Abstract
Many studies have reported the recovery ability of umbilical cord-derived mesenchymal stem cells (UC-MSCs) for neural diseases. In this study, the authors explored the roles of UC-MSCs to treat the traumatic brain injury. Umbilical cord-derived mesenchymal stem cells were isolated from healthy neonatal rat umbilical cord immediately after delivery. The traumatic brain injury (TBI) model was formed by the classical gravity method. The authors detected the behavior changes and measured the levels of inflammatory factors, such as interleukin-lβ and tumor necrosis factor-α by enzyme linked immunosorbent assay (ELISA) at 1, 2, 3, 4 weeks after transplantation between TBI treated and untreated with UC-MSCs. Simultaneously, the expression of glial cell line-derived neurotrophic factor (GDNF) and brain derived neurotrophic factor (BDNF) were measured by real-time–polymerase chain reaction and ELISA.
The authors found that the group of transplantation UC-MSCs has a significant improvement than other group treated by phosphate buffered saline. In the behavioral test, the Neurological Severity Scores of UC-MSCs + TBI group were lower than TBI group (P < 0.05), but not obviously higher than control group at 2, 3, and 4week, respectively. The inflammatory factors are significantly reduced comparison with TBI group (P < 0.05), but both GDNF and BDNF were higher than TBI group (P < 0.05). The results indicated that UC-MSCs might play an important role in TBI recovery through inhibiting the release of inflammatory factors and increasing the expression of GDNF and BDNF.
Keywords: Cell factors, traumatic brain injury, umbilical cord mesenchymal stem cell
Traumatic brain injury (TBI) is a high-incidence injury, which can cause severe brain damage or even disability. The patients suffered from TBI showed somewhat damaged neurological symptoms, such as intracranial hypertension, or lost the cognitive competence totally.1–3 But the process of the treatment is still unsatisfied. Thus, the TBI treatment is mainly focused on the survival of nerve in the injury domain.
Recently, umbilical cord-derived mesenchymal stem cells (UC-MSC) is a new focus of research in medicine and pharmacy. Numerous studies have reported that UC-MSCs, as a kind of pluripotent stem cells, can differentiate into microglial cells and oligodendrocytes, and which lead the new direction for cell therapy.4,5 Umbilical cord-derived mesenchymal stem cells might be used to treat a lot of neurodegenerative disease, such as Alzheimer disease, Parkinson disease, and stroke. Previous studies found that stem cells can replace the apoptotic cells and release some neurotrophic factors to restore the damage tissue.6–8 Further studies also showed that Stem cells and suppress inflammation by inhibiting the secretion of some proinflammatory cytokines.9,10 Traumatic brain injury is a complex pathological process, involving brain damage causing inflammation.11,12 Therefore, we expected to find more possible mechanism of UC-MSCs in TBI models to facilitate the treatment of TBI.
METHODS
Animal Model and Behavioral Measures
Thirty 6–8-week-old male SD rats, weighing 185 to 200 g and 20 pregnant rats were incorporated into our experiment. All animal experiments met the requirement of Animal ethics Committee. The method we used to make TBI model was conducted on the basis of previous studies.13,14 We divided the male rats into 3 groups (TBI group treat with phosphate buffered saline (PBS), TBI+UC-MSCs group, control group). All rats were anesthetized by intraperitoneal injection of 4% chloral hydrate. We performed this experiment and the behavioral measure according to the described as prevent research.15 Animals were assessed by Neurological Severity Scores (NSS).16
Isolation, Culturing, and Flow Cytometry Analysis of Umbilical Cord-Derived Mesenchymal Stem Cells
The total UC-MSCs fraction was obtained by digesting with 0.25% trypsin as described previously.17,18
The number of 1 × 106 cells were cultured with fresh medium contained DMEM/F12, N2, P/S, FGF2 for 7 days in cell incubator under 37°C and 5% CO2. Subsequently, we subcultured the cells when they reach 80% content, and as the same way, we subcultured the cells to the third generation.19–21 We analyzed the characteristics of cultured UC-MSCs with flow cytometry (BD Biosience, San Jose, CA). The UC-MSC surface marker included CD16, CD29, CD34, CD45, CD73, CD90 (BD Biosience).
Cell Transplantation
The UC-MSCs were diluted with PBS to a cell concentration of 5 × 105 cells/μL. And 6 μL of the cell suspension or PBS was immediately transplanted into the damage place of TBI+UC-MSCs group after TBI model structured. PBS was injected into TBI group and control group.
