Abstract
Yu et al. (Reports, 10 March 2017, p. 1072) state that contagious itch occurs in mice based on imitative scratching in normal mice observing excessive scratching in genetically modified demonstrator mice. However, despite employing multiple behavioral analysis approaches, we were unable to extend these findings to normal mice observing the well-established histamine model of acute itch in demonstrator mice.
Itch contagion is well known to occur in humans and nonhuman primates observing normal scratching behavior (1–6). Using a newly developed chronic itch mouse model characterized by excessive scratching, Yu et al. (7) recently reported the first evidence for contagious itch in mice observing these BRAFNaV1.8+/+ demonstrators (7, 8). In an independent line of research using the well-characterized histamine model, our group has also investigated contagious itch in mice observing more moderate levels of scratching. Despite employing three different behavioral quantification methods to assess contagious itch (Fig. 1), including the imitative scratching approach described by Yu et al., we were unable to find evidence for contagious itch in mice.
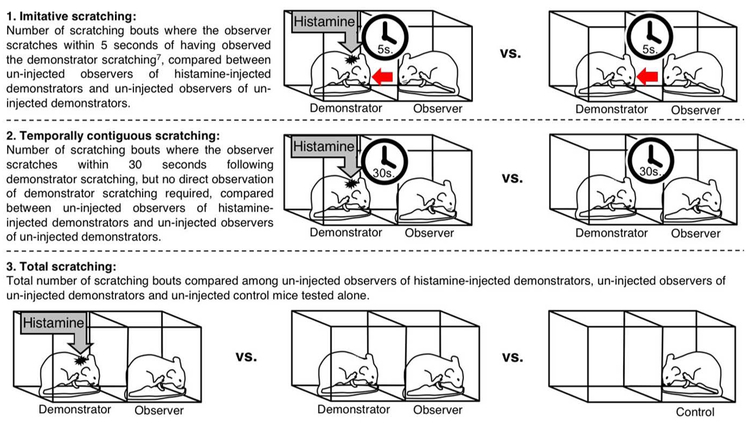
Fig. 1.
Three methods of assessing contagious itch in mice.
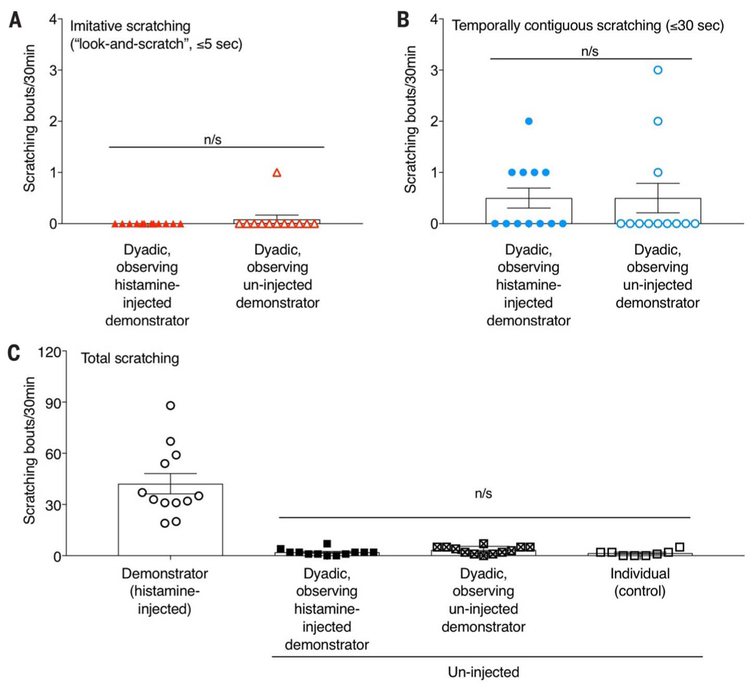
Using the identical imitative “look-and-scratch” definition described in (7), none of 12 uninjected observers of histamine-injected demonstrators exhibited scratching behavior within 5 s of looking in the direction of the scratching demonstrator (Fig. 2A). Similarly, only 1 of the 12 uninjected mice observing an uninjected demonstrator exhibited an imitative “look-and-scratch” behavior over the 30-min observation period (Fig. 2A). No significant difference between groups was found [χ2 = 1.04, 1(24); P = 0.307]. Even when using a far less stringent definition of temporally contiguous scratching behavior (i.e., within 30 s.), we did not observe contagious scratching in un-injected mice observing histamine-injected mice (0.5 ± 0.2 temporally contiguous scratches) compared with those observing uninjected mice (0.5 ± 0.3) [χ 2 = 0.75, 1(24); P = 0.387]. Finally, when examining the total number of scratching bouts during the entire 30-min observation period, the scratching behavior of uninjected observers next to histamine-injected demonstrators (2.0 ± 0.6 scratching bouts) was indistinguishable from either that of uninjected observers tested next to uninjected demonstrators (3.3 ± 0.6) or mice tested alone (1.5 ± 0.6) [H(3) = 4.372; P = 0.112] (Fig. 2C). Correlational analysis showed that the total number of scratching bouts in uninjected observers was not associated with that of histamine-injected demonstrators (r = −0.018; P = 0.958).
Fig. 2. No evidence for contagious itch in mice.
(A to C) In no case was scratching behavior in uninjected mice observing histamine-injected demonstrators significantly greater than that of uninjected mice observing uninjected demonstrators, indicating that contagious itch does not appear to occur in mice. Chi-square analysis was used for imitative (A) and temporally contiguous (B) scratching, whereas the Kruskal-Wallis H test was used to compare the three uninjected conditions in (C). P < 0.05 was considered significant in all cases. n/s, nonsignificant comparisons.
