Supplemental Digital Content is available in the text.
Key Words: tumor-infiltrating lymphocytes, adoptive immunotherapy, malignant melanoma, advanced therapy medicinal product, translational medical research
Abstract
Tumor-infiltrating lymphocytes (TIL)-therapy in advanced melanoma is an advanced therapy medicinal product (ATMP) which, despite promising results, has not been implemented widely. In a European setting, TIL-therapy has been in use since 2011 and is currently being evaluated in a randomized controlled trial. As clinical implementation of ATMPs is challenging, this study aims to evaluate early application of TIL-therapy, through the application of a constructive technology assessment (CTA). First the literature on ATMP barriers and facilitators in clinical translation was summarized. Subsequently, application of TIL-therapy was evaluated through semistructured interviews with 26 stakeholders according to 6 CTA domains: clinical, economic, patient-related, organizational, technical, and future. In addition, treatment costs were estimated. A number of barriers to clinical translation were identified in the literature, including: inadequate financial support, lack of regulatory knowledge, risks in using live tissues, and the complex path to market approval. Innovative reimbursement procedures could particularly facilitate translation. The CTA survey of TIL-therapy acknowledged these barriers, and revealed the following facilitators: the expected effectiveness resulting in institutional support for an internal pilot, the results of which led to the inclusion of TIL-therapy in a national coverage with evidence development program, the availability of an in-house pharmacist, quality assurance expertise and a TIL-skilled technician. Institutional and national implementation of TIL-therapy remains complex. The promising clinical effectiveness is expected to facilitate the adoption of TIL-therapy, especially when validated through a randomized controlled trial. Innovative and conditional reimbursement procedures, together with the organization of knowledge transfer, could support and improve clinical translation of TIL and ATMPs.
BACKGROUND
Advanced therapy medicinal products (ATMPs) are currently one of the most promising, personalized strategies for cancer treatment.1 These products are “medicines for human use that are based on genes, tissues or cells.”2 CAR-T cell treatment for leukemia is an example of such a product. Despite their promising nature, it remains a challenge to implement ATMPs into clinical practice.
In 2007, the European Medicines Agency (EMA) established a regulation concerning the path to market approval (MA) of ATMPs, namely No. 1394/2007.3 It mandates that ATMP production requires compliance with the Good Manufacturing Practices (GMP) guideline (2003/94/EC).4 This translates into a requirement for a solid quality system, suitable investments, and effective logistical preparation. Partly due to these regulations and necessary preparations, the clinical adoption of ATMPs has been limited.5–8 This may be explained by the few numbers of ATMPs (9 of nearly 300 submitted ATMPs) that have achieved MA thus far in Europe.9 In light of this, ongoing research has sought to identify potential solutions for translation of ATMPs into the clinic. Examples of this include gatekeeping flexibilities, for example, conditional coverage, simplification of ATMP regulations, and simplification of product development.10–13 Beyond this, the lack of evidence surrounding clinical benefit is likely an important factor hampering the wide clinical adoption of ATMPs.
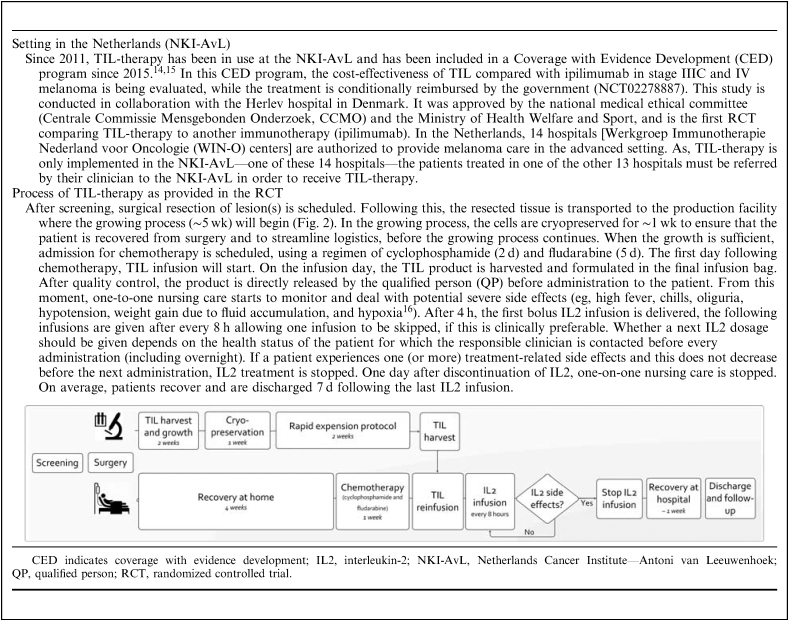
At the Netherlands Cancer Institute (NKI), TIL-therapy has been offered as an experimental treatment in patients with advanced melanoma since 2011 and is currently being evaluated in an international phase III randomized controlled trial (RCT) (Box 1). In this treatment, TILs residing within tumor material are isolated and expanded ex vivo to ∼1 billion cells, and are then infused into the patient. Results from phase II studies reveal 1- and 3-year survival rates following TIL-therapy of 55%–72% and 32%–55%, respectively,17,18 and complete responses in 10%–25% of highly advanced melanoma patients who were not responsive to previous treatments.19 Therefore, the treatment with TILs, which exists already for over 2 decades,20 appears to be a promising and complementary treatment option for advanced melanoma. Current standard treatment options in advanced melanoma, involve the utilization of antibodies which block checkpoint molecules such as CTLA-4 and PD-1. Despite, the promising results, TIL has not yet been adopted widely,21 which can be explained by a lack of robust clinical evidence.
Box 1.
Describes the setting in which TIL-therapy was applied in the Netherlands Cancer Institute—Antoni van Leeuwenhoek hospital, and the clinical process as followed in the randomized controlled trial
Health technology assessment (HTA) can play an important role in supporting new technologies from “bench to bedside.” HTA systematically evaluates social, clinical, economic, and ethical and legal aspects of new interventions to inform reimbursement and coverage decisions.22 Generally, these methods are introduced in mature technologies that have proved their efficacy and safety. However, when introduced alongside the basic, translational, and clinical research process it can steer technical development and even guide implementation by identifying barriers and facilitators while interacting with them. This process is described as “early HTA.”23–26 One of the early HTA methods is constructive technology assessment (CTA), which has its origin in the industry to inform technological development before and during the introduction of the technology.27,28
This study aims to evaluate early application of TIL-therapy in the Netherlands by means of a CTA including the 6 CTA domains—clinical, economic, patient-related, organizational, technical, and future in order to identify potential barriers and facilitators. Second, this study aims to review recent literature on ATMP implementation to compare findings from the TIL-therapy case to previously identified barriers and facilitators.
