Supplemental Digital Content is available in the text.
Keywords: critical care, musculoskeletal, physiotherapy, shoulder impairment, upper limb dysfunction
Abstract
Objectives:
Identify the prevalence of shoulder impairment in ICU survivors within 6 months of discharge from ICU. Evaluate the impact of shoulder impairment on upper limb functional status in patients treated on an ICU. Identify risk factors for the development of shoulder impairment.
Design:
Prospective cohort study.
Setting:
A tertiary care medical-surgical-trauma ICU at a U.K. hospital over 18 months, with a further 6-month follow-up after hospital discharge.
Subjects:
Adult patients with an ICU length of stay of greater than 72 hours with no preexisting or new neurologic or traumatic upper limb injury.
Interventions:
None.
Measurements and Main Results:
Patients underwent targeted shoulder assessments (pain, range of movement, Constant-Murley Score, shortened version of the disabilities of the arm, shoulder, and hand [DASH] score [QuickDASH] score) at hospital discharge, 3 and 6 months after hospital discharge. Assessments were undertaken on 96 patients, with 62 patients attending follow-up at 3 months and 61 patients at 6 months. Multivariate regression analysis was used to investigate risk factors for shoulder impairment. ICU-related shoulder impairment was present in 67% of patients at 6 months following discharge from hospital. Upper limb dysfunction occurred in 46%, with 16% having severe dysfunction (equivalent to shoulder dislocation). We were unable to identify specific risk factors for shoulder impairment.
Conclusions:
Shoulder impairment is a highly prevalent potential source of disability in ICU survivors. This persists at 6 months after discharge with a significant impact on upper limb function. More research is needed into potential mechanisms underlying shoulder impairment and potential targeted interventions to reduce the prevalence.
Survivors of ICU admission frequently experience long-term physical impairment and decreased health-related quality of life (HRQOL) (1, 2).The rate of unemployment following critical illness is very high, with approximately one third of patients not returning to work within 5 years of ICU discharge (3). Furthermore, survivors of critical illness in the United Kingdom commonly have a significant care requirement after discharge from hospital (4).
Shoulder impairment is a potentially serious musculoskeletal complication of ICU admission and is likely to contribute to poor physical function in survivors. Shoulder pain and reduced range of movement (ROM) are a significant problem in the general population with reported rates of frozen shoulder of 2–8% (5). Frozen shoulder is associated with other patient populations such as chronic obstructive pulmonary disease, ischemic heart disease, diabetes mellitus, and trauma (5)—all of which are frequently associated with ICU admission. Immobility is also associated with frozen shoulder (6). In some specific populations frequently admitted to ICU (e.g., spinal cord injury [SCI] and cerebral vascular accident [CVA]), a high prevalence of shoulder impairment has been reported (7, 8).
Where shoulder impairment has been found in ICU survivors, rates vary from 5% to 80% (1, 9–11). Limitations in physical function in ICU survivors have often been attributed to ICU-acquired weakness (ICUAW) (12, 13). However, long-term functional impairment in ICU survivors is likely to be multifactorial, and other musculoskeletal complications including joint pain and contractures may contribute (1, 9, 10). Two previous studies identified risk factors for the development of shoulder pain or reduced ROM (9, 10) in ICU patients but not shoulder impairment in general. Herridge et al (14) also reported bilateral frozen shoulder and limited shoulder ROM in patients attending their post-ICU disability program (RECOVER) but did not comment on the prevalence.
To date, no study has performed a longitudinal investigation of the prevalence and functional impact of ICU-related shoulder impairment. Such research is needed to help inform survivors and their families, identify at-risk populations, and tailor interventions to prevent shoulder impairment after ICU admission. Therefore, the aims of the study were 1) to identify the prevalence of shoulder impairment longitudinally in adult ICU survivors up to 6 months following discharge from hospital, 2) to evaluate the impact of shoulder impairment on upper limb function following discharge, and 3) to undertake a preliminary investigation of risk factors for the development of shoulder impairment.
METHODS
The study protocol was reviewed by the Oxford University Hospitals National Health Service Foundation Trust Research and Development department, and the requirement for ethical approval was waived.