Despite using mice of the same strain, age, sex, and supplier as in (7), we found no evidence for itch contagion in mice using either their definition of imitative scratching or other, less stringent, definitions. Nonetheless, a few methodological differences between the studies should be mentioned. For example, the mice used in (7) were tested in their home cages, whereas we used an observation box to which the mice were previously habituated. Also, whereas a 60-min test duration was used in (7), we used a 30-min duration due to the acute nature of histamine-induced scratching behavior (9, 10). However, it should be noted that Yu et al. reported that most scratching occurred before the 30-min mark and no scratching occurred after the 40-min mark. Finally, it is possible that differences between the more moderate histamine-induced itch and the pathological BRAFNaV1.8 chronic itch phenotype in the demonstrator mice could influence the degree of scratching in the observer. However, we found no association between the levels of scratching in the histamine-injected demonstrator and the uninjected observer, suggesting that contagious itch should not be a function of scratching intensity. Taken together, although our finding does not necessarily demonstrate irreproducibility of contagious itch in mice, it clearly limits the generalizability of this phenomenon across itch models.
In all, 44 adult male C57BL/6 mice (The Jackson Laboratory) between 8 and 10 weeks old were used. Mice were housed two per cage in a light-, humidity-, and temperature-controlled room with ad libitum access to water and food. Upon arrival, mice were visually inspected for any clinically relevant dermatological conditions. All protocols were reviewed and approved by the National Institute of Neurological Disorders and Stroke/National Institute on Deafness and other Communication Disorders Animal Care and Use Committee (NINDS/NIDCD ACUC). Experiments were in accordance with the NINDS/NIDCD ACUC and the International Association for the Study of Pain (IASP) guidelines for the care and use of experimental animals.
Before testing, mice were habituated to the room in which the behavioral recording took place for 30 min per day for 3 days before the experiment. Three experimental conditions were included (Fig. 1): 1) An uninjected observer (n = 12) adjacent to a histamine-injected demonstrator (n =12); 2) two uninjected mice adjacent to each other (n = 12); and 3) an uninjected control mouse tested alone (n = 8). In histamine-injected mice, histamine was injected subcutaneously in the nape of the neck with 500 mg histamine dissolved in 0.05 ml saline. Immediately after injection, mice were placed in the two-chambered transparent acrylic observation box measuring 10 by 10 cm per chamber. The test chambers were separated by a transparent acrylic wall allowing visual contact between mice. Transmission of auditory and ol-factory signals was also possible through the wire mesh floor. Behavioral testing occurred in a well-lit, temperature-controlled room (21°C). All animal handling, behavioral testing, and histamine injections were performed by trained experimenters (L.B. and M.H.P.).
Mouse behavior was recorded for a total of 30 min using a digital camera facing the test box. Videos were scored offline for scratching behavior. A scratching bout in histamine-injected mice was defined as lifting of either hind limb to scratch and replacing the paw onto the floor, regardless of the number of scratching strokes that occurred between the first lift and final lowering of the hind limb. A scratching bout in uninjected mice was defined as lifting of either hind limb to scratch at the face, neck, or side and replacing the paw onto the floor, regardless of the number of scratching strokes that occurred between the first lift and final lowering of the hind limb. Three different analysis strategies were used to assess contagious itch in uninjected observers: 1) bouts of imitative scratching, identical to the “look-and-scratch” method described in (7), where the observer scratches within 5 s of having paused (~1 s) and looked toward the demonstrator scratching; 2) temporally contiguous scratching, where the observer scratches within 30 s of the demonstrator scratching, without regard for “look-and-scratch” behavior; and 3) total scratching bouts during the 30-min observation period (Fig. 1). Prism 7 was used for chi-square, Kruskal-Wallis, and Spearman correlation analyses. In all cases, P < 0.05 was considered significant.
ACKNOWLEDGMENTS
We thank Y. Q. Yu and coauthors for their willingness to engage in constructive discussion. We are also grateful to A. Chesler for his helpful comments on this manuscript. This research was supported by the Division of Intramural Research of the National Center for Complementary and Integrative Health, National Institutes of Health. The authors have no conflicts of interest to declare. All data are stored in NIH servers and will be made available upon request. J.L. and M.H.P. designed the study, performed experiments, analyzed data, and wrote the manuscript. L.A.L. designed the study, performed experiments, and edited the manuscript. L.B. performed experiments. M.C.B. designed the study and edited the manuscript.
REFERENCES AND NOTES
- 1.Papoiu AD, Wang H, Coghill RC, Chan YH, Yosipovitch G, Br. J. Dermatol 164, 1299–1303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd DM, Hall E, Hall S, McGlone FP, Br. J. Dermatol 168, 106–111 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Ogden J, Zoukas S, Psychol. Health Med 14, 695–704 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Holle H, Warne K, Seth AK, Critchley HD, Ward J, Proc. Natl. Acad. Sci. U.S.A 109, 19816–19821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feneran AN et al. , Acta Derm. Venereol 93, 27–29 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Nakayama K, J. Comp. Psychol 118, 20–24 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Yu YQ, Barry DM, Hao Y, Liu XT, Chen ZF, Science 355, 1072–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao ZQ et al. , J. Clin. Invest 123, 4769–4780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han N, Zu JY, Chai J, Clin. Exp. Dermatol 37, 290–295 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Wang X et al. , Neuron 78, 312–324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]