METHODS
The methodology in this analysis is 2-fold: a literature overview on ATMP barriers and facilitators and a CTA on the early application of TIL-therapy in the Netherlands.
Literature Review of ATMP Barriers and Facilitators
Literature published between 2012 and 2017 was screened for barriers and facilitators in implementing ATMPs into the clinic, using the search terms: “advanced therapy medicinal products” and “implementation or regulation or translation.” In addition, “snowballing” was used to identify other relevant articles that had been missed using this search strategy. In this analysis, snowballing entailed: (i) screening of the reference lists of the included articles, (ii) using suggestions from journal websites after reading an included article, and (iii) screening reference lists in governmental documents (gray literature) related to ATMP implementation. We included barriers and facilitators on the translational pathway from a basic research concept which demonstrated promise for clinical use until MA; hence we left out fundamental barriers commonly related to basic research such as unsuitable mouse models in preclinical testing. Supplement 1, Supplemental Digital Content 1 (http://links.lww.com/JIT/A514) describes the search strategy and reasons for in and exclusion.
CTA Framework
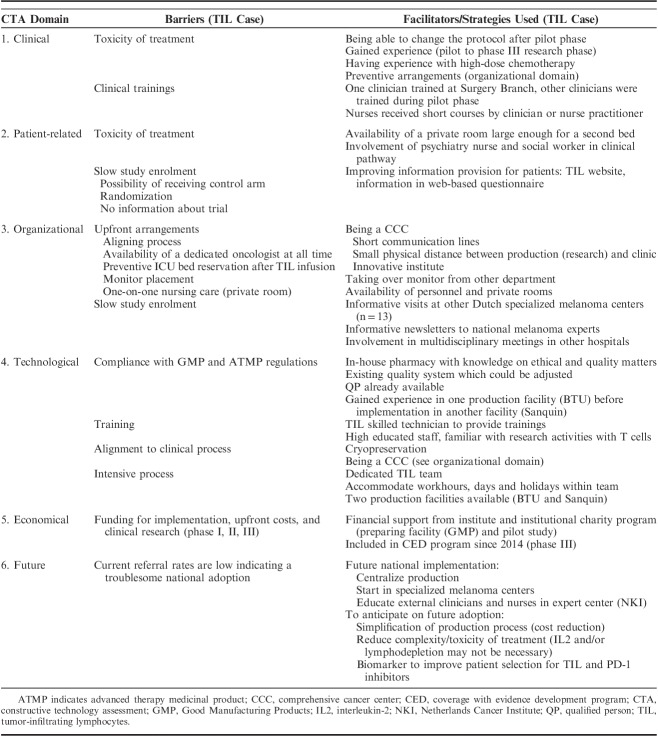
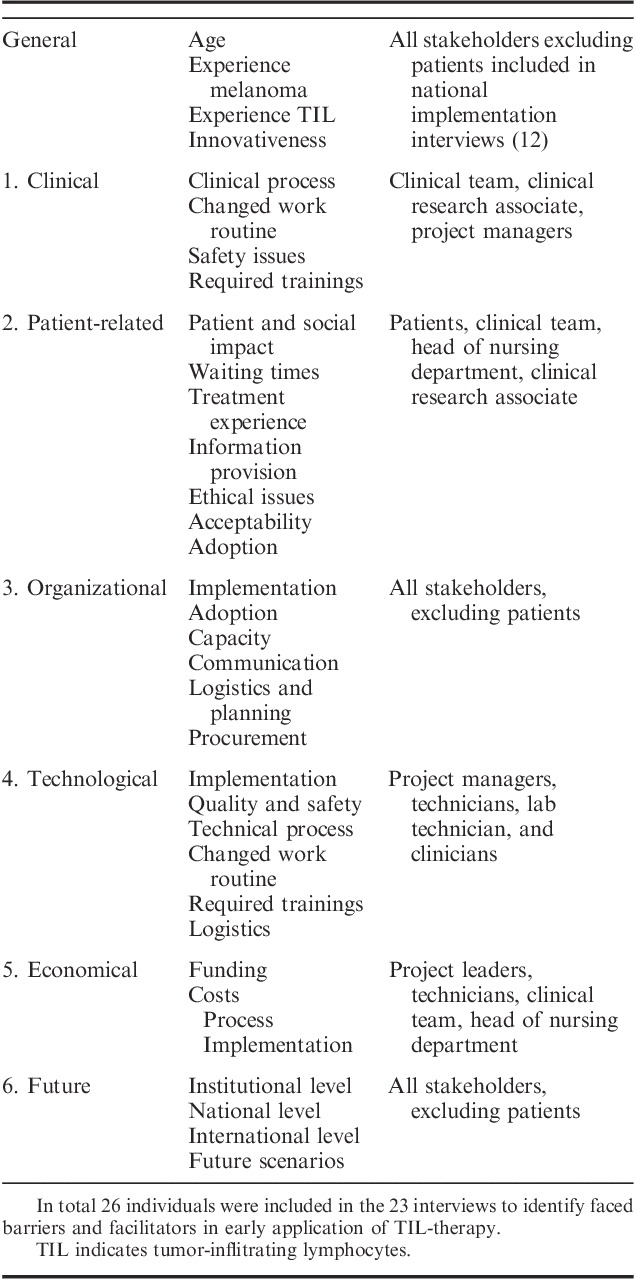
The exact methodology used within CTA depends on the nature of the technology in question, but consists mainly of accepted methods from social sciences and health services research. In this analysis, a TIL-therapy specific CTA framework was designed comprising the 4 domains proposed by Douma et al28: (1) clinical, (2) economic, (3) patient-related, and (4) organizational. Two parameters relevant for the technology were added: (5) technological, referring to the production of TILs, and (6) the future perspective, since the treatment is implemented early in a treatment setting which evolves quickly. Evaluating all these domains enabled identification of a comprehensive range of barriers (Table 1).
TABLE 1.
CTA Framework Used in the Semistructured Interviews (23) Describing Which Domain and Parameters Were Discussed With Which Stakeholders
Semistructured Interviews
Each CTA domain was evaluated by using semistructured face to face interviews with all stakeholders of the TIL-therapy process during the pilot phase (2012) and 1.5 years after the start of the phase III trial (2016). Stakeholders from the hospital included: 2 clinicians, a nurse-practitioner, 4 nurses, 6 patients, the head of the nursing department and 1 clinical research associate. From the specialized production sites [BioTherapeutics Unit (BTU) and Sanquin] the following stakeholders were included: a head of production facility, 2 project managers, 7 technicians, and a labworker involved in leukapheresis. For each interview, a tailored interview protocol was constructed to reflect the areas of interest specific to the role of the stakeholder. The domains and related parameters discussed with each stakeholder are listed in Table 1. In the national implementation phase, interviewees were also asked about: age, experience with TILs (years), and respondents’ judgment on their level of innovativeness. Innovativeness was characterized according to the theory of Rogers.29
All interviews were recorded, fully transcribed and labelled using NVivo30 according to the CTA framework. This labeling step was verified by a second researcher (V.P.R.), independently labelling the transcribed interviews of key stakeholders. In cases of discrepancy, labels were discussed (V.P.R., M.A.L.). Labelled information was summarized and first discussed with the second researcher (V.P.R.) and afterwards discussed with the stakeholders to check whether the most relevant elements were extracted from the interviews.