All consecutive patients over the age of 18 admitted to a general ICU in an university teaching hospital between February 2013 and September 2014 with an ICU length of stay (LOS) of greater than 72 hours were assessed for inclusion to the study. Patients were excluded if they were unable to give informed consent to assessment, had an acute upper limb injury, SCI, or hemiplegia due to upper motor neuron injury/comorbidity, or had a palliative diagnosis/treatment pathway.
Data were collected by the physiotherapy team as part of the patients’ routine daily review. Risk factors previously identified as potentially contributing to the presence of decreased shoulder ROM, pain, or frozen shoulder (9, 10, 15) were collected in addition to other potential critical care–associated risks (Supplemental Tables 1 and 2, Supplemental Digital Content 1, http://links.lww.com/CCM/D902).
Shoulder Assessments
All patients undertook routine critical care rehabilitation while on ICU. Following discharge from ICU, patients underwent shoulder assessments immediately prior to discharge from hospital, at 3 months and at 6 months after discharge from hospital. Shoulder ROM and pain were assessed using a standard goniometer and the Visual Analogue Score, respectively. All patients also underwent a cervical spine assessment to rule out any referred symptoms (16). In addition, at 3 and 6 months after hospital discharge, the Constant-Murley Score (CMS) and the shortened version of the disabilities of the arm, shoulder, and hand (DASH) score (QuickDASH) were collected (17, 18). Further details are available in Description 1, 2, and 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/D902).
All patients presenting with shoulder impairment at 3 months after hospital discharge were given basic shoulder ROM exercises as appropriate and advised to attend their general practitioner. Patients were given the option of being referred to orthopedic services if shoulder impairment persisted at 6 months.
ICU-Related Shoulder Impairment and Upper Limb Function
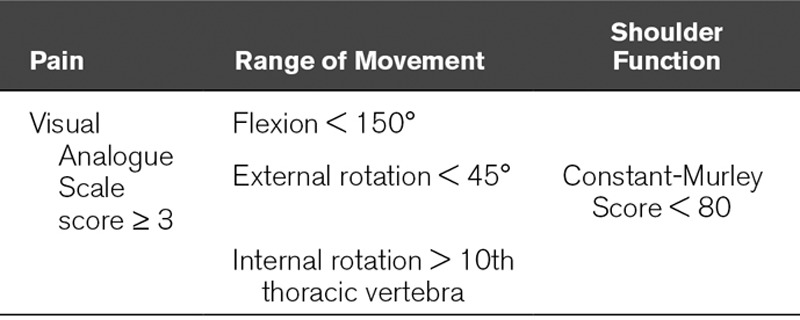
A definition of ICU-related shoulder impairment was developed for the purposes of this study. ICU-related shoulder impairment was deemed to be present if any of the measurements seen in Table 1 were achieved. Upper limb dysfunction was deemed present with a QuickDASH score of greater than or equal to 16, and severe dysfunction a score of greater than or equal to 40 (19–21). Further details are included in Description 4(Supplemental Digital Content 1, http://links.lww.com/CCM/D902)
TABLE 1.
Definition of Shoulder Impairment
Statistical Analysis
All statistical analyses were performed using SPSS Version 22.0 (IBM Corp., Armonk, NY). Prevalence of shoulder impairment and impact on upper limb function were presented as number of patients and percentages. Categorical variables were also presented as number of patients and percentages. Continuous variables that were normally distributed were presented as means and sds, with medians and interquartile ranges (IQRs) presented where data were not normally distributed. Univariate analyses were undertaken where an independent samples t test was used for normally distributed continuous variables and the Mann-Whitney U test for ordinal variables and not normally distributed continuous variables. Nominal variables were analyzed using the chi-square test for association, except where cells had a count of less than five where Fisher exact test was used. Adjusted odds ratio and the 95% CIs were calculated where possible for each risk factor. Variables reaching a statistical significance of p value of less than 0.15 in the univariate analysis were included in the multivariate analysis to assess for independent association with shoulder impairment (22). Multivariate analysis was performed using binomial logistic regression analysis for overall prevalence of shoulder dysfunction and deemed statistically significant at p value of less than 0.05.