Immunohistochemistry
The rats were sacrificed after UC-MSCs were transplanted 1, 2, 3, 4 weeks and at the same time they were perfused with 4% paraformaldehyde. We obtained and fixed the whole rat brains in 4% paraformaldehyde for 24 hours. Subsequently, we performed the protocol of immunohistochemistry as described previously to stain the Glial cells’ marker (GFAP, Dako rabbit 1:500). We used the fluorescence microscope (Nikon, Ti) to take all images of the brain slices what were stained.22,23
Real-Time Quantitative Polymerase Chain Reaction and Enzyme Linked Immunosorbent Assay
We isolated total RNA with Trizol (Sigma-Aldrich, St Louis, MO), and reversed transcripted with PrimeScript real-time reagent Kit with genomic DNA Eraser (Takara Bio, Kusatsu, Japan). The reactive buffer for real-time quantitative polymerase chain reaction (PCR) was Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA). The primer sequence was described as follows.
GDNF-F: TTATTCAAGCCACCATCA
GDNF-R: AGGAACCGCTACAATATC
BDNF-F: CTGATAGTTCTGTCCATTC
BDNF-R: TCCACTCCTAAGATGAAG
GAPDH-F: GCCTTCCGTGTTCCTACC
GAPDH-R: CCTGCTTCACCACCTTCTT
We vibrated the damaged or cell transplanted tissue obtained from 3 groups with radio immunoprecipitation assay lysis buffer, and the antibodies to GDNF and BDNF were used to detect the expression of GDNF or BDNF.
Data Analysis
The data were analyzed with the Students t test (SPSS statistics software 20.1, Chicago, IL).
RESULTS
Isolation and Cultured of Umbilical Cord-Derived Mesenchymal Stem Cells
The total UC-MSCs fraction was obtained by digesting with 0.25% trypsin.
After isolation of UC-MSCs, we immediately cultured them with fresh medium. And then a part of cells were detected in the characterization with flow cytometry. As a result, the average expression of CD16, CD29, CD34, CD45, CD73, and CD90 accounts for 32.10% ± 2.97%, 72.10% ± 0.63%, 3.52% ± 0.86%, 92.25% ± 8.95%, 19.30% ± 4.13%, and 42.36% ± 0.32% of the UC-MSCs respectively as shown in Figure 1.
FIGURE 1.
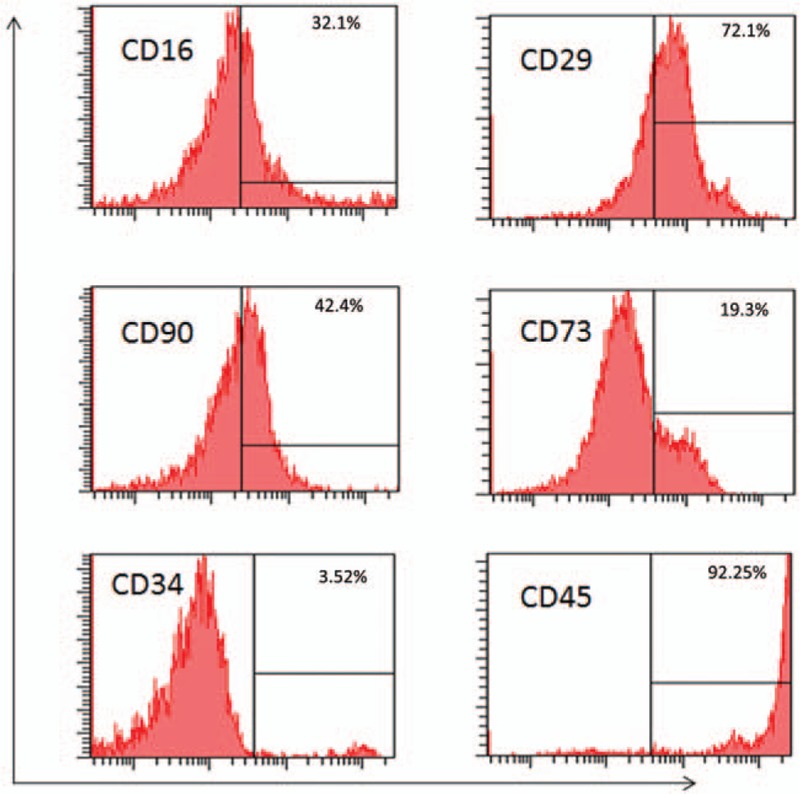
Flow cytometry analysis of the UC-MSCs. (A) Expression of surface markers of UC-MSCs. (B) The average rate of CD16, CD29, CD34, CD45, CD73, CD90. UC-MSCs, umbilical cord-derived mesenchymal stem cells.