Clinical Domain
Interviews in this domain focused on (i) the clinical process, (ii) training required, and (iii) safety priorities. When comparing the interviews during the pilot phase to those in the national implementation phase (during RCT), changes in work routines were identified.
Patient-related Domain
There were 3 measures used in this domain. First, patient interviews were held during the recovery phase of TIL-therapy focusing on waiting times experienced, information provision, treatment experience in each treatment phase, and reasoning behind participation in the TIL trial. Second, the clinical team was asked to explain their experiences related to social and patient impact and their role in providing supportive activities. Third, a web-based questionnaire was developed to analyze factors influencing TIL trial participation. This was based on (i) aspects described in the accept/decline questionnaire of Penman and colleagues and the adapted version by Jenkins and colleagues, (ii) the Attitudes on Randomized Trials Questionnaire,31 and (iii) factors described by Kaur and colleagues.32–34 The questionnaire (Supplement 2, Supplemental Digital Content 1, http://links.lww.com/JIT/A514) was distributed via the Dutch patient association, “stichting Melanoom,” and aimed at advanced melanoma patients. In addition, during the ongoing RCT, quality of life is measured by means of the QLQ-C15, EQ5D-3L and the Impact of Event Scale, the results are, however, not included in this research article as the clinical trial is still ongoing.
Organizational Domain
This domain focused on the clinical implementation, and includes: logistical alignment, communication, procurement, and planning and capacity.
Technological Domain
Interviews within the production facilities evaluated the technical implementation process including required training, and quality and safety regulations. Furthermore, capacity and the production process itself were discussed. When comparing the interviews during the pilot phase to those during the national implementation phase, changes in the process were identified.
Economical Domain
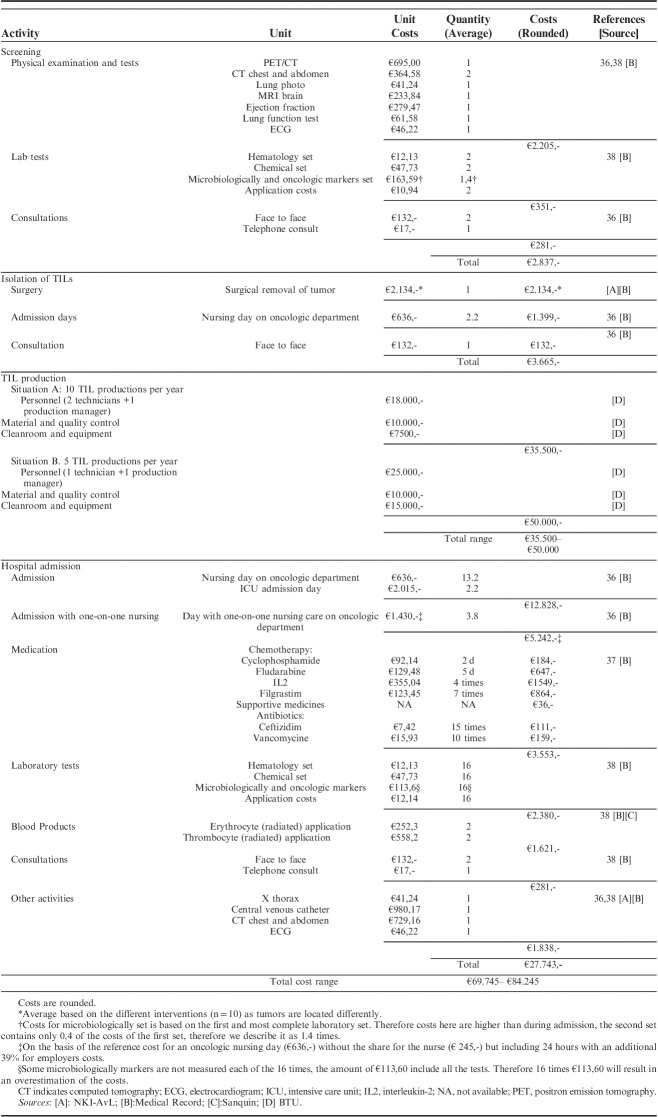
In this domain, the process of obtaining funding for research and achieving coverage under health insurance schemes was discussed. Furthermore, a bottom-up costing approach was used to estimate the economic burden per patient from screening until the first follow-up appointment. In this approach, resource use is identified per patient resulting in patient-specific unit cost.35 Therefore, the process of TIL treatment was observed in real-time and verified in the semistructured interviews during the phase III trial. TIL treatment consists of: (1) screening; (2) isolation of TILs; and (3) hospital admission. For each step, activities were described such as the duration of hospital admission, diagnostic activities, medicine use, laboratory tests, blood products, surgery, and consultations. This information was retrieved from the medical record for the first 10 patients treated in the phase III trial, and was linked to unit prices obtained from recent Dutch reference prices to calculate the costs of every process step.36–38 The production costs were estimated before the start of the RCT by the production facilities.
Future Perspectives
In this domain, estimates of the expected uptake, implementation and potential process changes at the institutional, national, and international levels were discussed. For various aspects, possible but feasible scenarios for the coming 5 years were identified. These aspects, such as the degree of effectiveness, the emergence of competing therapies, and the attitudes of clinicians toward the technology, can be used to describe their possible influence on adoption and diffusion.39
RESULTS
Literature Overview: ATMP Barriers and Facilitators
Of the 65 identified articles, 12 articles were selected.5–7,10–12,40–45 The 2 main barriers identified were: (1) inadequate financial support for both the required investments for GMP manufacturing and for setting up first pilot series and clinical trials (described in 8/12 articles) and (2) obtaining the required efficacy results and demonstrating long-term effectiveness data, toward market access and implementation in clinical practice. This was hampered by for example: a lack of harmonization in the hospital exemption clause, and difficulties in setting-up and receiving approval for clinical trials with ATMPs (8/12). Other barriers described were: compliance with GMP regulation which requires specific standard operating procedures, and specific documentation (7/12), potential therapeutic and technical risks in using live tissues as a basis for a treatment strategy (5/12), and a lack of regulatory knowledge to build a full product dossier for obtaining MA (2/12). The main facilitators or suggested solutions were (described in 11/12 articles): using adaptive licensing approaches such as coverage with evidence development (CED) programs, applying risk-sharing principles, or the use of accelerated assessment (5/11 articles), the organization of a (national) knowledge platform for information on GMP compliance and route to MA (2/11), securing engagement of HTA organizations alongside ATMP development to estimate the added value of a new ATMP in a certain field (eg, headroom analysis, cost-effectiveness analysis) (2/11), stimulating harmonization of ATMP and hospital exemption definitions and their procedures across Europe (2/11), and the use of a clinical implementation model in which the trained personnel is responsible for integrating a new therapy into routine clinical practice (2/11). The complete overview of barriers and facilitators, categorized according to the CTA domains, is listed in Supplement 3 (Supplemental Digital Content 1, http://links.lww.com/JIT/A514).