RESULTS
Over the 18-month recruitment period, 330 patients had an ICU LOS greater than 3 days. One-hundred thirty-nine (42%) were judged eligible for recruitment. Ninety-six eligible patients (69%) were recruited and underwent at least one shoulder assessment. Study recruitment and retention, with reasons for exclusion, are outlined in Figure 1. Of patients recruited, the follow-up rate at 6 months was 64% (61/96), representing 44% (61/139) of eligible patients. Only 26% underwent assessment prior to hospital discharge due to difficulties in scheduling assessments around existing clinical interventions. Patient demographic characteristics are presented in Table 2.
Figure 1.
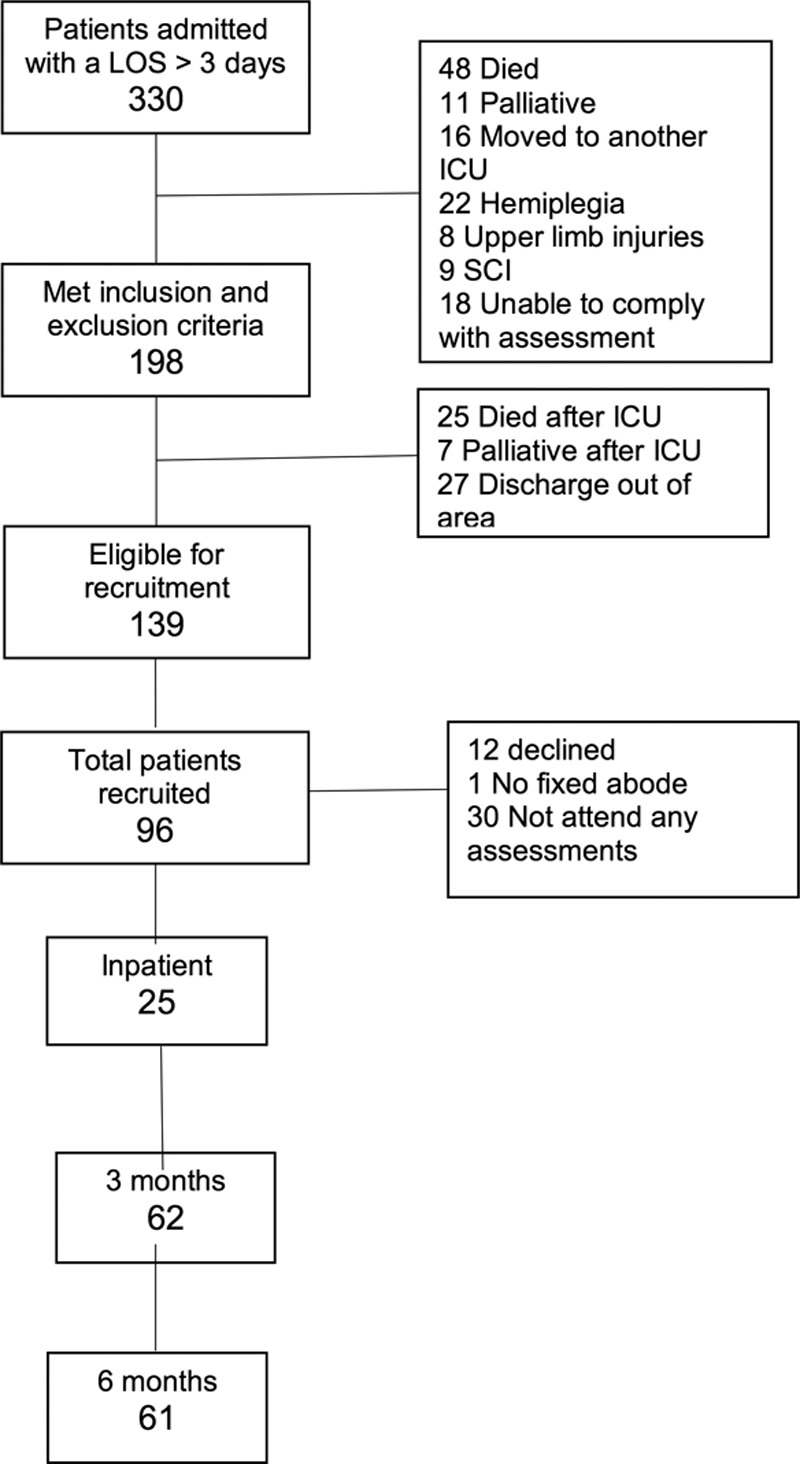
Study flow diagram for recruitment. LOS = length of stay, SCI = spinal cord injury.