Behavioral Measures of Traumatic Brain Injury Rats Transplanted Umbilical Cord-Derived Mesenchymal Stem Cells
To investigate whether UC-MSCs have an impact on respiration to the damage neuron. we tested the behavior after cells were transplanted 1, 2, 3, 4 weeks, respectively, by evaluating NSS in each group (Fig. 2). We found that the score of TBI + UC-MSCs group was significantly lower than TBI group (P < 0.05).
FIGURE 2.
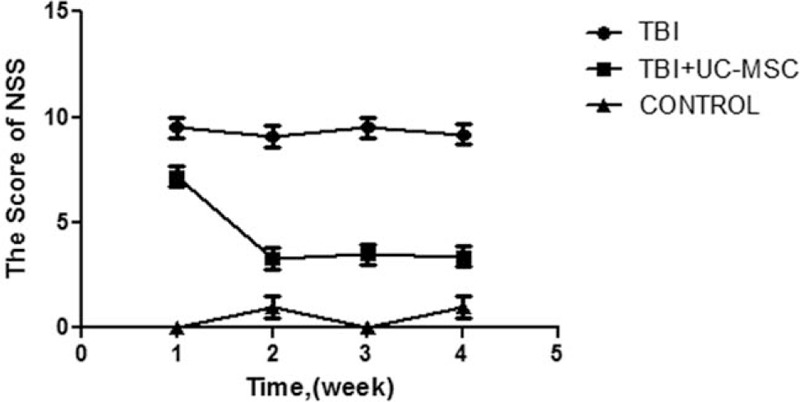
The score of Neurological Severity Scores (NSS) of the groups. The scores of TBI+UC-MSCs group were lower than TBI group after UC-MSCs were transplanted 2, 3, 4 weeks (∗P < 0.05), 2-way ANOVA with post hoc test. TBI, traumatic brain injury; UC-MSCs, umbilical cord-derived mesenchymal stem cells.
The Differentiation of Umbilical Cord-Derived Mesenchymal Stem Cells In Vivo
To investigate the survival and the differentiation of UC-MSCs in vivo, we performed immunohistochemistry after UC-MSCs were transplanted 1, 2, 3, 4 weeks. Nerve-specific antibody of rat was used to label UC-MSCs. The images of immunohistochemistry were obtained with fluorescence microscope. We performed this experiment with the antibody GFAP (gliocyte's marker) to label UC-MSCs-derived gliocyte. Images showed that the morphological changes of the transplanted UC-MSCs differentiated toward the glial cells (GFAP staining, Fig. 3).
FIGURE 3.
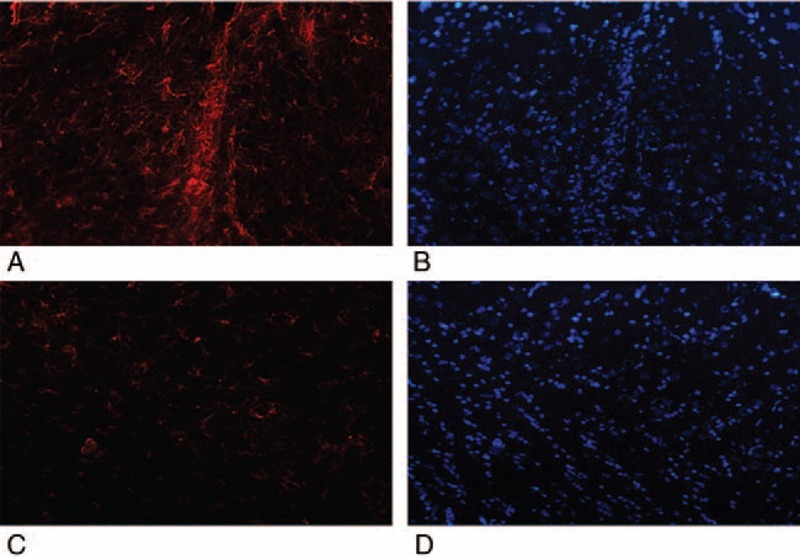
Immunohistochemistry analysis of TBI rats what transplanted UC-MSCs or not. Transplanted UC-MSCs were stained with GFAP (red) and DAPI (blue) 4 weeks after transplantation. (A, B) Immunostaining of UC+TBI group rats after UC-MSCs transplantation. (C, D) Immunostaining of TBI group rats after PBS transplantation. TBI, traumatic brain injury; UC-MSCs, umbilical cord-derived mesenchymal stem cells.