CTA on TIL-therapy: Results of Semistructured Interviews
The barriers and facilitators revealed by the CTA are summarized in Table 2. The following subsection is structured according to the 6 CTA domains.
TABLE 2.
Barriers and Facilitators in Early Application of TIL-therapy in the Netherlands Cancer Institute, Identified by Semistructured Interviews With 26 Stakeholders in the Constructive Technology Assessment Using the Following CTA Domains: Clinical, Patient-related, Organizational, Technical, Economical, and Future
Characteristics of Participants
In total, 26 stakeholders participated in the semistructured interviews during the pilot study phase and the national implementation phase. We included 2 medical oncologists, 6 patients, 4 nurses, 1 nurse practitioner, the head of the nursing department and 1 clinical research associate. From the 2 production facilities 3 project managers, 7 technicians, and 1 lab scientist took part. The average age was 47 (range: 32–59) years, having on average 6 (range: 3–9) years of experience with aspects of TIL-therapy, and for the clinically involved stakeholders (7/12) average experience with melanoma was 18 (range: 5–31) years. The participants showed different levels of innovativeness: 5 judged themselves as “innovator”/“early adopter”, 5 as “early majority”, one as “late majority” and one as a “laggard”,29 supporting the presence of critical respondents.
Clinical Domain
A facilitator for the implementation of TIL-therapy in the clinic was the clinical training of a clinician from the NKI-AvL at an expert center (Surgery Branch of the National Cancer Institute). This clinician trained the other oncologists during the pilot study in the NKI-AvL (skills training). Subsequently, the nurses were regularly trained and informed by a clinician or nurse-practitioner on the treatment itself and the treatment effects that could be expected. One of the barriers was the observed high toxicity (eg, cold shivers, high fever; Box 1), during the pilot phase after TIL and IL2 infusion. To decrease this toxicity, the clinical process was adapted before entering the RCT phase through the addition of further inclusion criteria: WHO status (≤1), a less stringent number of Interleukin-2 (IL2) doses, and providing supportive treatment (eg, pethidine) at an earlier stage. In addition the apheresis process step, which was used to harvest feeder cells for the production process, was left out as a specific blood product (allogenic buffycoats) could be used instead, showing similar results in the growth of TILs. These changes and the experience of the clinical team by treating the first 10 patients in the pilot, resulted in an improved acceptance by patients. A decrease in the average length of stay (from 22 to 19 d), and less frequent intensive care unit admissions (a reduction of 40%–10% in the first 10 patients enrolled in the RCT) were demonstrated. The ability to change the protocol based on experiences from the pilot study was a facilitator for further clinical implementation. From a nursing perspective the adjustment of the number of IL2 infusions was seen as a significant improvement regarding treatment intensity. The adjusted clinical process was felt to be more patient-centered. Clinical results of the current RCT study are expected in 2020 (NCT02278887) which will give more insight into the effect of these adaptations.
Patient-related Domain
To anticipate the expected toxicity of TIL-therapy and IL2 infusions (facilitator in implementing TIL-therapy), a psychiatry nurse and social worker were included in the clinical pathway to support patients and their families. Patients reported the complete TIL-therapy process to be physically and mentally burdensome, though acceptable. As a result of the intensified nursing care, closely involved physicians, and the possibility for family members to stay overnight during the TIL and IL2 treatment, patients felt safe. The interviewed nurses considered the intensity of the treatment to be acceptable, and emphasized the importance of the increased contact with health professionals. This was the case especially during the nights in the period of the TIL reinfusion and IL2 infusion to ensure adequate treatment of potential side effects that may arise. As the severity of the period after the IL2 infusions was underestimated by patients, the clinical team adapted the information provided to better prepare the patients for the potential adverse events that can occur after the IL2 infusions.
Study enrollment of the clinical phase III trial was slow and therefore evaluated as a barrier. The web-based questionnaire analyzing factors related to trial participation was completed by 11 stage IV melanoma patients. Figure 1 shows the results of this questionnaire. General RCT aspects, for example, receiving additional investigations or treatments, travel distance, and switching to another hospital showed limited or no influence on a patient’s decision to participate. Expected side effects appeared to impede participation, whereas recommendation by a clinician and the expected improved results demonstrated a positive influence on participation. The statements revealed that the idea of randomization, the probability of not receiving TIL-therapy but ipilimumab, and the fear of becoming more sick over time, have a negative influence on trial participation. Conversely, the idea that participating would be beneficial to other patients and clinicians shows a positive influence on trial participation (Fig. 1). The majority of respondents (6/11) were not informed about the TIL-therapy trial. Of the informed patients, one patient participated in the TIL-trial, 4 patients reported to consider participation but still had other effective standard treatment options, and the last patient suffered from an autoimmune disease and was therefore not eligible for the trial.
FIGURE 1.
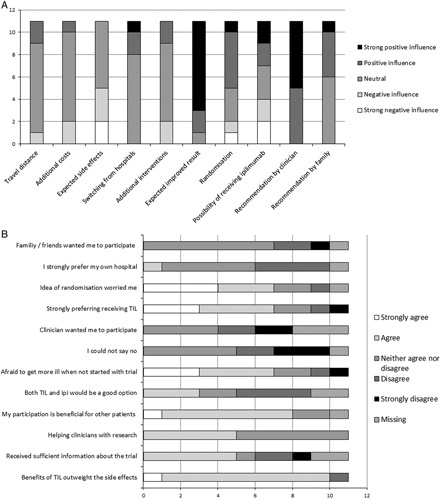
Results of the web-based questionnaire aimed at patients with advanced melanoma distributed via the patient association (n=11). A, Shows the level of influence of each aspect (positive or negative) in deciding to take part in the TIL trial. B, shows the level of agreement with several statements. The majority of questions does not sum up to 11, this is because of missing values. TIL indicates tumor-infiltrating lymphocytes.