TABLE 2.
Demographic Characteristics of the Participants
ICU- Related Shoulder Impairment Was Highly Prevalent up to 6 Months After Hospital Discharge
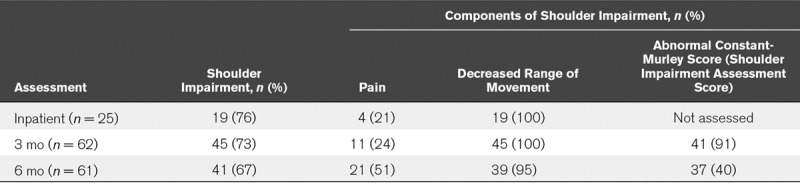
The overall prevalence of shoulder impairment among ICU survivors assessed at 3 and 6 months was 45 of 62 (73%) and 41 of 61 (67%), respectively. This was consistent with the prevalence of 76% (19/25) seen in the smaller number of patients assessed prior to hospital discharge. The prevalence of shoulder impairment and the different components assessed as part of the definition are presented in Table 3. Of the patients presenting with shoulder impairment, 100% had decreased shoulder ROM at inpatient assessment (19/19) and at 3 months (45/45), decreasing to 95% (39/41) at 6 months. The proportion of patients with shoulder impairment presenting with shoulder pain was similar at inpatient (4/19; 21%) and 3 months (11/45, 24%); however, this more than doubled at 6 months (21/41; 51%). Of those patients with shoulder impairment, the majority had a bilateral presentation, constituting 31 of 41 patients (76%) at 6 months. Of the 45 patients with shoulder impairment at 6 months who were given the option of referral to orthopedic services, seven underwent follow-up imaging of the shoulder. Common pathology reported were bursitis, tendonitis, tendinopathy, and subluxation of the humeral head. Further details of the imaging reports are available in Supplemental Table 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/D902).
TABLE 3.
Prevalence of Shoulder Impairment and Its Separate Components
Upper Limb Dysfunction Was Present in Patients With ICU-Related Shoulder Impairment
The proportion of patients presenting with upper limb dysfunction was similar at 3 and 6 months, 48% (30/62) and 46% (28/61), respectively. Of the patients assessed at 6 months, 16% (10/62) presented with severe upper limb dysfunction (Table 4). At 6 months, upper limb dysfunction was significantly worse (p < 0.001) in those with shoulder impairment (median QuickDASH, 25; IQR, 12-38) compared with those without (median QuickDASH, 2; IQR, 1-3).
TABLE 4.
Upper Limb Dysfunction as Measured by the QuickDASH

ICU-Related Shoulder Impairment Was Not Associated With Any of the Risk Factors Analyzed
Participants with shoulder impairment were significantly older (66 vs 57 median yr; p = 0.008) and weaker (Medical Research Council Sum Score 48 vs 52; p = 0.042). There was no significant difference in Acute Physiology and Chronic Health Evaluation (APACHE) score at admission (APACHE II 19.7 vs 16.8; p = 0.062). There was a statistically significant difference in the presence of infection (defined as microbiologically confirmed infection and/or treatment with antibiotics) (97% vs 83%; p = 0.028) and a past medical history of hypertension (28% vs 13%; p = 0.039) between participants with and without shoulder impairment. The results of the univariate analyses of variables that did and did not undergo multivariate analysis are available in Supplemental Tables 4 and 5 (Supplemental Digital Content 1, http://links.lww.com/CCM/D902). None of the predictor variables that underwent multivariate analysis reached statistical significance for independent association with shoulder impairment (Supplemental Table 6, Supplemental Digital Content 1, http://links.lww.com/CCM/D902).
DISCUSSION
To our knowledge, this is the first study to investigate ICU-related shoulder impairment in patients surviving to hospital discharge. Unlike studies investigating other musculoskeletal disorders in ICU survivors (1, 9, 10), ICU-related shoulder impairment was accurately and reliably identified using a standardized definition that encompassed several previously validated outcome measures (18, 23, 24). Our results demonstrate a high prevalence of shoulder impairment in the acute period following ICU discharge which persists up to 6 months following hospital discharge resulting in a significant adverse impact on upper limb function.