The Secretion of the Neurotrophic Factors and Inflammatory Factors
To measure the role of the transplanted UC-MSCs in vivo, we used real-time quantitation PCR or enzyme linked immunosorbent assay (ELISA) to analyze the expression of neurotrophic factors, such as GDNF and BDGF from the tissue of damage or UC-MSCs transplantation. As the results described in Figure 4A and B, we found that the expression of GDNF and BDNF in TBI+UC-MSCs group was significantly higher than TBI group (P < 0.05).
FIGURE 4.
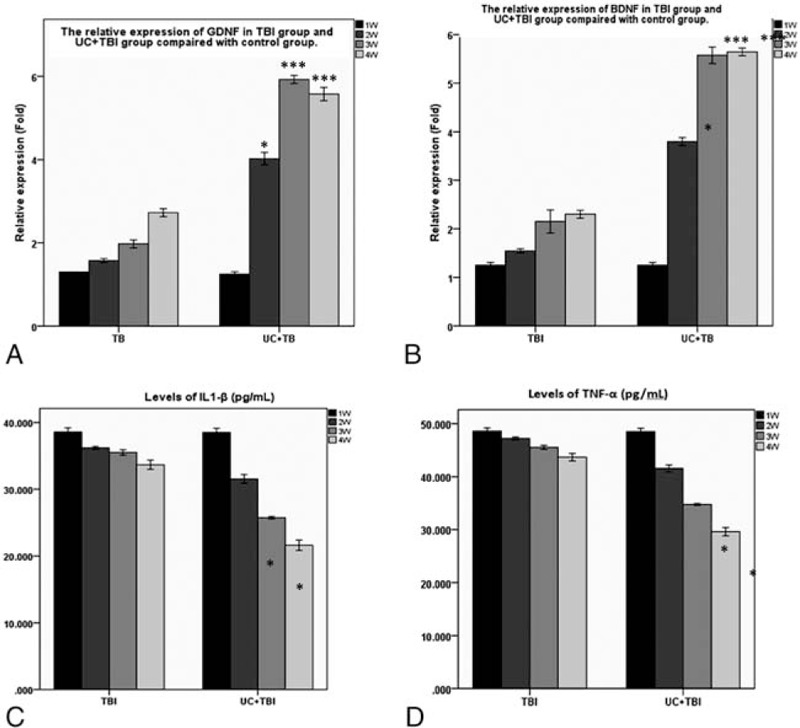
The expression of the neurotrophic growth factors and the inflammatory factors. (A) The relative expression of GDNF in UC+TBI group and TBI group. (B) The relative expression of BDNF in TBI group and UC+TBI group. (C) The content of IL-1β in TBI group and UC+TBI group. (D) The content of TNF-αin TBI group and UC+TBI group.∗ P < 0.05, ∗∗∗P < 0.001; 2-tailed Student t test. TBI, traumatic brain injury; UC-MSCs, umbilical cord-derived mesenchymal stem cells; IL-1β, interleukin-lβ.
A lot of researches indicated that the survival of neuron after damage was closely connected with inflammatory factors in the surrounding microenvironment. So, we detected the content of inflammatory factors, such as interleukin-lβ (IL-1β) and tumor necrosis factor (TNF)-α by ELISA (Fig. 4C, D). Compared with TBI group, the content of inflammatory factors was lower in TBI+UC-MSCs group (P < 0.05).
DISCUSSION
Recent studies showed that the UC-MSCs could be induced into gliocyte-like cells, such as astrocytes and microglia cells which can secrete some neurotrophic factors to promote the survival of damaged neurons.24,25 A large amount of cytokines were released in the damaged section, and played important roles in the cell death, recovery or survival, especially IL-1β and TNF-α, both are major inflammatory factors that can induce immune response. Meanwhile, GDNF and BDNF are neurotrophic factors which can promote nerve survival and recovery.