Organizational Domain
Before the first application of the TIL-therapy, the following logistics were necessary: (i) agreements with surgical planning to align various steps with TIL production; (ii) during TIL and IL2 phase: arrangement of one-to-one nursing care, 24 hour availability of a trained oncologist (on-site or via telephone); and (iii) preventive intensive care unit bed reservation after the TIL infusion. These arrangements were in the NKI-AvL case not evaluated as a barrier due to being a Comprehensive Cancer Center (CCC) in which research (TIL production) and clinic are already well integrated. The clinic is already well accustomed to adapting clinical processes to research projects.
A barrier that was seen in this domain was the slow adoption of TIL-therapy in the Netherlands, resulting from the low number of referrals to our study. To improve this, the 13 specialized melanoma hospitals in the Netherlands (Box 1) were visited to provide more information on the treatment and on the trial. In addition, a dedicated webpage was created for both physicians and patients, and finally social media platforms from the hospital and patient association were used for promotion. This resulted in twice as many referrals over the following months (May to November 2017).
Technological Domain
Figure 2 shows the implementation timeline of TIL-therapy in our institute (NKI-AvL). This paragraph explains some of these processes. Before TILs may be produced, a manufacturing license is required which demonstrates compliance with ATMP and GMP guidelines.3,4 The process of obtaining approval for this specific product by the Dutch health care inspectorate (despite the production facility already holding a GMP permit for other products) took ∼2 years. Approval by the Dutch health care inspectorate also allows for the acceptance to the entire European market, provided that EU regulations are followed. Two factors in our case facilitated this process: (1) the availability of an in-house pharmacy with regulatory knowledge for advice; and (2) existing quality management system into which TIL production could be integrated. Examples of additions to the quality system were: generation of product-specific production runs and quality control protocols, general T-cell related SOPs, validation plans and reports, assessing suppliers and their materials for GMP use, and creating the investigators’ medicinal product dossier required for MA. Unless these facilitators, the technical preparation was very time consuming and is therefore categorized as a barrier for clinical implementation of TIL-therapy.
FIGURE 2.

Implementation timeline of TIL-therapy in the Netherlands Cancer Institute—Antoni van Leeuwenhoek hospital. CEA indicates cost-effectiveness analysis; CED, coverage with evidence development; IL2, interleukin-2; IMPD, investigational medicinal product dossier; NCI, National Cancer Institute; RCT, randomized controlled trial; TIL, tumor-infiltrating lymphocytes.
Training and acquiring suitable staff for the production process was another barrier as the TIL process deviates from both a research and a standard production setting. A technician familiar with producing TILs (trained in an expert center, ie, National Cancer Institute) was involved in the implementation process, facilitating the training of new employees. The learning curve for TIL production is strongly dependent on the frequency of TIL patients presenting and the frequency of similar research projects. Training of a new technician would still take at least 1 year to be able to work independently. The challenging nature of the training and staff acquisition is mainly due to compliance with GMP guidelines which require: (i) regular quality assurance checks (eg, on sterility, growth, and viability of the TILs); (ii) creating an auditable process (eg, all critical process steps performed by 2 operators) and; (iii) writing a report on the proceedings and potential deviations per TIL product. All the results from testing an individual batch are transcribed in a patient-specific Batch Record which is reviewed by the manufacturing department or quality assurance (dependent on local procedures) and qualified person (QP) after which the QP can release the product. Figure 3 shows the TIL growth process schematically, which is described in more detail by Donia et al.46
FIGURE 3.
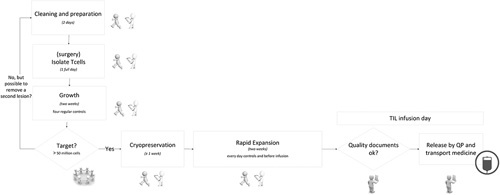
Visualization of technical process of generating TILs in a production facility. This visualization includes all quality controls and the duration of steps. The icons reflect the technicians involved, in all process steps: one executes and one monitors. QP indicates qualified person; TIL, tumor-infiltrating lymphocytes.
For the alignment of the technical process to the clinical process, a cryopreservation step of ∼1 week was included to control the start of chemotherapy and, thus, the day of TIL infusion. This is to ensure the availability of a complete medical team when TIL and IL2 side-effects are expected. This required a strict planning and regular communication between the various disciplines. A final barrier identified in this domain is that TIL production is time-consuming, especially on certain production days, such as the initial TIL isolation, initiation of the Rapid Expansion Protocol and the harvesting day. Any deviation in the growing process will affect the schedule and work routine, potentially resulting in irregular working days or hours. Although adequate, in a small academic production facility, with only a few trained technicians (which is often the case in such specific ATMPs) this could cause problems related to the availability of staff and logistics in the production cycle.
Economical Domain
The clinicians reported that gaining financial support was seen as a hurdle by hospitals and laboratories around the world aiming to implement TIL-therapy. In our case, implementation and the pilot study were financially supported by charitable and institutional funding because of the therapy’s promising effectiveness results in phase I/II trials. On our pilot results, the national Dutch CED program was granted which enabled a phase III trial and thus facilitated clinical implementation.
Treatment costs. The bottom-up costing approach resulted in a comprehensive insight into the financial impact of TIL-therapy from a hospital perspective. The TIL process was divided into 4 steps: screening, isolation of TILs, TIL production, and admission costs. For all monitored activities, costs, and their respective averages are listed in Table 3. Screening included several physical scans, blood tests and consultations, resulting in a total average cost of €2.837. Isolation of TILs consisted of surgery, admission day(s) and consultations, resulting in a total average cost of €3.665. The TIL production costs consisted of personnel, materials, quality control and cleanroom use costs, resulting in a total cost between €35.500 and €50.000, depending on the number of productions per year (10 or 5 patients). These costs were estimated before the start of the CED program, assuming a nonprofit production base and should therefore be interpreted with caution. Admission costs included hospital admission, medication, one-on-one nursing care, blood tests, imaging, consultations and complication-control, which resulted in a total average cost of €27.743. Summing up those steps gives a total estimated costs per patient between €69.745 and €84.245 depending on the number of patients that should receive TIL-therapy.
TABLE 3.
Bottom-up Costing Approach to Estimating Costs for TIL-therapy Based on 10 Patients Treated in CED Phase
Future Domain
From a technical perspective, the production process could be simplified in the future, for example by means of a more automated process or the use of a closed bioreactor. For the clinical process, it remains unclear whether lymphodepletion and additional IL2 treatment are necessary for its effectiveness. Decreasing the intensity of the nonmyeoloablative chemotherapy or changing to a lower dose schedule of IL2 or even removal of these steps could ease clinical adoption (clinical application and clinicians attitude). For national clinical implementation, the stakeholders recommend to first start in specialized melanoma centers due to their experience in treating patients with high dose chemotherapy. Furthermore, other hospitals could receive training from clinicians and nurses at the NKI-AvL. Finally, centralization of the production of TILs seems advisable for the years to come as technical implementation is both highly challenging and time-consuming.