The prevalence of shoulder impairment found in this study was higher than most of the previous studies identifying this problem in ICU survivors (1, 9, 10). This is likely due to the use of a clear definition of shoulder impairment that encompassed pain, ROM, and the CMS, resulting in increased sensitivity. This study confirms the persistence of shoulder impairment at 3 and 6 months following hospital discharge. Shoulder impairment was also a bilateral presentation in over 75% of patients. These novel findings confirm that shoulder impairment following critical illness is not simply a short-term problem but rather an impairment that persists in patients months following hospital discharge.
There was a high prevalence of severe upper limb dysfunction in patients with shoulder impairment that persisted following ICU discharge. At 6 months, 16% of patients had upper limb dysfunction severe enough to have a debilitating effect on everyday tasks such as washing and dressing and likely to prevent return to work (19). When combined with the finding that over 50% of patients presenting with persisting shoulder impairment had moderate or severe pain, it is clear that shoulder impairment is a cause of significant morbidity in ICU survivors and is likely to limit longer term functional recovery.
The high rates of poor physical function scores in HRQOL outcomes and high levels of unemployment in ICU survivors have been increasingly reported (3, 14, 25, 26) However, to date, there has been little discussion as to the specific reasons for poor physical function. Given the prevalence and severity of upper limb dysfunction demonstrated in our study, it is reasonable to consider shoulder impairment as a likely contributory cause.
The cause of shoulder impairment in ICU survivors is likely to be multifactorial. Although several variables (age, weakness, infection, and hypertension) were associated with shoulder impairment on univariate analysis in this study, we were unable to identify any risk factors that were independently associated with shoulder impairment on multivariate analysis. Other patient populations have highlighted potential risk factors that may be relevant to the ICU population however. Immobilization of the shoulder is associated with joint contracture and frozen shoulder, the effects of which are exacerbated in the elderly (5, 27, 28). Patients with diabetes have an increased risk of developing frozen shoulder compared with the general population (29, 30). It is unclear if this increased risk extends to patients with abnormal blood glucose levels in the absence of diabetes as commonly seen in ICU. The widespread inflammatory changes seen in critical illness and infection are similar to the local inflammatory process present in frozen shoulder (31).
In this study, patients who developed shoulder impairment were significantly weaker than those who did not. The rate of muscle mass loss demonstrated in ICU patients can be rapid and severe (32). It is likely that this puts the glenohumeral joint (GHJ) at risk of impairment as much of the GHJ stability arises from dynamic muscle stabilizers. Weakness and atrophy of the rotator cuff group will result in displacement of the humeral head and shoulder dysfunction. This is evident in patients with hemiplegia following CVA (33). In the upper limb, the presentation of ICUAW can mimic the paresis seen in hemiplegia. A comparable loss of GHJ stability may also occur with prolonged use of neuromuscular blocking agents (NMBAs). The negative impact of weakness and joint immobility on shoulder dysfunction in ICU survivors suggest that early rehabilitation may be protective. This was not obviously the case in our study as participants with shoulder dysfunction started rehabilitation a day earlier than participants without shoulder dysfunction (Supplemental Table 4, Supplemental Digital Content 1, http://links.lww.com/CCM/D902). However, it is likely that these patients spent more time in an upright position during rehabilitation and mobilization with little emphasis on maintaining GHJ position as previously highlighted. Early rehabilitation with an emphasis on GHJ stability may be protective and warrants further investigation.
There is an increasing body of evidence surrounding early rehabilitation in ICU but little specific detail of the content of the rehabilitation practices (34). The high prevalence of shoulder impairment has several implications for rehabilitation and mobilization in ICU. Healthcare professionals should have an increased awareness of GHJ position when handling and positioning patients to ensure that repetitive joint subluxation is avoided. Importantly, early rehabilitation of the upper limb in patients with severe ICUAW should mirror the rehabilitation provided to hemiplegic patients, with an increased focus on GHJ stability prior to wide ranging movements.