Heo et al11 reported that the transplanted stem cells can secrete some nerve growth factors, such as GDNF, BDNF, and NGF what could affect the survival of the ischemic nerve. The transplanted mesenchymal stem cell could mediate the immunomodulation by secreting the inflammatory factors.26 Interleukin-lβ and TNF-α play an significant role in the immune response by recruiting macrophage and nature kill cells, increasing the cytotoxicity to damaged issue, and promoting cell apoptosis. But the roles of UC-MSCs have not been reported in TBI animal models. In our current study we investigated the roles of UC-MSCs after they were transplanted into TBI rats from the scores of NSS; the pathological changes between TBI group and UC+TBI group; the secretion of neurotrophic factors (GDNF and BDNF) and inflammatory factors (IL-1β and TNF-α).
We isolated the UC-MSCs from umbilical cord of pregnant rats and cultured. To detect the characteristic of isolated UC-MSCs, we measured the biomarkers by flow cytometry. In our result, UC-MSCs were high expressed CD45 and CD29, which is consistent to the previous reports.
Both behavior of TBI rats, and the morphology and density of neuron in damage area had a substantial improvement after UC-MSCs were transplanted, we detected the change of the behavior at 2 weeks after UC-MSCs were transplanted. We also detected the secretion of cytokines at the same time. The transplanted stem cells could secrete neurotrophic growth factors such as BDNF, GDNF to play the roles of therapy.27,28 We found the expression of BDNF and GDNF were significantly increased. The expression level of the BDNF and GDNF in the UC-MSCs transplanted rat brains were observed by using ELISA kits from Promega Corporation, which demonstrated that the increase of neurotrophic factors in the area of transplanted UC-MSCs could improve the repair and regeneration of the neuron significantly.29–31
The inflammation of damage area is a crucial factor in neuron regeneration. Interleukin-lβ is a member of the interleukin 1 family of inflammatory factors. Recent studies have implied that IL-1β could play a key role in mediating the inflammatory response after injury. Interleukin-lβ is also involved in many bioreactions in the central nervous system after damage; TNF-α is one of cytokines protein of signaling pathway involved in inflammation and apoptosis in damaged tissue. Both of them could recruit macrophage and nature killer cells and enhance the cytotoxicity to damaged issue and promote cell apoptosis, and they could be involved in cell proliferation, cell differentiation, and apoptosis.32
To observe the importance of the inflammatory factors in TBI, we measured the content of IL-1β and TNF-α in the different groups by ELISA and immunohistochemistry. Both results showed that the level of the inflammatory factors in UC-MSCs TBI group was significantly lower than TBI group treated without UC-MSCs, which suggest that transplanted UC-MSCs can reduce the inflammatory response by downregulation of IL-1β and TNF-α expression.
In conclusion, this study demonstrated that the transplanted UC-MSCs have a significant therapeutic effect in TBI models by upregulating the neurotrophic factors and downregulating the inflammatory factors. The increased neurotrophic factors might play an important role in the repair function by promoting the survival and cell proliferation in damaged area. Simultaneously, the decreased inflammatory factors could reduce immunoresponse and cell apoptosis. This study may provide a new therapeutic strategy in the nerve injury.
Acknowledgments
The authors thank Dr BS, as there were a lot of theories and methods from his lab. Thanks to XX, JS, and QW for their contribution to this experiment.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Fuller GW, Ransom J, Mandrekar J, et al. Long-term survival following traumatic brain injury: a population-based parametric survival analysis. Neuroepidemiology 2016; 47:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salehpour F, Bazzazi AM, Aghazadeh J, et al. Can serum glucose level in early admission predict outcome in patients with severe head trauma? World Neurosurg 2016; 87:132–135. [DOI] [PubMed] [Google Scholar]
- 3.McCredie VA, Alali AS, Xiong W, et al. Timing of withdrawal of life-sustaining therapies in severe traumatic brain injury: impact on overall mortality. J Trauma Acute Care Surg 2016; 80:484–491. [DOI] [PubMed] [Google Scholar]
- 4.Noh YH, Yim YS, Kim DH, et al. Correlation between chemokines released from umbilical cord blood-derived mesenchymal stem cells and engraftment of hematopoietic stem cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Pediatr Hematol Oncol 2011; 28:682–690. [DOI] [PubMed] [Google Scholar]
- 5.Kim DS, Kim JH, Lee JK, et al. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev 2009; 18:511–519. [DOI] [PubMed] [Google Scholar]