DISCUSSION
To the best of our knowledge, this is the first study that comprehensively evaluates local and national implementation of a specific ATMP; in our case, early application of TIL-therapy. Our analyses showed that clinical implementation remains complex—mostly explained by the general ATMP barriers identified in the literature. Despite these barriers, a clinical implementation may become feasible when financial support, regulatory knowledge (GMP and route to market access), and both clinical and technical experience is available.
The CTA survey identified the following barriers: toxicity of TIL-therapy (clinical and patient-related), the need for trainings (clinical, technical), limited patient accrual (patient-related, organizational, future), upfront clinical arrangements (organizational), compliance with GMP and ATMP regulations (technical), and funding for upfront investments and following clinical studies (economic). Nonetheless, TIL-therapy was implemented in the NKI as part of the immunotherapy research program as a result of certain facilitators. The main facilitator was gaining financial support. First, from the institute to create the production facility and to start a pilot study to evaluate the feasibility of providing TIL-therapy. Second from the Dutch CED program which was received in 2014 based on the promising results in the pilot study (n=10).14 Furthermore, implementation was facilitated by: (i) the availability of an in-house pharmacist with regulatory knowledge and quality assurance department for advice on GMP regulations and quality assurance, (ii) having a TIL skilled technician (from an expert center), (iii) upfront clinical training in an expert center, and (iv) being a CCC. For future adoption, the costs of TIL-therapy is likely to be a facilitator as it is predicted to be lower compared with ipilimumab and other standard treatments in advanced melanoma. A first model which assessed the cost-effectiveness of TIL-therapy compared with ipilimumab demonstrated that TIL-therapy is dominant over ipilimumab, hence showing higher quality adjusted life years correlated to lower treatment costs.47,48
Limited patient accrual in the RCT remained one of the barriers which may be explained by recent developments in treating advanced melanoma.49 As these newly developed treatments show at least similar response rates over the study period compared with TIL-therapy, but are easier to apply (not personalized, available off the shelf).50 This could negatively influence the attitude of clinicians toward the potential of TIL-therapy, as the speed of adoption is related to the complexity, relative advantage, visibility, trialability, and compatibility29 of a new treatment strategy which might affect the choice of treatment/participation in the trial by the patient.
Of all the identified barriers in the literature (discussed in Supplement 3, Supplemental Digital Content 1, http://links.lww.com/JIT/A514) on translating ATMPs, gaining financial support, the route to MA, and compliance to GMP regulations were seen as the main hurdles. Gaining financial support is especially challenging as small and medium academic facilities face the biggest financial risks related to ATMP development and demonstrating treatment efficacy (eg, high upfront and manufacture costs, to show treatments’ efficacy).11,51 It has been recognized that in translating innovative, and personalized technologies into the clinic, existing generic regulatory assessments may be unsuitable.42,52 Therefore, the suitability of the NICE (the National Institute for Health and Care Excellence) appraisal methodology for regenerative and cell-based therapies was investigated.42 They recognized that evidence for the efficacy of regenerative medicines can be associated with high uncertainty levels around long-term costs and benefits. Using existing methods to estimate the implications of this uncertainty—such as calculating Cost-Effectiveness Acceptability Curves, Expected Value of Perfect Information and expected opportunity losses—were considered as sufficient. Yet, the NICE appraisal mentioned recommendations to gather the required data for this regulatory assessment. For instance, (i) use of surrogate endpoints, which should first be validated by systematic reviews; (ii) use of alternative trial designs in rare diseases, for example single arm trials or responsive-adaptive randomization; and (iii) innovative reimbursement programs aiming to find a balance between shorter approval times and ensuring a flow to gain efficacy and safety data for promising medicines in patient categories with high unmet needs. This final aspect of risk-sharing seems to be one of the key recommendations as this also enabled application of TIL-therapy in the NKI-AvL case. The ADAPT SMART project, funded by the EU Innovative Medicines Initiative, seeks for solutions to develop such Medicines Adaptive Pathways to Patients.42,53,54
Limitations
In our analysis, several limitations emerged. All semistructured interviews were conducted by one researcher to create uniformity in the several interviews; however, this potentially resulted in interview bias. Therefore, we discussed our results with the stakeholders and a second researcher verifying the labels given to the interviews. Second, to optimally use the CTA methodology for steering development before application, we should have started the CTA survey before any clinical implementation. However, our first series of interviews were held during the pilot study. Furthermore, in terms of content, we were unable to effectively address the patient impact of TIL-therapy by means of the Impact of Event scale and EQ5D as we included a limited number of patients in the interviews (n=6). In addition, the analysis would have been strengthened, especially toward the patient and clinical domain, by including the first clinical results of the TIL trial. Unfortunately, as the trial is still ongoing, the results could not be analyzed and published. Moreover, the external validity of the cost estimation is limited as Dutch reference prices were used and only a limited number of patients were included as a basis for our analysis (n=10).55 However, Table 3 and the specification of the activities can be used by other hospitals to estimate the costs for their situation using country specific unit prices. To have a sense of the treatment costs for providing TIL-therapy (noncommercially) in other countries such as Canada, the United Kingdom, and the United States we translated our results by using a recent article that compared the health care costs across countries and using the GDP per capita of 2015 for these countries.56 The average conversion rates of 2015 were used to generate a country specific range of the costs. For example, based on the results of Papanicolas et al56 total health care costs in the United States seem to be ∼80% higher than in the Netherlands. The treatment costs were increased with 80% which resulted in a cost range of $139.448–$168.440 per patient. Using the difference in GDP per capita between the United States and the Netherlands the costs were multiplied by 1.25 which resulted in a range of $97.600–$117.891. Thus, the estimated cost range for providing TIL treatment in the United States is $97.600–$168.440 per patient. For Canada and the United Kingdom a similar translation resulted in cost ranges of: C$89.072–C$116.295 and £32.945–£60.608, respectively. We acknowledge that using these ratios results in a rough estimation. It is, however, a more accurate indication than only using a conversion rate which neglects the differences in health care costs per country at all. In addition, the costs for the production of TILs in our analysis could be an underestimation as the initial costs were estimated on a higher throughput of patients (10–20 patients per year). Therefore the costs for TIL production with a smaller number of patients was estimated and included in our cost analysis. A cost-effectiveness analysis could be an informative approach to estimate the minimal throughput of patients per year required to result in a cost-effective alternative to the standard of care. Finally, we may not have included all relevant literature because our literature overview was not a systematic literature search. However, we feel that by using snowballing methods we included the most important documents as we found several similarities between the articles regarding the identified barriers/facilitators, and these similarities were endorsed by the technical staff of the specialized production sites.