The provision of rehabilitation for ICU survivors after they leave hospital is variable (35) and rarely focused on specific musculoskeletal disorders. Given the high prevalence and persistence of shoulder impairment and upper limb dysfunction in this study, it seems likely that significant shoulder impairment is currently both underrecognized and undertreated. An increased awareness of this problem among healthcare professionals in contact with ICU survivors after hospital discharge could improve their access to rehabilitation and musculoskeletal services.
Our study has a number of limitations. Participants were selected from a single center general adult ICU using a consecutive sampling method. As such, the findings may not be generalizable to other ICU populations. However, patients in this study are unlikely to be fundamentally different to ICU survivors in general as the patient characteristics in our study (age, APACHE score, comorbidities) were similar to previous studies identifying shoulder impairment in ICU survivors and other longitudinal follow-up studies (1, 4, 9, 10, 25, 36).
Although the overall sample size is comparable with other single-center follow-up studies of ICU survivors, the number of participants presenting with several of the variables (e.g., diabetes, use of NMBA) analyzed for association was low. Ten of the variables that underwent univariate analysis for association with shoulder impairment presented in 15 participants or less, with several not present in participants without shoulder impairment.
One of the major difficulties with a follow-up study of ICU survivors is the loss to follow-up rate. In this study, the loss to follow-up rate at 6 months after hospital discharge was 36%. This is similar, or better, than the reported rates in other longitudinal ICU follow-up studies (4, 37).
CONCLUSIONS
In conclusion, to our knowledge, this is the first cohort study specifically investigating the prevalence and impact of shoulder impairment in ICU survivors. Our results demonstrate a high prevalence of bilateral shoulder impairment persisting in patients at least 6 months after discharge. The prevalence and severity of upper limb dysfunction seen in our study demonstrates a potential source of long-term functional limitation in ICU survivors. Further studies will focus on interventions for the prevention and treatment of ICU-related shoulder impairment.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Physiotherapy and Critical Care departments at the John Radcliffe Hospital, the Statistics department at the University of Oxford, and the advice of Christine Carpenter, Toby Thomas, and George Hadjipavlou.
Supplementary Material
Footnotes
Drs. McKechnie and Igo contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Mr. Gustafson’s institution received funding from the Intensive Care Foundation (United Kingdom). Dr. Watkinson’s institution received funding from Drayson Health, National Institute for Health Research Biomedical Research Centre, Oxford, United Kingdom, and Innovate UK, and he disclosed that he has developed an electronic observations application for which Drayson Health has purchased a sole license. The company has a research agreement with the University of Oxford and may in the future pay him personal fees. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group: Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364:1293–1304. [DOI] [PubMed] [Google Scholar]
- 2.Kamdar BB, Huang M, Dinglas VD, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network: Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Respir Crit Care Med 2017; 196:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamdar BB, Sepulveda KA, Chong A, et al. Return to work and lost earnings after acute respiratory distress syndrome: A 5-year prospective, longitudinal study of long-term survivors. Thorax 2018; 73:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths J, Hatch RA, Bishop J, et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: A 12-month follow-up study. Crit Care 2013; 17:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson CM, Seah KT, Chee YH, et al. Frozen shoulder. J Bone Joint Surg Br 2012; 94:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Hand GC, Athanasou NA, Matthews T, et al. The pathology of frozen shoulder. J Bone Joint Surg Br 2007; 89:928–932. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao JV, Klopfstein J, Turpin R.Prevalence and impact of wrist and shoulder pain in patients with spinal cord injury. J Spinal Cord Med 1995; 18:9–13. [DOI] [PubMed] [Google Scholar]
- 8.Lindgren I, Jönsson AC, Norrving B, et al. Shoulder pain after stroke: A prospective population-based study. Stroke 2007; 38:343–348. [DOI] [PubMed] [Google Scholar]