- 6.Rafieemehr H, Kheyrandish M, Soleimani M. Neuroprotective effects of transplanted mesenchymal stromal cells-derived human umbilical cord blood neural progenitor cells in EAE. Iran J Allergy Asthma Immunol 2015; 14:596–604. [PubMed] [Google Scholar]
- 7.Bae JS, Han HS, Youn DH, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells 2007; 25:1307–1316. [DOI] [PubMed] [Google Scholar]
- 8.Bossolasco P, Cova L, Calzarossa C, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol 2005; 193:312–325. [DOI] [PubMed] [Google Scholar]
- 9.Cerri S, Greco R, Levandis G, et al. Intracarotid infusion of mesenchymal stem cells in an animal model of Parkinson's disease, focusing on cell distribution and neuroprotective and behavioral effects. Stem Cells Transl Med 2015; 4:1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Wang S, Cao W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol 2017; 326:8–14. [DOI] [PubMed] [Google Scholar]
- 11.Heo JS, Choi SM, Kim HO, et al. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience 2013; 238:305–318. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, Tabata Y, Gao JQ. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release 2012; 162:464–473. [DOI] [PubMed] [Google Scholar]
- 13.Uruma G, Hashimoto K, Abo M. A new method for evaluation of mild traumatic brain injury with neuropsychological impairment using statistical imaging analysis for Tc-ECD SPECT. Ann Nucl Med 2013; 27:187–202. [DOI] [PubMed] [Google Scholar]
- 14.Argenta LC, Zheng Z, Bryant A, et al. A new method for modulating traumatic brain injury with mechanical tissue resuscitation. Neurosurgery 2012; 70:1281–1295. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther 2011; 130:106–113. [DOI] [PubMed] [Google Scholar]
- 16.Udupa AN, Ravindra MN, Chandrika YR, et al. Comparison of pediatric perioperative risk assessment by ASA physical status and by NARCO-SS (neurological, airway, respiratory, cardiovascular, other-surgical severity) scores. Paediatr Anaesth 2015; 25:309–316. [DOI] [PubMed] [Google Scholar]
- 17.Cai XZ, Ni J, Li Z, et al. Regulation of UC-MSC on immune inflammatory thrombophilia in MRL/lpr Mice. [in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015; 23:1697–1701. [DOI] [PubMed] [Google Scholar]
- 18.Gu J, Lin CM, Gu W, et al. Immunomodulatory effect of UC-MSC on function of immunocytes of rats with collagen type II induced arthritis. [in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014; 22:166–170. [DOI] [PubMed] [Google Scholar]
- 19.Bieback K, Netsch P. Isolation, culture, and characterization of human umbilical cord blood-derived mesenchymal stromal cells. Methods Mol Biol 2016; 1416:245–258. [DOI] [PubMed] [Google Scholar]
- 20.Barilani M, Lavazza C, Viganò M, et al. Dissection of the cord blood stromal component reveals predictive parameters for culture outcome. Stem Cells Dev 2015; 24:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham PV, Vu NB, Pham VM, et al. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med 2014; 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boltze J, Schmidt UR, Reich DM, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant 2012; 21:1199–1211. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, McHugh J, Tork C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One 2007; 2:e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronig M, Walter M, Drendel V, et al. Cell type specific gene expression analysis of prostate needle biopsies resolves tumor tissue heterogeneity. Oncotarget 2015; 6:1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Li J, Sheng W, et al. Astrocyte-like cells differentiated from a novel population of CD45-positive cells in adult human peripheral blood. Cell Biol Int 2015; 39:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Wang S, Cao W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol 2018; 326:8–14. [DOI] [PubMed] [Google Scholar]
- 27.Wei N, Yu SP, Gu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 2013; 22:977–991. [DOI] [PubMed] [Google Scholar]
- 28.Danielyan L, Schäfer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res 2011; 14:3–16. [DOI] [PubMed] [Google Scholar]
- 29.Spielman LJ, Gibson DL, Klegeris A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur J Cell Biol 2017; 96:240–253. [DOI] [PubMed] [Google Scholar]
- 30.Benito C, Davis CM, Gomez-Sanchez JA, et al. STAT3 controls the long-term survival and phenotype of repair schwann cells during nerve regeneration. J Neurosci 2017; 37:4255–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortazavi Y, Sheikhsaran F, Khamisipour GK, et al. The evaluation of nerve growth factor over expression on neural lineage specific genes in human mesenchymal stem cells. Cell J 2016; 18:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano S, Uchida K, Miyagi M, et al. Nerve growth factor regulation by TNF-alpha and IL-1beta in synovial macrophages and fibroblasts in osteoarthritic mice. J Immunol Res 2016; 2016:5706359. [DOI] [PMC free article] [PubMed] [Google Scholar]