Future Perspective
Implementation of TIL-therapy would have even been more challenging if the NKI-AvL had not enjoyed the financial support of the CED program. This shows that applying financial risk-mitigating principles have a big influence on patient access. Especially for ATMPs, it would be valuable to develop financing strategies with government, industry, research institutes and/or insurance companies to share risks and facilitate uptake of ATMPs. In these strategies, involvement of HTA bodies at an early stage of development would be beneficial as these methods (eg, headroom analysis, multidecision criteria analysis and value if information) can help to estimate the potential of the proposed ATMP11 and give an estimate of the uncertainty surrounding outcome measures. Future research should focus on the effect of using these risk-sharing principles for ATMPs on patient access. Beyond this, identifying the most valuable early HTA method per translational phase would be worthwhile as these not only assist in identifying critical aspects in the early development process, but also help in gaining financial support and continue on the route to MA by defining and accordingly reducing uncertainty. For TIL-therapy specifically, further research should focus on identifying aspects—such as clinician’s attitudes and perceptions on (side) effects—influencing future adoption of TIL-therapy as this was evaluated as a hurdle in the early application of TIL-therapy.
CONCLUSIONS
On the basis of this comprehensive evaluation of early application of TIL-therapy we conclude that implementation is complex and—in at least the preparatory steps—is expensive, but feasible under optimal circumstances (ie, sufficient financial support, and experience with TIL and GMP). As TIL-therapy seems to be a potentially cost-effective alternative to ipilimumab47,48 other institutes may want to consider TIL-therapy as a standard treatment option. For the TIL case, implementation in an already GMP-certified production facility would facilitate effective technical implementation and minimize some of the financial risks. In addition, for clinical application, a medical team should be thoroughly trained at a specialist cancer center. Finally, for national implementation it was suggested to start first in the specialized, high volume melanoma centers and to centralize production at the specialized production sites. When these strategies are used, the likelihood of implementing TIL-therapy in clinical practice will increase, and thus provide increased patient access to this promising treatment.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
ACKNOWLEDGMENTS
The authors want to thank all the stakeholders that participated in the semistructured interviews. Besides, the authors thank all patients that participated in the TIL-trial, the ones that completed the web-based questionnaire on factors related to participation, and especially the authors thank the patients that participated in the semistructured interviews.
Conflicts of Interest/Financial Disclosures
Supported by the Netherlands Organisation for Health Research and Development, dossier number: 837004011.
All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
M.A.L.: conducted the semistructured interviews, cost estimation, literature search, and analyzed the results. She drafted the paper and adapted the paper according the feedback received from co-authors. V.P.R.: assisted in preparing the semistructured interviews, cost estimation, and web-based questionnaire. She checked the labels of the semistructured interviews, and read and gave feedback on the manuscript. J.H.v.d.B., M.H.G.F., and J.B.H.: participated in one of the semistructured interviews, assisted with the literature search and structuring the results regarding ATMP barriers and facilitators. Furthermore, they read and gave feedback on the manuscript. W.H.v.H., M.A.L., and V.P.R.: drafted the research concept W.H.v.H: assisted with clarifying the results and drafting the manuscript. Furthermore, he read, edited and gave feedback on the manuscript.
REFERENCES
- 1.Department for Business Innovation & Skills. Eight great technologies. 2013. Available at: www.gov.uk/government/speeches/eight-great-technologies. Accessed July 11, 2017.
- 2.European Medicines Agency. Advanced therapy medicinal products. Available at: www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000294.jsp&mid=WC0b01ac05800241e0. Accessed May 30, 2018.
- 3.The European Parliament and the Council of the European Union. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending directive 2001/83/EC and regulation (EC) No 726/2004. 2007. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:0121:0137:en:PDF. Accessed July 10, 2017.
- 4.EudraLex. Volume 4—good manufacturing practice (GMP) guidelines. European Commission, Public Health. 2016. Available at: https://ec.europa.eu/health/documents/eudralex/vol-4_en. Accessed July 24, 2017.
- 5.de Wilde S, Veltrop-Duits L, Hoozemans-Strik M, et al. Hurdles in clinical implementation of academic advanced therapy medicinal products: a national evaluation. Cytotherapy. 2016;18:797–805. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann-Fritsch F, Marino D, Reichmann E. About ATMPs, SOPs and GMP: the hurdles to produce novel skin grafts for clinical use. Transfus Med Hemother. 2016;43:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce KF, Hildebrandt M, Greinix H, et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy. 2014;16:289–297. [DOI] [PubMed] [Google Scholar]
- 8.Belardelli F, Rizza P, Moretti F, et al. Translational research on advanced therapies. Ann Ist Super Sanita. 2011;47:72–78. [DOI] [PubMed] [Google Scholar]
- 9.Committee for Advanced Therapies. CAT monthly report of application procedures, guidelines and related documents on advanced therapies. 2017. Available at: www.ema.europa.eu/docs/en_GB/document_library/Committee_meeting_report/2017/11/WC500238885.pdf. Accessed July 10, 2017.
- 10.Faulkner A. Opening the gateways to market and adoption of regenerative medicine? The UK case in context. Regen Med. 2016;11:321–330. [DOI] [PubMed] [Google Scholar]
- 11.Bubela T, McCabe C, Archibald P, et al. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. Regen Med. 2015;10:897–911. [DOI] [PubMed] [Google Scholar]
- 12.Heathman TRJ, Nienow AW, McCall MJ, et al. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. 2015;10:49–64. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Advanced therapy medicines: exploring solutions to foster development and expand patient access in Europe. 2016. Available at: www.ema.europa.eu/docs/en_GB/document_library/Report/2016/06/WC500208080.pdf. Accessed July 24, 2017.
- 14.van Harten WH, Retèl VP. Innovations that reach the patient: early health technology assessment and improving the chances of coverage and implementation. Ecancermedicalscience. 2016;10:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zorginstituut Nederland. Voorwaardelijke toelating tot het basispakket Voortgangsrapportage 2017 [Conditional approval to the basic insurance package Progress report 2017] (Dutch report). 2017. Available at: www.zorginstituutnederland.nl/binaries/zinl/documenten/rapport/2017/3/27/voorwaardelijke-toelating-tot-het-basispakket-voortgangsrapportage-2017/Voorwaardelijke+toelating+tot+het+basispakket+%28Voortgangsrapportage+2017%29.pdf. Accessed August 16, 2017.
