS. Processing and reactivity of T cell epitopes containing two cysteine residues from hen egg-white lysozyme (HEL74-90) J Immunol. 2000;164:1775. doi: 10.4049/jimmunol.164.4.1775. [DOI] [PubMed] [Google Scholar]
- 37.Li P, Haque MA, Blum JS. Role of disulfide bonds in regulating antigen processing and epitope selection. J Immunol. 2002;169:2444. doi: 10.4049/jimmunol.169.5.2444. [DOI] [PubMed] [Google Scholar]
- 38.Meadows L, Wang W, den Haan JM, Blokland E, Reinhardus C, Drijfhout JW, Shabanowitz J, Pierce R, Agulnik AI, Bishop CE, Hunt DF, Goulmy E, Engelhard VH. The HLA-A*0201-restricted H-Y antigen contains a posttranslationally modified cysteine that significantly affects T cell recognition. Immunity. 1997;6:273. doi: 10.1016/s1074-7613(00)80330-1. [DOI] [PubMed] [Google Scholar]
- 39.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Z, Liu Y, Savas L, Smith L, Bonkovsky H, Baker S, Banner B. Frequency and distribution of DNA fragmentation as a marker of cell death in chronic liver diseases. Virchows Arch. 1997;431:189. doi: 10.1007/s004280050087. [DOI] [PubMed] [Google Scholar]
- 41.Narumoto K, Saibara T, Maeda T, Onishi S, Hayashi Y, Miyazaki E, Hiroi M, Enzan H, Kobayashi N. Transforming growth factor-beta 1 derived from biliary epithelial cells may attenuate alloantigen-specific immune responses. Transpl Int. 2000;13:21. doi: 10.1007/s001470050003. [DOI] [PubMed] [Google Scholar]
- 42.Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, Handa M, Ishibashi H, Gershwin ME, Harada M. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology. 2005;41:151. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 43.Cruickshank SM, Southgate J, Selby PJ, Trejdosiewicz LK. Inhibition of T cell activation by normal human biliary epithelial cells. J Hepatol. 1999;31:1026. doi: 10.1016/s0168-8278(99)80315-8. [DOI] [PubMed] [Google Scholar]
- 44.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO, Flavell RA, Kronenberg M, Mackay IR, Gershwin ME. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 45.Ekdahl KN, Loof L, Nyberg A, Nilsson UR, Nilsson B. Defective Fc receptor-mediated clearance in patients with primary biliary cirrhosis. Gastroenterology. 1991;101:1076. doi: 10.1016/0016-5085(91)90736-5. [DOI] [PubMed] [Google Scholar]
- 46.Wallaert B, Bonniere P, Prin L, Cortot A, Tonnel AB, Voisin C. Primary biliary cirrhosis. Subclinical inflammatory alveolitis in patients with normal chest roentgenograms. Chest. 1986;90:842. doi: 10.1378/chest.90.6.842. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson Ekdahl K, Loof L, Ahrenstedt O, Nilsson UR, Nilsson B. Defective elimination of C3b/iC3b-coated autologous erythrocytes in patients with primary biliary cirrhosis, alcoholic cirrhosis, and ulcerative colitis. J Lab Clin Med. 1997;130:285. doi: 10.1016/s0022-2143(97)90023-8. [DOI] [PubMed] [Google Scholar]
- 48.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 49.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 50.Sheriff A, Gaipl US, Voll RE, Kalden JR, Herrmann M. Apoptosis and systemic lupus erythematosus. Rheum Dis Clin North Am. 2004;30:505. doi: 10.1016/j.rdc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 52.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281. [PubMed] [Google Scholar]
- 53.Galati G, Rovere P, Citterio G, Bondanza A, Scagliette U, Bucci E, Heltai S, Fascio U, Rugarli C, Manfredi AA. In vivo administration of GM-CSF promotes the clearance of apoptotic cells: effects on monocytes and polymorphonuclear leukocytes. J Leukoc Biol. 2000;67:174. doi: 10.1002/jlb.67.2.174. [DOI] [PubMed] [Google Scholar]
- 54.Giles KM, Ross K, Rossi AG, Hotchin NA, Haslett C, Dransfield I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J Immunol. 2001;167:976. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- 55.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice. J Cell Biol. 2004;167:1161. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding H, Peng R, Reed E, Li QQ. Effects of Kupffer cell inhibition on liver function and hepatocellular activity in mice. Int J Mol Med. 2003;12:549. [PubMed] [Google Scholar]
- 59.Calmus Y, Guechot J, Podevin P, Bonnefis MT, Giboudeau J, Poupon R. Differential effects of chenodeoxycholic and ursodeoxycholic acids on interleukin 1, interleukin 6 and tumor necrosis factor-alpha production by monocytes. Hepatology. 1992;16:719. doi: 10.1002/hep.1840160317. [DOI] [PubMed] [Google Scholar]
- 60.Hattori Y, Murakami Y, Hattori S, Kuroda H, Kasai K, Shimoda S. Ursodeoxycholic acid inhibits the induction of nitric oxide synthase. Eur J Pharmacol. 1996;300:147. doi: 10.1016/0014-2999(96)00012-x. [DOI] [PubMed] [Google Scholar]
- 61.Ljubuncic P, Fuhrman B, Oiknine J, Aviram M, Bomzon A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut. 1996;39:475. doi: 10.1136/gut.39.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergamini A, Dini L, Baiocchi L, Cappannoli L, Falasca L, Bolacchi F, Capozzi M, Faggioli E, Nistri A, Salanitro A, Ventura L, Rocchi G, Angelico M. Bile acids with differing hydrophilic-hydrophobic properties do not influence cytokine production by human monocytes and murine Kupffer cells. Hepatology. 1997;25:927. doi: 10.1002/hep.510250423. [DOI] [PubMed] [Google Scholar]
- 63.Funaoka M, Komatsu M, Toyoshima I, Mikami K, Ono T, Hoshino T, Kato J, Kuramitsu T, Ishii T, Masamune O. Tauroursodeoxycholic acid enhances phagocytosis of the cultured rat Kupffer cell. J Gastroenterol Hepatol. 1999;14:652. doi: 10.1046/j.1440-1746.1999.01931.x. [DOI] [PubMed] [Google Scholar]
- 64.Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1998;29:409. doi: 10.1016/s0168-8278(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 65.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]