- 9.Clavet H, Hébert PC, Fergusson D, et al. Joint contracture following prolonged stay in the intensive care unit. CMAJ 2008; 178:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battle CE, Lovett S, Hutchings H.Chronic pain in survivors of critical illness: A retrospective analysis of incidence and risk factors. Crit Care 2013; 17:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson O.The incidence of shoulder dysfunction in intensive care survivors. Intensive Care Med 2012; 38:S91–S91. [Google Scholar]
- 12.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit Care Med 2014; 42:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, et al. Impact of ICU-acquired weakness on post-ICU physical functioning: A follow-up study. Crit Care 2015; 19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016; 42:725–738. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth CT.Frozen shoulder. Phys Ther 1986; 66:1878–1883. [DOI] [PubMed] [Google Scholar]
- 16.Bullock MP, Foster NE, Wright CC.Shoulder impingement: The effect of sitting posture on shoulder pain and range of motion. Man Ther 2005; 10:28–37. [DOI] [PubMed] [Google Scholar]
- 17.Kolber MJ, Salamh PA, Hanney WJ, et al. Clinimetric evaluation of the disabilities of the arm, shoulder, and hand (DASH) and Quick DASH questionnaires for patients with shoulder disorders. Phys Ther Rev 2014; 19:163–73. [Google Scholar]
- 18.Rocourt MH, Radlinger L, Kalberer F, et al. Evaluation of intratester and intertester reliability of the Constant-Murley shoulder assessment. J Shoulder Elbow Surg 2008; 17:364–369. [DOI] [PubMed] [Google Scholar]
- 19.Angst F, Schwyzer HK, Aeschlimann A, et al. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res (Hoboken) 2011; 63(Suppl 11):S174–S188. [DOI] [PubMed] [Google Scholar]
- 20.Hunsaker FG, Cioffi DA, Amadio PC, et al. The American academy of orthopaedic surgeons outcomes instruments: Normative values from the general population. J Bone Joint Surg Am 2002; 84-A:208–215. [DOI] [PubMed] [Google Scholar]
- 21.Aasheim T, Finsen V.The DASH and the QuickDASH instruments. Normative values in the general population in Norway. J Hand Surg Eur Vol 2014; 39:140–144. [DOI] [PubMed] [Google Scholar]
- 22.Sauerbrei W, Meier-Hirmer C, Benner A, et al. Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Comput Stat Data Anal 2006; 50:3464–85. [Google Scholar]
- 23.Bijur PE, Silver W, Gallagher EJ.Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 2001; 8:1153–1157. [DOI] [PubMed] [Google Scholar]
- 24.Mullaney MJ, McHugh MP, Johnson CP, et al. Reliability of shoulder range of motion comparing a goniometer to a digital level. Physiother Theory Pract 2010; 26:327–333. [DOI] [PubMed] [Google Scholar]
- 25.Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: A cohort study. Crit Care 2010; 14:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgson CL, Udy AA, Bailey M, et al. The impact of disability in survivors of critical illness. Intensive Care Med 2017; 43:992–1001. [DOI] [PubMed] [Google Scholar]
- 27.van de Laar S, van der Zwaal P.Management of the frozen shoulder. Orthop Res Rev 2014; 6:81–90. [Google Scholar]
- 28.Roy JS, Macdermid JC, Boyd KU, et al. Rotational strength, range of motion, and function in people with unaffected shoulders from various stages of life. Sports Med Arthrosc Rehabil Ther Technol 2009; 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anton HA.Frozen shoulder. Can Fam Physician 1993; 39:1773–1778. [PMC free article] [PubMed] [Google Scholar]
- 30.Tighe CB, Oakley WS., JrThe prevalence of a diabetic condition and adhesive capsulitis of the shoulder. South Med J 2008; 101:591–595. [DOI] [PubMed] [Google Scholar]
- 31.Loew M, Heichel TO, Lehner B.Intraarticular lesions in primary frozen shoulder after manipulation under general anesthesia. J Shoulder Elbow Surg 2005; 14:16–21. [DOI] [PubMed] [Google Scholar]
- 32.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013; 310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 33.Carr EK, Kenney FD.Positioning of the stroke patient: A review of the literature. Int J Nurs Stud 1992; 29:355–369. [DOI] [PubMed] [Google Scholar]
- 34.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009; 373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly B, Salisbury L, O’Neill B, et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev 2015; (6):CD008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vesz PS, Costanzi M, Stolnik D, et al. Functional and psychological features immediately after discharge from an intensive care unit: Prospective cohort study. Rev Bras Ter Intensiva 2013; 25:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eddleston JM, White P, Guthrie E.Survival, morbidity, and quality of life after discharge from intensive care. Crit Care Med 2000; 28:2293–2299. [DOI] [PubMed] [Google Scholar]