- 16.Geukes Foppen MH, Donia M, Svane IM, et al. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol. 2015;9:1918–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen R, Donia M, Ellebaek E, et al. Long-Lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated il2 regimen. Clin Cancer Res. 2016;22:3734–3745. [DOI] [PubMed] [Google Scholar]
- 19.Merhavi-Shoham E, Itzhaki O, Markel G, et al. Adoptive cell therapy for metastatic melanoma. Cancer J. 2017;23:48–53. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med. 1988;319:1676–1680. [DOI] [PubMed] [Google Scholar]
- 21.Svane IM, Verdegaal EM. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care? Cancer Immunol Immunother. 2014;63:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Health technology assessment. Available at: www.who.int/medical_devices/assessment/en/. Accessed July 24, 2017.
- 23.IJzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Heal Econ Heal Policy. 2011;9:331–347. [DOI] [PubMed] [Google Scholar]
- 24.IJzerman MJ, Koffijberg H, Fenwick E, et al. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics. 2017;35:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husereau D, Henshall C, Sampietro-Colom L, et al. Changing health technology assessment paradigms? Int J Technol Assess Health Care. 2016;32:191–199. [DOI] [PubMed] [Google Scholar]
- 26.Miquel-Cases A, Schouten PC, Steuten LMG, et al. (Very) Early technology assessment and translation of predictive biomarkers in breast cancer. Cancer Treat Rev. 2017;52:117–127. [DOI] [PubMed] [Google Scholar]
- 27.Schot J, Rip A. The past and future of constructive technology assessment. Technol Forecast Soc Change. 1997;54:251–268. [Google Scholar]
- 28.Douma KFL, Karsenberg K, Hummel MJM, et al. Methodology of constructive technology assessment in health care. Int J Technol Assess Health Care. 2007;23:162–168. [DOI] [PubMed] [Google Scholar]
- 29.Rogers EM. The diffusion of innovations. 5th ed. Simon & Schuster; 2003:576.
- 30.NVivo Qualitative Data Analysis Software. QSR International Pty LTd (version 10). 2012. Available at: http://www.qsrinternational.com/nvivo/support-overview/faqs/how-do-i-cite-nvivo-for-mac,-nvivo-11-for-windows.
- 31.Igwe E, Woodburn J, Davolos J, et al. Patient perceptions and willingness to participate in clinical trials. Gynecol Oncol. 2016;142:520–524. [DOI] [PubMed] [Google Scholar]
- 32.Penman DT, Holland JC, Bahna GF, et al. Informed consent for investigational chemotherapy: patients’ and physicians’ perceptions. J Clin Oncol. 1984;2:849–855. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins V, Farewell V, Farewell D, et al. Drivers and barriers to patient participation in RCTs. Br J Cancer. 2013;108:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur Rosalind L, Williamson, Paula GS. Developing a survey of barriers and facilitators to recruitment in randomized controlled trials. Trials. 2012;13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapko MK, Liu CF, Perkins M, et al. Equivalence of two healthcare costing methods: bottom-up and top-down. Health Econ. 2009;18:1188–1201. [DOI] [PubMed] [Google Scholar]
- 36.Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Manual for cost research: methods and standard cost prices for economic evaluations in health care. Diemen; 2015.
- 37.Zorginstituut Nederland. Medicijnkosten. 2017. Available at: www.medicijnkosten.nl/. Accessed February 1, 2017.
- 38.Dutch Healthcare Authority (NZa). DBC product finder for tariffs. 2017. Available at: http://dbc-zorgproducten-tarieven.nza.nl. Accessed February 2, 2017.
- 39.Retèl VP, Joore MA, Linn SC, et al. Scenario drafting to anticipate future developments in technology assessment. BMC Res Notes. 2012;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner J, Faulkner A, Mahalatchimy A, et al. Are there specific translational challenges in regenerative medicine? Lessons from other fields. Regen Med. 2015;10:885–895. [DOI] [PubMed] [Google Scholar]
- 41.Ali R, Hollander A, Kemp P, et al. Regulating cell-based regenerative medicine: the challenges ahead. Regen Med. 2014;9:81–87. [DOI] [PubMed] [Google Scholar]
- 42.Corbett MS, Webster A, Hawkins R, et al. Innovative regenerative medicines in the EU: a better future in evidence? BMC Med. 2017;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boran T, Menezes-Ferreira M, Reischl I, et al. Clinical development and commercialization of advanced therapy medicinal products in the European Union: how are the product pipeline and regulatory framework evolving? Hum Gene Ther Clin Dev. 2017;28:126–135. [DOI] [PubMed] [Google Scholar]
- 44.Galli MC. ATMPs for cancer immunotherapy: a regulatory overview. Methods Mol Biol. 2016;1393:1–9. [DOI] [PubMed] [Google Scholar]
- 45.Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell. 2016;19:293–297. [DOI] [PubMed] [Google Scholar]
- 46.Donia M, Larsen SM, Met Ö, et al. Simplified protocol for clinical-grade tumor-infiltrating lymphocyte manufacturing with use of the Wave bioreactor. Cytotherapy. 2014;16:1117–1120. [DOI] [PubMed] [Google Scholar]
- 47.Retel VP, Steuten LMG, Mewes JC, et al. Early cost-effectiveness modeling for tumor infiltrating lymphocytes (TIL)—treatment versus ipilimumab in metastatic melanoma patients. Value Health. 2014;17:A640. [DOI] [PubMed] [Google Scholar]
- 48.Retel VP, Steuten LMG, Geukes Foppen MH, et al. Early cost-effectiveness of tumor infiltrating lymphocytes (TIL) for second line treatment in advanced melanoma: a model-based economic evaluation. BMC Cancer. 2018;18:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sznol M. Challenges in conducting clinical research on patients with advanced melanoma. Cancer J. 2017;23:75–78. [DOI] [PubMed] [Google Scholar]
- 50.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.House of Lords Science and Technology Committee. Regenerative medicine report. 2017. Available at: www.publications.parliament.uk/pa/cm201617/cmselect/cmsctech/275/27502.htm. Accessed July 24, 2017.
- 52.Faulkner E, Annemans L, Garrison L, et al. Challenges in the development and reimbursement of personalized medicine-payer and manufacturer perspectives and implications for health economics and outcomes research: a report of the ISPOR personalized medicine special interest group. Value Health. 2012;15:1162–1171. [DOI] [PubMed] [Google Scholar]
- 53.ADAPT SMART. Accelerated development of appropriate patient therapies. Available at: http://adaptsmart.eu/. Accessed August 8, 2017.
- 54.Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21:1–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welte R, Feenstra T, Jager H, et al. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22:857–876. [DOI] [PubMed] [Google Scholar]
- 56.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024–1039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.